Abstract
Introduction
Common medications used to treat mild persistent asthma are glucocorticoids, leukotriene receptor antagonists and theophylline. The aim of the study was to evaluate monotherapy with either inhaled steroids, oral leukotriene receptor antagonist or theophylline in Egyptian children with mild persistent asthma by determining their clinical, laboratory and spirometric responses to treatment.
Material and methods
Thirty-nine mild asthmatic children between 8 and 13 years of age were included in the study. Patients were classified according to therapy received into four groups: oral leukotriene receptor antagonist (montelukast), inhaled corticosteroid (fluticasone propionate), sustained-release (SR) theophylline, and no treatment. Pulmonary function testing was performed at the start of therapy and 8 weeks later using spirometry. Eosinophil count and serum nitric oxide were estimated in the blood. Minitab statistical package was used for analysis of data.
Results
Follow-up after 8 weeks revealed significant improvement in FEV1% in groups 1 (p < 0.01) and 3 (p < 0.05), significant improvement in PEFR in groups 1 (p < 0.05) and 2 (p < 0.01), significant decline in serum NO levels in groups 1 (p < 0.05) and 2 (p < 0.05), as well as significant improvement in eosinophil count in groups 1, 2 and 3 (p < 0.01, < 0.001, < 0.01 respectively). There was a statistically significant positive correlation between the decline in serum NO and the decline in blood eosinophil % in group 2 (p < 0.05).
Conclusions
Inhaled corticosteroids and montelukast have a significant role in controlling the pulmonary functions and the inflammatory process in children with mild persistent asthma, although inhaled corticosteroids seem to yield a better response. Children with mild persistent asthma should receive a controller medication, and SR theophylline may be a good cost-benefit alternative for low socio-economic groups of patients.
Keywords: asthma, inhaled corticosteroids, montelukast, slow-release theophylline
Introduction
Asthma is a chronic inflammatory condition of the lung airways resulting in episodic airflow obstruction. This chronic inflammation heightens the airways’ hyper-responsiveness (AHR) to provocative exposures [1].
Asthma is a relatively common chronic disease, affecting 3% to 38% of children worldwide [2]. Moreover, it is the highest ranking chronic condition causing hospitalization as well as being the primary chronic illness resulting in school absences [3]. Throughout the world, asthma morbidity and mortality appear to be increasing [4, 5].
According to GINA guidelines for asthma therapy, the daily controller medication for mild persistent asthma is low dose inhaled glucocorticoid [6]. Other treatment options include sustained-release theophylline, chromone, and leukotriene modifiers.
Corticosteroids are the most potent and consistently effective long-term-control medication for asthma. Their broad action on the inflammatory process may account for their efficacy as preventive therapy. Their clinical effects include reduction in severity of symptoms, improvement in peak expiratory flow and spirometry, diminished airway hyper-responsiveness, prevention of exacerbations, and possibly the prevention of airway wall remodelling [7].
Leukotrienes are released by several types of cells and can cause bronchoconstriction and inflammation [8]. Leukotriene modifiers comprise two pharmacological classes of compounds: 5-lipoxygenase pathway inhibitors (e.g., zileuton), and leukotriene receptor antagonists (LTRAs), e.g., zafirlukast and montelukast [9]. Montelukast can be used for children as young as 6 months of age [10], whereas zafirlukast can be used only for children older than 7 years [11].
Leukotriene receptor antagonists (LTRAs) inhibit the effects of cysteinyl leukotrienes, which are chemical mediators of asthma. LTRAs competitively block leukotriene receptors on bronchial smooth muscle and elsewhere [12]. Thus, they exert anti-inflammatory properties and can reduce or prevent airway eosinophilia [13], in addition to their ability to attenuate AHR [14]. They can improve asthma symptoms and airway function, and reduce asthma exacerbations [15]. As a result, leukotriene antagonists have been acknowledged by some guidelines as acceptable first line long-term controller therapies for mild persistent asthma [7, 16].
Previous studies have shown that theophylline could be effective as monotherapy and as add-on treatment to inhaled or oral glucocorticosteroids in children older than 5 years. It is significantly more effective than placebo at controlling day and night symptoms and improving lung function. Maintenance treatment offers a marginal protective effect against exercise-induced bronchoconstriction. Sustained-release products are preferable for maintenance therapy, since they enable twice-daily dosing [17].
