Abstract
Neuroinflammation is a local tissue response to injurious stimuli in the central nervous system (CNS) and is characterized by glial reactivity, induction of cytokines and chemokines, and vascular permeability. The cytokine interleukin (IL)-1β is rapidly induced following CNS insult, and is chronically expressed in neurodegenerative disorders such as Alzheimer’s disease (AD). We recently developed a novel method of sustained IL-1β production in the brain to study the link between IL-1β and AD pathogenesis. Utilizing this model, we have previously demonstrated reduction of plaque size and frequency accompanied by a robust neuroinflammatory response. These observations were limited to a single early time point in the course of AD plaque deposition and did not investigate other neurodegenerative endpoints. To extend these observations to other stages of disease progression and evaluate additional pathologic markers, we investigated the effects of age and duration of IL-1β overexpression in the APPswe/PS-1dE9 AD model on a congenic C57BL/6 background. We now report that IL1β overexpression leads to decreased 6E10 immunopositive plaque pathology regardless of age or duration. We also investigated whether IL-1β overexpression led to neuronal apoptosis or cholinergic axonal degeneration in the context of this AD model. Although we could demonstrate apoptosis of infiltrating inflammatory cells, we found no evidence for IL-1 associated apoptosis of neurons or cholinergic axon degeneration even after five months of chronic neuroinflammation. Together, these observations point to a neuroprotective role for IL-1β in AD neuropathogenesis.
Keywords: Interleukin-1, Alzheimer’s disease, microglia, chronic neuroinflammation
INTRODUCTION
The proinflammatory cytokine interleukin(IL)-1β has been extensively studied as a potent mediator of acute neuroinflammatory responses (Basu et al., 2004). However, its role in chronic pathophysiological conditions is less well known. IL-1 is upregulated in many degenerative diseases and has long been considered to promote neuronal toxicity in these settings (Griffin et al., 1989; Mogi et al., 1996; McGuinness et al., 1997); however, current knowledge regarding the contribution of IL-1 to chronic neurodegeneration is mostly indirect. Research has largely focused on the role of IL-1 in Alzheimer’s disease (AD), where the cytokine is upregulated in cells associated with plaques in post-mortem brains (Griffin et al., 1989; Griffin et al., 1995; Benzing et al., 1999). Speculation for a pathogenic role emerged from the observation that the extent of neuroinflammation in AD brains appeared to parallel pathology (Sheng et al., 1997). This hypothesis was strengthened by epidemiological studies showing lowered AD prevalence in chronic users of non-steroidal anti-inflammatory drugs (NSAIDs) (McGeer and McGeer, 2007; Vlad et al., 2008).
The central nervous system (CNS) innate immune response may become dysfunctional with age, which may explain the inability of microglia to remove aggregated amyloid-β despite chronic activation (Giunta et al., 2008). A growing body of literature has shown that manipulating the neuroinflammatory response toward enhanced microglial activation can lead to phagocytosis of amyloid-β (Aβ) plaques in mouse models of AD (DiCarlo et al., 2001; Jantzen et al., 2002; Malm et al., 2005; Stahl et al., 2006; Lemere and Masliah, 2010). Our laboratory has recently shown that chronic overexpression of IL-1β can produce similar effects (Shaftel et al., 2007a). These results suggest that neuroinflammation, and particularly IL-1, might play an adaptive role in Alzheimer’s disease whereby amyloid plaque deposition leads to increased cytokine production, which in turn enhances microglial clearance (Shaftel et al., 2008).
Using the previously described IL-1β excisional activation transgenic (XAT) mouse model which allows for temporally and spatially controlled chronic IL-1β overexpression (Shaftel et al., 2007a), we investigated how duration of IL-1β induction and age at induction affects pathology in the APPswe/PS1dE9 AD mouse model. Our results indicate that IL-1β overexpression leads to plaque clearance regardless of age or duration of cytokine expression. In addition, sustained IL-1β did not cause overt apoptosis or cholinergic axonal degeneration in the hippocampus, as measured by terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) and acetylcholinesterase (AChE) staining, respectively.