Monitoring of airway inflammation in bronchial asthma could be done by measurement of nitric oxide (NO), which is assessed directly by sputum examination, and indirectly by its measurement in peripheral blood [18].
Nitric oxide, a small molecule and a strong free radical, influences many aspects of pulmonary functions in healthy subjects and patients. It is synthesized from the amino acid L-arginine by nitric oxide synthase (NOS), which exists in three forms. After production, NO can be exhaled, metabolized to nitrite and nitrate, or interacts with superoxide to form peroxynitrite. Determination of NO itself is difficult because of its radical nature and very short half-life. Therefore, determination of the stable end products of the NO radical in the plasma such as nitrite and nitrate is the most frequently used method to measure the production of the NO radical [19].
The aim of the present study is to evaluate monotherapy with different drugs (inhaled steroids, oral leukotriene receptor antagonist or SR theophylline) in Egyptian children with mild persistent asthma by determining the clinical and laboratory responses as well as their effect on spirometric parameters.
Material and methods
Study design
A randomized parallel group study was conducted to compare the effect of anti-asthmatic monotherapy (inhaled corticosteroids [ICS], leukotriene receptor antagonists [LTRAs] and SR theophylline) on mild asthmatic Egyptian children.
The study included 39 asthmatic children (20 females and 19 males) between the ages of 8 and 13 years. They all completed an 8-week study period. All patients were diagnosed as having mild persistent asthma based on the classification of asthma severity according to GINA Guidelines (2009) [17] and were attending the Allergy Clinic of Children’s Hospital, Cairo University for routine visits. The selection was based on the following inclusion and exclusion criteria:
Inclusion criteria: The study group included children aged 8-13 years who were able to perform pulmonary function tests efficiently; had a history of mild persistent asthma; were healthy, non-smokers (active smoking); were not in acute exacerbation; did not receive any anti-asthmatic therapy before the study (1 month for steroids, 2 weeks for theophylline and 1 week for long acting β 2 agonist).
Exclusion criteria: children who had any respiratory disorder other than asthma; children who had symptoms and signs of upper respiratory tract infection within 3 weeks of the study.
The patients were randomly distributed into four groups:
Group 1: 11 patients (4 females and 7 males) were receiving a selective and orally active leukotriene receptor antagonist (montelukast sodium 5 mg per day chewable tablets once daily, at bedtime)
Group 2: 11 patients (6 females and 5 males) were receiving inhaled corticosteroids (fluticasone propionate, at a dose of 100 µg twice daily, supplied as an Evohaler, a pressurized inhalation suspension, delivering 50 µg per action as oral inhalation) for 8 weeks.
Group 3: 10 patients (7 females and 3 males) were receiving sustained-release theophylline (theophylline anhydrous, oral tablets in a dose of 15 mg/kg/day divided into two doses every 12 hours) for 8 weeks.
Group 4: 7 patients (3 females and 4 males) were receiving no regular medication for 8 weeks, and were considered as a control group.
Asthma was stable and considered clinically well controlled in all patients; treatment remained unchanged throughout the whole study period.
The following steps were performed for each child at the beginning of the study, and 8 weeks later.
Thorough medical history was taken with emphasis upon symptoms of asthma, precipitating factors, family history, and impact of asthma on patient and family.
Clinical examination included recording of vital signs, anthropometric measurements, and detailed physical examination
Pulmonary function testing was performed at the beginning of the study and 8 weeks later using a spirometer (Fukuda Denshi, Spirosift SP5000). At least three technically accepted manoeuvres were performed and the highest value was recorded.
Measurement of eosinophil count %: blood samples were collected at the first visit and 8 weeks later. Blood films were prepared from EDTA anticoagulated blood and then stained with Leishman stain. The blood films were examined and recorded as a percentage where eosinophils were counted in 100 white blood cells.
Measurement of serum nitric oxide: nitric oxide was assessed in the serum of patients at the onset of the study and 8 weeks later using Total NO/Nitrite/Nitrate Assay Kit, for the quantitative determination of nitric oxide concentration in serum, supplied by R&D systems Europe, Ltd. Abingdon, OX14 3NB United Kingdom.