MATERIALS AND METHODS
Animals
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee (University of Rochester Committee on Animal Resources) for compliance with federal regulations before the initiation of this study. Creation and genotyping of the IL-1βXAT mice on the C57BL/6 background has been described previously (Shaftel et al., 2007a). IL-1βXAT mice carry a transgenic construct containing a glial fibrillary acidic protein (GFAP) promoter, a loxP flanked transcriptional stop sequence, and downstream ssIL-1β transgene coding for the signal sequence from the human IL-1 receptor antagonist (75 bp) fused to the cDNA sequence of human mature IL-1β (464 bp). To achieve transcriptional activation of the transgene, a feline immunodeficiency virus encoding for Cre protein is directed to the area of interest for Cre-mediated excision of the transcriptional stop at the loxP elements. Heterozygous APPswe/PS1dE9 (APP/PS1) mice (stock no. 005864) on a congenic C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME) and genotyped according to the manufacturer’s suggested protocol. APP/PS1 mice were crossed to the IL-1βXAT mice to produce the APP/PS1-IL-1βXAT mice used in most experiments.
Stereotactic Injections
Intrahippocampal stereotactic injections of feline immunodeficiency virus (FIV) were performed using FIV-Cre or FIV-LacZ (Invitrogen, Carlsbad, CA). The construction and packaging of FIV-Cre and LacZ have been described previously (Lai et al., 2006). FIV-Cre and FIV-LacZ include a V5 epitope tag under the control of a cytomegalovirus promoter and were packaged to a final titer of ~1×107 infectious viral particles (IVP) per ml. Our intrahippocampal injection protocol was detailed previously (Shaftel et al., 2007a). Briefly, mice were anesthetized with 1.75% isoflurane in 30/70% oxygen/nitrogen gas and secured in a Kopf stereotax. A 0.5 mm burr hole was drilled in the skull at −1.8 mm caudal and 1.8 mm horizontal from bregma and a 33 GA needle pre-loaded with virus was lowered 1.8 mm from the brain surface over 2 minutes. A Micro-1 microsyringe pump controller (World Precision Instruments, Sarasota, FL) was used to inject 1.5 µl of virus (~1.5×104 IVP) at a constant rate over 10 minutes. Following a five-minute delay to allow viral diffusion, the needle was raised slowly over 2 minutes, the burr hole sealed with Ethicon bone wax and soft tissues sutured using 6-0 Dermalon suture (Ethicon, Somerville, NJ). For time course experiments, injections were performed bilaterally at either 3 or 8 months of age in APP/PS1-IL-1βXAT mice with FIV-Cre in one hemisphere and FIV-LacZ in the other. A total of 51 APP/PS1-IL-1βXAT mice were used in this study.
Immunohistochemistry
For all immunohistochemical analyses, mice were anesthetized with ketamine and xylazine (i.p., 60–90 and 4–8 mg/kg, respectively) and intracardially flushed with a 0.15 M phosphate buffer (PB) solution containing 0.5% w/v sodium nitrite and 2 IU/ml heparin followed immediately by an ice cold 4% paraformaldehyde solution. Brains were post-fixed for 3 h in 4% paraformaldehyde then immersed in 30% sucrose, snap frozen in isopentane and sectioned into 30 µm free floating slices. Antibody binding was visualized using either Elite avidin-biotin and 3,3-diaminodenzidine (Vector Laboratories, Burlingame, CA) or secondary antibodies bound to Alexa 488, 594, or 647 fluorophores (Invitrogen, Carlsbad, CA). Primary antibodies used were as follows: 6E10 (Covance, Princeton, NJ; Catalog # SIG-39300; 1:2000), GFAP (Dako, Carpinteria, CA; Catalog # Z0334; 1:5000), ionized calcium binding adaptor molecule 1 (iba-1) (Wako Chemicals, Richmond, VA; Catalog # 016-20001; 1:5000), Neu-N (Chemicon, Temecula, CA; Catalog # MAB377B; 1:2000), and 7/4 (Serotec, Oxford, UK; Catalog # MCA771A700; 1:2000). Congo red (Sigma), Hoechst (Invitrogen) and terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) (ApopTag; Chemicon) were used according to manufacturer’s protocols. Acetylcholinesterase staining was performed as described previously (Hedreen et al., 1985) with the addition of 10 mM tetraisopropyl pyrophosphoramide to the incubation solution. For all such procedures, groups of tissue were processed and stained in parallel under identical conditions.