Sample Preparation: blood samples were collected in plain tubes and then centrifuged for 15 min at 100 revolutions per min (rpm) to obtain serum. Serum aliquots were collected and stored at ≤ 20°C until assay was performed. Repeated freeze-thaw cycles were avoided. Prior to assay, serum samples were filtered through a 10 or 30 kDa molecular weight cut-off filter.
Written consent to participate in the study was obtained from every child’s care-taker according to guidelines approved by the ethical committee of the National Research Centre.
Statistical analysis
Minitab statistical package was used for analysis of data. All data were expressed as mean and standard deviation of the mean (mean ± SD). Different data were analysed by paired t-test. One-way analysis of variance (ANOVA) was performed to test the equality of the means of different treatments. Tukey’s method was used to compare all pairwise differences between level means. The relationship between studied parameters was assessed using Pearson’s linear correlation coefficient (r). A p value < 0.05 was considered significant.
Results
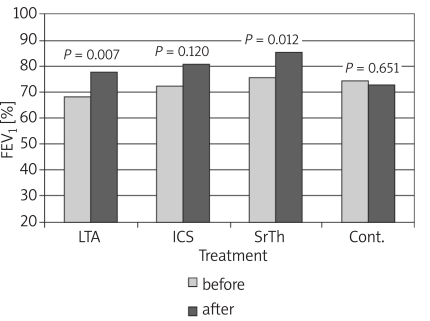
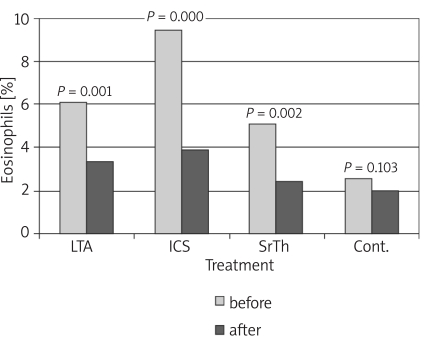
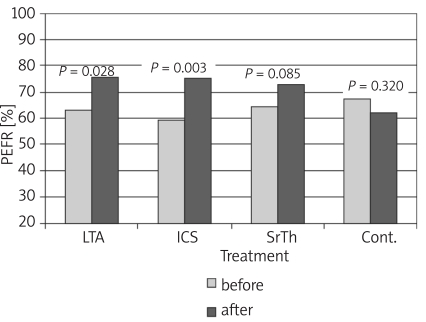
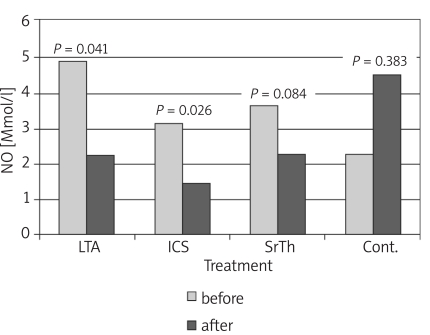
Table I shows the values of the different studied parameters in the four groups before and 8 weeks after treatment. Values show improvement in pulmonary functions and nitric oxide in patients receiving treatment by the different modalities. Figures 1-4 show the changes that occurred in each parameter, namely, FEV1%, PEFR%, NO and Eosin%, respectively, before and 8 weeks after treatment with a different modality. The improvement in FEV1% after treatment was statistically significant in groups 1 and 3 (p < 0.01, p < 0.05 respectively), whereas FEV1% became worse in group 4 (Figure 1). As regards the peak expiratory flow rate (PEFR), it showed improvement in the three groups, which reached statistical significance in groups 1 and 2 (p < 0.05, p < 0.01 respectively), whereas PEFR became worse in group 4 (Figure 2). The evaluation of mean serum levels of nitric oxide (NO) revealed a significant decrease in groups 1 and 2 after treatment (p < 0.05), while there was a non-significant decrease in group 3 (Figure 3). Patients in group 4 who received no treatment showed an increase in mean NO levels and worsening of their condition. As for the percentage of blood eosinophils (Eosin%), our results showed significant improvement after treatment in the first three groups (p < 0.01) (Figure 4). Table II shows the different correlations between the changes in NO versus the changes in FEV1, PEFR and Eosin% in the four studied groups, where there was a statistically significant (p = 0.014) positive correlation (r = 0.714) between the decline in the serum nitric oxide and the decline in the blood eosinophil % in group 2, which received inhaled fluticasone for 8 weeks.
Table I.