ELISA and western blot
At various timepoints following FIV-Cre/FIV-LacZ injections, mice were anesthetized with ketamine and xylazine (i.p., 60–90 and 4–8 mg/kg, respectively) and perfused with 0.15 M PB containing 2 IU/ml heparin and 0.5% w/v sodium nitrite. Brains were removed and hippocampi microdissected, snap frozen in ice-cold isopentane, and stored at −80 °C. Frozen hippocampi were homogenized, vortexed, and sonicated in 20 µl tissue protein extraction reagent (Pierce, Rockford, IL) per milligram of tissue weight with protease (EMD Biosciences, San Diego, CA) and phosphatase (Sigma-Aldrich, St. Louis, MO) inhibitors. To generate samples for Aβ peptide measurements, homogenates were centrifuged for 1 h at 100,000 g, and supernatants containing soluble Aβ fraction were stored at −80 °C. Pellets were washed with 1 ml PB, re-suspended in an equal volume of 70% formic acid (Sigma), and centrifuged for 1 h at 100,000 × g. Supernatants containing Aβ fractions were stored at −80 °C until Aβ peptide concentrations were determined by ELISA (Covance) according to the manufacturer’s protocol. For Western blot, lysates from homogenized hippocampi were subject to bicinchoninic assay (Pierce) to determine protein concentration. Samples were then diluted in 2× sample buffer containing 125 mM Tris-HCl, 4% SDS, and 20% glycerol, and 15 µg of each sample was electrophoresed on either Tris-HCl (for amyloid precursor protein (APP)) or Tris-Tricine (for beta-site APP cleaving enzyme (BACE)-1) polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose or PVDF membranes (Bio-Rad), respectively. Membranes were blocked for 1 h in Western blocking reagent (Roche Diagnostics, Indianapolis, IN) and incubated overnight at 4 °C in primary antibody to APP (clone 6E10, Covance; 1:1000), BACE-1 (Calbiochem, San Diego, CA; Catalog # 195111; 1:2000) or GAPDH (Ambion, Austin, TX; Catalog # AM4300; 1:5,000). Blots were incubated with peroxidase-linked secondary antibodies (Supersignal West Dura Kit; Pierce) and visualized with X-AR film or the Image Station 440 CF (Kodak, Rochester, NY).
Data analysis and image capture
Confocal images were obtained using an Olympus FV1000 laser scanning confocal microscope (Center Valley, PA) in the Confocal and Conventional Microscopy Core of the University of Rochester Medical Center Core Facility Program. All images were acquired using sequential scanning and oversaturation was prevented by using the hi-lo feature of the FV1000 software. UPLAN objectives were used to acquire the images. Light microscopic images were acquired on an Axioplan IIi (Carl Zeiss, Oberkochen, Germany) microscope equipped with a Spot RT camera and software (version 4.5.9.8; Diagnostic Instruments, Burroughs, MI). For area fraction quantification of Congo red or 6E10 staining, images were captured from 3 hippocampal sections per animal using a 5 × objective. Hippocampal boundaries were defined using ImageJ (http://rsb.info.nih.gov/ij/) to determine the area fraction of staining using a low threshold for plaque area of 10 µm to minimize artifacts. Quantification of acetylcholinesterase immunostaining was performed using ImageJ. Two to three sections per animal (N=5–8) were subject to blinded mean gray value measurements. Constant threshold levels were maintained between control and IL-1β expressing hemispheres. For Western blot analysis, signals were captured and quantified using the Image Station 440 CF (Kodak, Rochester, NY).
Statistical analyses
All results are expressed as mean ± SEM. Graphing and statistical analyses were performed using Prism (GraphPad Software, San Diego, CA). Either paired t-tests or 2-way analyses of variance (ANOVAs) were used to determine significance comparing the IL-1β overexpressing hemisphere to control viral injection in the opposite hemisphere of the same animal. Bonferroni post-hoc tests were used to further analyze significant findings in ANOVA analyses. Significance was established by p-values < 0.05.