Comparative data of FEV %, PEFR%, serum nitric oxide (NO) and Eosin% at baseline and 8 weeks after treatment
| Group 1 (n = 11) (Montelukast) | Group 2 (n = 11) Fluticasone) | Group 3 (n = 10) (SR-Theophylline) | Group 4 (n = 7) (No medication) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 56 | Baseline | Day 56 | Baseline | Day 56 | Baseline | Day 56 | |
| ± SD | ± SD | ± SD | ± SD | ± SD | ± SD | ± SD | ± SD | |
| Age | 10.0 ±1.95 | 9.7 ±2.01 | 9.6 ±0.97 | 9.9 ±2.91 | ||||
| FEV1% | 68 ±10* | 78 ±13* | 72 ±15 | 81 ±11 | 76 ±9* | 86 ±11* | 75 ±14 | 73 ±21 |
| PEFR% | 63 ±17* | 76 ±11* | 59 ±15* | 75 ±15* | 65 ±18 | 73 ±11 | 67 ±19 | 62 ±17 |
| NO [μmol/l] | 4.9 ±3.9* | 2.2 ±2.1* | 3.2 ±2.5* | 1.4 ±1.7* | 3.7 ±2.4 | 2.3 ±2.7 | 2.4 ±3.6 | 4.5 ±5.2 |
| Eosin% | 6.0 ±2.0* | 3.0 ±1.0* | 9.0 ±4.0* | 4.0 ±3.0* | 5.0 ±3.0* | 2.0 ±2.0* | 3.0 ±2.0 | 2.0 ±1.0 |
* p = significant (p < 0.05) improvement in parameter after 8 weeks of treatment; for FEV % p = 0.007 and 0.012 (in groups 1 and 3 respectively for PEFR% p = 0.028 and 0.003 (in groups 1 and 2 respectively); for NO p = 0.041 and 0.026 (in groups 1 and 2 respectively); and for Eosin% p = 0.001, 0.000 and 0.002 (in groups 1, 2 and 3 respectively)
FEV1%–forced expiratory flow rate at first second as percentage of that predicted, PEFR%–peak expiratory flow rate as percentage of tha predicted, Eosin%–eosinophil count as percentage of eosinophils
Figure 1.
Changes in forced expiratory volume in 1st second (FEV1) before and after treatment in the different studied groups
LTA− leukotriene antagonist, ICS − inhaled corticosteroid, Sr-Th − theophylline, cont. − control
Figure 4.
Changes in blood eosinophils (%) before and after treatment in the different studied groups
Figure 2.
Changes in peak expiratory flow rate (PEFR) before and after treatment in the different studied groups
Figure 3.
Changes in serum nitric oxide (NO) level before and after treatment in the different studied groups
Table II.
Correlation of changes in serum nitric oxide versus changes in FEV1, PEFR and Eosin% in the four studied groups
| Group | Nitric oxide | Correlation coefficient (r) | p-value |
|---|---|---|---|
| 1 | FEV1% | −0.124 | 0.72 |
| PEFR% | 0.563 | 0.07 | |
| Eosin% | 0.287 | 0.39 | |
| 2 | FEV1% | 0.210 | 0.54 |
| PEFR% | −0.218 | 0.52 | |
| Eosin% | 0.714 | 0.014* | |
| 3 | FEV1% | 0.022 | 0.95 |
| PEFR% | −0.125 | 0.73 | |
| Eosin% | −0.219 | 0.54 | |
| 4 | FEV1% | −0.040 | 0.93 |
| PEFR% | 0.061 | 0.90 | |
| Eosin% | 0.213 | 0.65 |
significant p-value (< 0.05)
Group 1: receiving montelukast; Group 2: receiving fluticasone; Group 3: receiving sustained-release theophylline; Group 4: receiving no medication
FEV1% – forced expiratory flow rate at first second as percentage of that predicted, PEFR%-peak expiratory flow rate as percentage of that predicted, Eosin% – eosinophil count as percentage of eosinophils
Discussion
In the present work, different modes of monotherapy for mild persistent asthma were evaluated: namely a leukotriene receptor antagonist (montelukast), inhaled corticosteroid (ICS) (fluticasone), and SR theophylline. The control group consisted of a group of patients who did not receive any preventive medication during the study period.
The results of pulmonary function tests in the studied groups (Table I) showed that there was improvement in FEV1% after 8 weeks of treatment, which was statistically significant in groups 1 and 3 (Figure 1). As regards the peak expiratory flow rate (PEFR), improvement was statistically significant in groups 1 and 2 (Figure 2).
Published data comparing the effects of ICS versus montelukast on pulmonary functions are inconsistent. Concerning the improvement in FEV1%, our results are consistent with those of Mandeep et al. [20], who demonstrated marked improvement in FEV1 in those receiving montelukast compared to placebo, yet it was not superior to ICS.
As regards % PEFR change, similar results were obtained in different studies [21, 22], which revealed improvement in % PEFR in two groups of patients receiving oral montelukast and fluticasone inhaler for a period of 12 weeks with no significant difference between the groups. In a prospective 12-month observational analysis [23], and in a randomized double-blind 6-week study [24], similar results were obtained.
In contrast, a multicentre, randomized, double-blind study by Busse and Lemanske [25] concluded that treatment with fluticasone resulted in significantly greater improvement in FEV1, FVC and PEFR compared with montelukast. These results agree with other previous studies [26-28].
The evaluation of mean serum levels of nitric oxide (NO) in the present work revealed a significant decrease in groups 1 and 2 eight weeks after treatment (Figure 3). Patients in group 4 who received no treatment showed an increase in mean NO levels and worsening of their condition. Although measurement of NO can be performed directly by its estimation in exhaled air, due to technical problems encountered in this study, NO was measured in serum by an indirect method. Nitric oxide is synthesized mainly through NO synthase enzymes. In particular, NO derived from the inducible isoform of NOS (iNOS) seems to be implicated in inflammatory diseases such as asthma, where it is supposed to be a pro-inflammatory mediator with immunomodulatory effects [29]. Several studies have shown that the secreted pro-inflammatory cytokines lead to an increase of NO levels in exhaled air, plasma and serum of patients with asthma compared with healthy individuals [30-32].
The present results showed a non-significant (p = 0.06) decline in the mean NO levels in group 1 (montelukast) and group 2 (fluticasone); however, the lower mean level of NO attained with ICS could affirm their role as better controller medications. Ricciardolo et al. [29] postulated that asthma treatment with corticosteroids resulted in a reduction of expired NO levels due to both the reducing effects of steroids on the underlying airway inflammation in asthma and the inhibitory effects on iNOS expression itself. Since our main concern was to assess the effect of the studied drugs on the levels of NO, we referred to other publications confirming the decline in NO levels in exhaled air following treatment with inhaled steroids [33, 34].
As for the percentage of blood eosinophils (Eosin%), our results showed significant improvement in all studied patients receiving medications (Figure 4). Eosin% showed a statistically significant difference among the 4 groups at the end of the study period, where fluticasone was superior to montelukast and theophylline. This might be due to the mode of action of corticosteroids causing a reduction in eosinophil count in addition to their role as anti-inflammatory agents.
Our results were confirmed by another comparative study which demonstrated a reduction in blood eosinophils with both montelukast and fluticasone but with greater significant reduction with fluticasone, after 12-month therapy in children with mild persistent asthma [26].
On the other hand, a randomized, double-blind 6-week study [35] compared the effect of montelukast and inhaled beclomethasone on blood eosinophils, and concluded that eosinophils decreased with both modalities, but with more decline in favour of montelukast rather than inhaled beclomethasone.
In a study conducted by Hassan et al. [21], the mean absolute eosinophil count showed a significant reduction in both the montelukast group and in the inhaled steroid group, with no statistically significant difference between the two groups.
The results of the present study showed a significant positive correlation (p = 0.014; r = 0.714) between the decline in serum nitric oxide and the decline in blood eosinophils in group 2 patients who received inhaled fluticasone for 8 weeks (Table II).
In contrast, in a study by Jang and Choi [36], comparing the role of nitric oxide metabolites in induced sputum versus blood, they found no correlation between serum nitric oxide and eosinophils in asthmatic patients.
In conclusion, this study shows that both ICS (fluticasone) and LTA (montelukast) have a significant role in controlling the pulmonary functions and the inflammatory process in children with mild persistent asthma; however, ICS seems to yield a better response for most of the studied parameters. Eosinophil count follow-up has an important role in the evaluation of therapy. Mild persistent asthmatic children should receive a controller medication, and SR theophylline may be a good cost-benefit alternative for low socio-economic groups of patients.