RESULTS
Reduction of amyloid plaques following one or five months of IL-1β overexpression
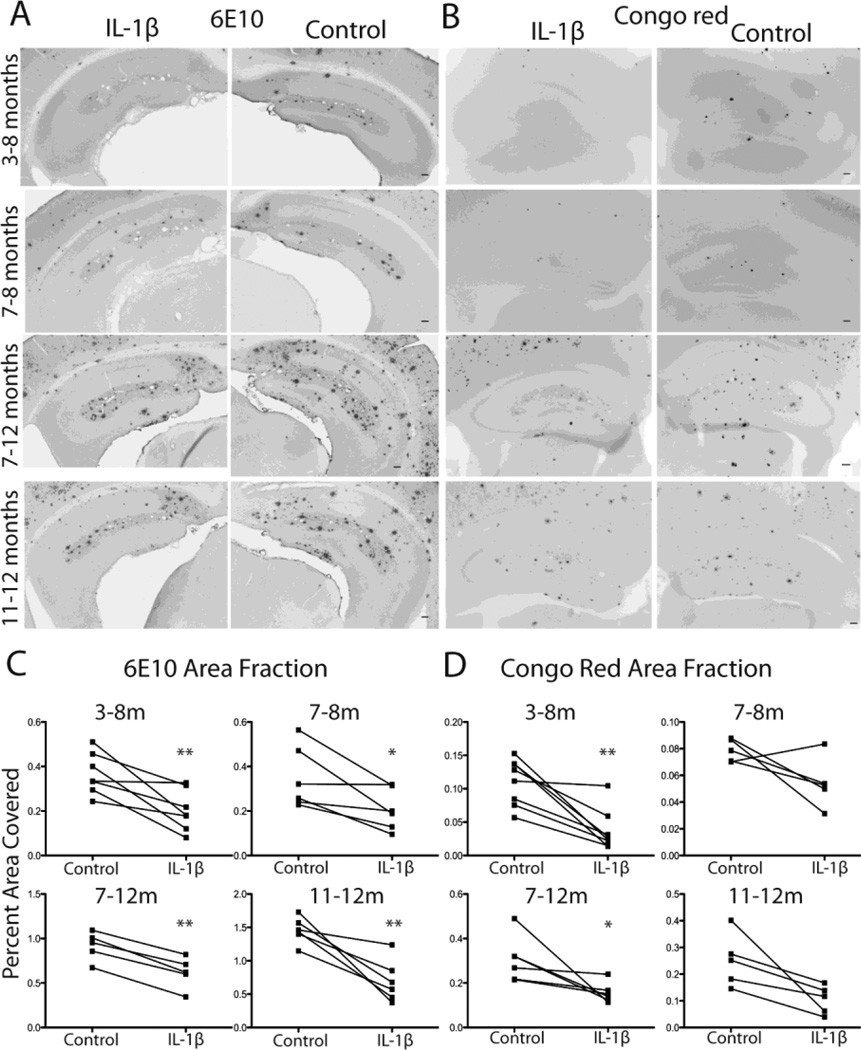
Previous studies in our laboratory suggested that IL-1β may play an adaptive role in AD (Shaftel et al., 2007a). In order to examine this possibility in more detail, we tested whether upregulation of IL-1β contributes differently to plaque clearance at varied ages and duration of IL-1β upregulation. We crossed the IL-1βXAT mouse model to the APPswe/PS1dE9 (APP/PS1) model of AD on the C57BL/6 background. Resulting APP/PS1-IL-1βXAT mice were injected with FIV-Cre in one hemisphere (for IL-1β overexpression) and FIV-LacZ in the other (as a viral control) at 3 or 7 months of age and sacrificed at 8 or 12 months of age, resulting in either 1 or 5 months of unilateral IL-1β upregulation. Brains were sectioned at 30 µm and stained for 6E10 or Congo red to visualize plaques. Representative images of 6E10 (Fig. 1A) and Congo red (Fig. 1B) staining indicate unilateral plaque reduction at all timepoints. Plaque indices were measured with ImageJ (Fig. 1C–D). Two-way ANOVA showed a significant effect of IL-1β on 6E10 area fraction (F(3,19)=52.62, p <0.0001) (Fig. 1C). Individual paired t-tests revealed significance at every timepoint. Two-way ANOVA also showed a main effect of IL-1β on Congo red area fractions with significant reductions in plaque volume observed for the 3–8 month (p =0.002) and 7–12 month (p =0.04) treatment periods. The shorter IL-1β induction lengths from 7–8 months (p =0.09) and 11–12 months (p =0.09) showed trends toward significant reductions in Congophilic plaques, indicating that longer periods of inflammation may be more effective for amyloid clearance (Fig. 1D).
Figure 1.
Plaque indices following 1 or 5 months of IL-1β upregulation. A,B, Representative photomicrographs of 6E10 (A) and Congo red (B) labeling in hippocampus following 1 or 5 months of unilateral IL-1β upregulation at varied ages. Bar = 100 µm. C,D, Quantitative individual dot plot analysis of 6E10 (C) and Congo red (D) measured as percent area in hippocampus occupied by plaque. n = 5–8 per group. Data displayed as mean ± SEM. *p <0.05, **p <0.01 compared to control (FIV-LacZ injected) hemisphere. C=control, I=IL-1β, m=months of IL-1β overexpression.