Further studies including a larger sample size are required to validate the relationship between the pulmonary functions and nitric oxide, and also to assess the effect of the genetic background and gender as well as environmental factors of our country on the results achieved.
References
- 1.Liu AH, Spahn JD, Leung DYM. Childhood asthma: Allergic disorders. In: Kliegman R, Jenson H, Behrman R, editors. Nelson Textbook of Pediatrics. 17th. Pub. Elsevier Science; 2004. pp. 760–74. [Google Scholar]
- 2.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control (CDC) Forecasted state-specific estimates of self-reported asthma prevalence − United States, 1998. MMWR Morb Mortal Wkly Rep. 1998;47:1022–5. [PubMed] [Google Scholar]
- 4.Sears MR. Worldwide trends in asthma mortality. Bull Int Union Tuberc Lung Dis. 1991;66:79–83. [PubMed] [Google Scholar]
- 5.Woolcock AJ. Worldwide trends in asthma morbidity and mortality: Explanation of trends. Bull Int Union Tuberc Lung Dis. 1991;66:85–9. [PubMed] [Google Scholar]
- 6.Global Initiative For Asthma. A pocket guide for physicians and nurses ( Updated 2009). Based on the Global Strategy for Asthma Management and Prevention. pp. 9–16. Available from http://www.ginasthma.org .
- 7.Jayaram L, Pizzichini E, Lemie`re C, et al. Steroid naive eosinophilic asthma: anti-inflammatory effects of fluticasone and montelukast. Thorax. 2005;60:100–5. doi: 10.1136/thx.2004.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson A, Holgate S. Leukotriene modifiers in the treatment of asthma. BMJ. 1998;316:1257–8. doi: 10.1136/bmj.316.7140.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearlman DS, Lampl KL, Dowling PJ, Jr, Miller CJ, Bonuccelli CM. Effectiveness and tolerability of zafirlukast for the treatment of asthma in children. Clin Ther. 2000;22:732–47. doi: 10.1016/S0149-2918(00)90007-9. [DOI] [PubMed] [Google Scholar]
- 10.Migoya E, Kearns GL, Hartford A, et al. Pharmacokinetics of Montelukast in asthmatic patients 6 to 24 months old. J Clin Pharmacol. 2004;44:487–94. doi: 10.1177/0091270004264970. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger M. Zafirlukast and cromolyn are effective first-line therapies for child asthma. Ann Allergy Asthma Immunol. 2000;84:638–9. doi: 10.1016/s1081-1206(10)62419-2. [DOI] [PubMed] [Google Scholar]
- 12.Renzi P. Antileukotriene agents in asthma: The dart that kills the elephant? CMAJ. 1999;160:217–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Leigh R, Vethanayagam D, Yoshida M, et al. Effects of montelukast and budesonide on airway responses and airway inflammation in asthma. Am J Respir Crit Care Med. 2002;166:1212–7. doi: 10.1164/rccm.200206-509OC. [DOI] [PubMed] [Google Scholar]
- 14.Currie GP, Lee DKC, Srivastava P. Long-acting bronchodilator or leukotriene modifier as add-on therapy to inhaled corticosteroids in persistent asthma? Chest. 2005;128:2954–62. doi: 10.1378/chest.128.4.2954. [DOI] [PubMed] [Google Scholar]
- 15.Bisgaard H for the Study Group on Montelukast and Respiratory Syncytial Virus. A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med. 2003;167:379–83. doi: 10.1164/rccm.200207-747OC. [DOI] [PubMed] [Google Scholar]
- 16.Zeidler MR, Kleerup EC, Goldin JG, et al. Montelukast improves regional air trapping due to small airways obstruction in asthma. Eur Respir J. 2006;27:307–15. doi: 10.1183/09031936.06.00005605. [DOI] [PubMed] [Google Scholar]
- 17.Global Initiative for Asthma (GINA): Global Strategy for Asthma Management and Prevention, (Updated 2009): Chapter 2: Diagnosis and Classification. pp. 15–26. Available on www.ginasthma.org .