Insoluble amyloid-β peptides are reduced following IL-1β upregulation
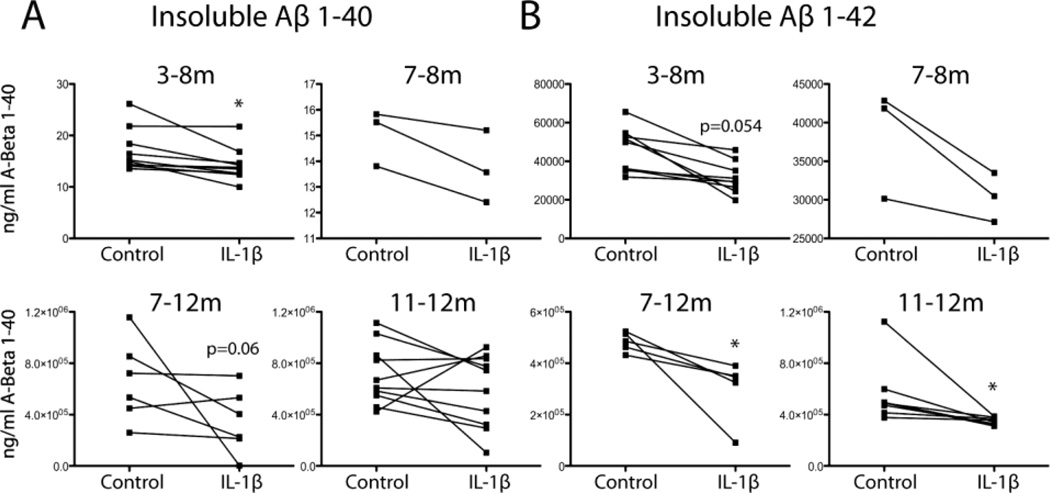
APP/PS1-IL-1βXAT mice were injected in the same manner as the previous experiment resulting in either 1 or 5 months of unilateral IL-1β upregulation in the hippocampus. Hippocampi were processed for analysis by Aβ ELISA and western blot. Reduction in hippocampal insoluble Aβ1–40 was observed only in the 3–8 month group (p =0.015) although the 7–12 month group approached significance (p =0.06) (Fig. 2A). The two groups that were subjected to IL-1β overexpression for only 1 month showed no change in Aβ1–40 peptides (Fig. 2A). Two-way ANOVA showed an overall effect of IL-1β on levels of insoluble Aβ1–42 peptide (F(1,21)=12.73, p =0.0018). Individual t-tests indicated that IL-1β overexpression mediated significant reductions in insoluble Aβ1–42 peptide levels in both the 7–12 month and 11–12 month groups (p <0.05), while the 3–8 month group showed a trend towards significance (p=0.054) (Fig. 2B). These observed decreases in insoluble Aβ peptides are consistent with the decreased hippocampal loads of 6E10-positive and Congophillic plaques observed in histological sections.
Figure 2.
Insoluble Aβ levels following chronic IL-1β overexpression in APP/PS1 mice. A,B, Quantitative individual dot plot analysis of insoluble Aβ1–40 (A) and Aβ1–42 (B) measured by ELISA. n = 3–8 per group. *p <0.05 compared to control hemisphere at same time point. C=control, I=IL-1β, m=months of IL-1β overexpression.
IL-1β does not alter APP production or Aβ peptide generation
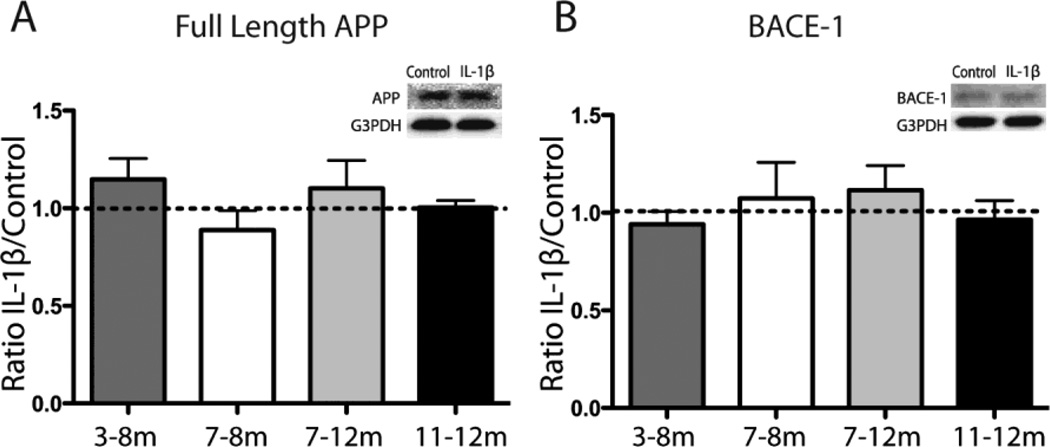
Prior research has suggested a role for IL-1β in regulating APP expression or BACE-1 cleavage of APP in certain experimental paradigms (Goldgaber et al., 1989; Buxbaum et al., 1992; Heneka et al., 2005). In order to determine whether altered production or processing of APP may be responsible for our observed changes in amyloid pathology, levels of APP and BACE-1 were measured by western blot and levels of soluble Aβ peptides by ELISA. IL-1β induction did not significantly alter levels of soluble amyloid-β (data not shown), full length APP (Fig. 3A), or BACE-1 (Fig. 3B), which is consistent with prior work in our laboratory (Shaftel et al., 2007a). These findings suggest that IL-1β facilitates removal of insoluble amyloid plaques rather than inhibiting or slowing their production.
Figure 3.
APP and BACE-1 levels after IL-1β induction in APP/PS1 mice. A,B, Western blot for full length APP (A) and BACE-1 (B) with representative image as inset. Data displayed as ratio of IL-1β to control hemisphere at each timepoint normalized to G3PDH. n = 3–8 per group. C=control, I=IL-1β, m=months of IL-1β overexpression.
Neuronal and axonal viability was maintained following one or five months of IL-1β activation
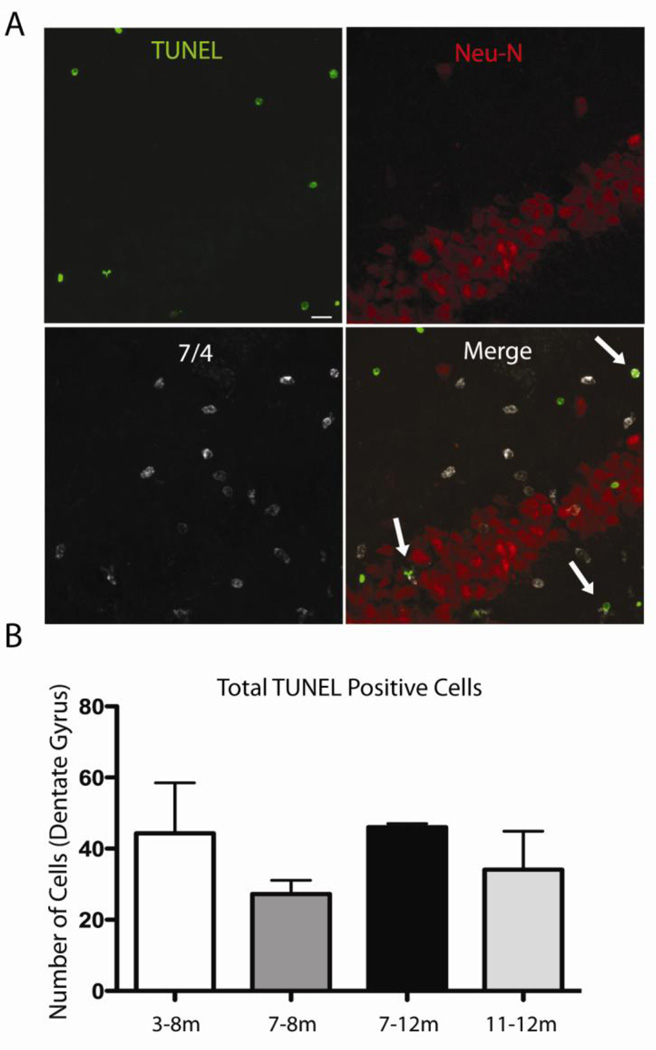
We previously found that upregulation of IL-1 for up to two months in IL-1βXAT mice did not lead to overt signs of neuronal toxicity as measured by neuronal cell counts, synaptophysin, and acetylcholinesterase (AChE) staining (Shaftel et al., 2007b). However, long-term IL-1β expression in a mouse model of Alzheimer’s disease may lead to neuronal degeneration. To assess whether one or five months of IL-1β overexpression caused neuronal apoptosis we used TUNEL as a marker of cellular apoptosis in APP/PS1-IL-1βXAT mice that received FIV-Cre in one hemisphere and FIV-LacZ in the control hemisphere. While many TUNEL positive cells could be seen in the dentate gyrus of the hippocampus following IL-1β upregulation at all timepoints, no cellular co-localization was observed with the neuronal marker Neu-N, even in mice