- 18.Jang AS, Yeum CH, Choi IS. Nitric oxide metabolites, eosinophils and eosinophilic cationic protein in patients with asthma: sputum versus blood. J Korean Med Sci. 2003;18:489–93. doi: 10.3346/jkms.2003.18.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ÇalIkog?lu M, Tamer L, ÇaIkog?lu I, AtI S, Uluba B, Ercan B. Oxidative Stress and products of nitric oxide metabolism in chronic obstructive pulmonary disease and in healthy smokers. Turkish Resp J. 2002;3:24–7. [Google Scholar]
- 20.Mandeep W, Rakesh L, Kabra SK. Montelukast in pediatric asthma management. Indian J Pediatr. 2006;73:275–82. doi: 10.1007/BF02825818. [DOI] [PubMed] [Google Scholar]
- 21.Hassan NF, Wali SG, El-Helaly NS, Mostafa HW. Oral montelukast versus fluticasone propionate inhaler in persistent athma. Thesis submitted for fulfillment of master degree, Faculty of Medicine, Cairo University, 2005 [Google Scholar]
- 22.Karaman O, Sunneli L, Uzuner N, et al. Evaluation of montelukast in 8-14 year old children with mild persistent asthma and compared with inhaled corticosteroids. Allergol Immunopathol (Madr) 2004;32:21–7. doi: 10.1016/s0301-0546(04)79219-8. [DOI] [PubMed] [Google Scholar]
- 23.Bukstein DA, Luskin AT, Bernstein A. “Real-world” effectiveness of daily controller medicine in children with mild persistent asthma. Ann Allergy Asthma Immunol. 2003;91:585–7. doi: 10.1016/S1081-1206(10)61848-0. [DOI] [PubMed] [Google Scholar]
- 24.Balzano G, Fuscillo S, Gaudiosi C. Leukotriene receptor antagonists in the treatment of asthma: an update. J Allergy. 2002;57:16–9. doi: 10.1034/j.1398-9995.57.s72.2.x. [DOI] [PubMed] [Google Scholar]
- 25.Busse WW, Lemanske RF. Asthma. New England Journal Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 26.Garcia ML, Wahn U, Gilles L, Swern A, Tozzi CA, Polos P. Montelukast compared with fluticasone for control of asthma among 6 to 14 year old patients with mild asthma: The MOSAIC study. Pediatrics. 2005;116:360–9. doi: 10.1542/peds.2004-1172. [DOI] [PubMed] [Google Scholar]
- 27.Ostrom N, Decotis B, Lincourt W, et al. Comparative efficacy and safety of l0w-dose fluticasone propionate and montelukast in children with persistent asthma. J Pediatr. 2005;147:213–20. doi: 10.1016/j.jpeds.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 28.Meltzer EO, Lockey RF, Friedman BF, et al. Efficacy and safety of low-dose fluticasone propionate compared with montelukast for maintenance treatment of persistent asthma. Mayo Clin Proc. 2002;77:437–45. [PubMed] [Google Scholar]
- 29.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–65. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 30.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 31.Kocyigit A, Zeyrek D, Keles H, Koylu A. Relationship among manganese, arginase, and nitric oxide in childhood asthma. Biol Trace Elem Res. 2004;102:11–8. doi: 10.1385/bter:102:1-3:011. [DOI] [PubMed] [Google Scholar]
- 32.Batra J, Singh TP, Mabalirajan U, Sinha A, Prasad R, Ghosh B. Association of inducible nitric oxide synthase with asthma severity, total serum immunoglobulin E and blood eosinophil levels. Thorax. 2007;62:16–22. doi: 10.1136/thx.2006.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berlyne GS, Parameswaran K, Kamada D, Efthimiadis A, Hargreave FE. A comparison of exhaled nitric oxide and induced sputum as markers of airway inflammation. J Allergy Clin Immunol. 2000;106:638–44. doi: 10.1067/mai.2000.109622. [DOI] [PubMed] [Google Scholar]
- 34.Colon-Semidey AJ, Marshik P, Crowley M, Katz R, Kelly HW. Correlation between reversibility of airway obstruction and exhaled nitric oxide levels in children with stable bronchial asthma. Pediatr Pulmonol. 2000;30:385–92. doi: 10.1002/1099-0496(200011)30:5<385::aid-ppul4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–22. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 36.Jang AS, Choi IS. Nitric oxide metabolites in patients with asthma: induced sputum versus blood. Respir Med. 1999;93:912–8. doi: 10.1016/s0954-6111(99)90059-8. [DOI] [PubMed] [Google Scholar]