that had experienced five months of IL-1β upregulation, indicating that IL-1β does not mediate neuronal apoptosis in these mice. Some TUNEL positive cells co-localized with the neutrophil marker 7/4, as indicated by arrows in the representative image of the dentate gyrus from a mouse with IL-1β upregulation from 7–8 months (Fig. 4A). Although we suspect that most of the other TUNEL positive cells also represent infiltrating inflammatory cells, additional co-localization experiments with other markers, including ones specific for glia, are required to fully characterize apoptosis in our model. Quantitative data from three sections per animal showed no difference in total numbers of TUNEL positive cells (Fig. 4B) or TUNEL positive neutrophils (data not shown) in the IL-1β-upregulated dentate gyrus among the different timepoints. Importantly, no TUNEL positive cells were observed in the hippocampus that received control viral injections.
Figure 4.
TUNEL as marker of apoptosis following IL-1β overexpression in the dentate gyrus. A, Immunohistochemistry in the IL-1β upregulated dentate gyrus of APP/PS1-XAT mouse (representative images are from 7–8 month timepoint). Sections are labeled for TUNEL (green), the neuronal marker Neu-N (red) and the neutrophil marker 7/4 (white). Double-labeled neutrophils are depicted by arrows in merged image. Bar = 10 µm. B, Quantification of total TUNEL positive cells showed no significant difference between timepoints.
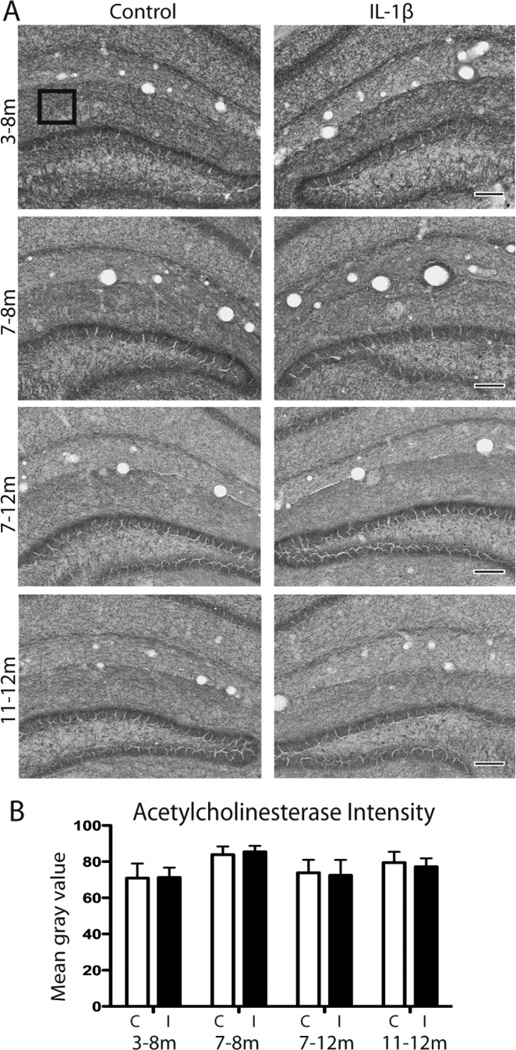
Severe cholinergic depletion is a well-known pathologic feature of AD (Tago et al., 1987; Geula and Mesulam, 1989). Some mouse models of AD exhibit overall reduction in cholinergic fibers measured by AChE staining and IL-1β has been shown to increase AChE in neurons, indicating a role for potentiating acetylcholine degradation in AD (Li et al., 2000; Sturchler-Pierrat and Staufenbiel, 2000). In order to explore the relationship of IL-1β to cholinergic neurons in our chronic inflammatory paradigm, we performed AChE staining on APP/PS1-IL-1βXAT animals that received either 1 or 5 months of IL-1β upregulation unilaterally. Representative images of AChE histochemistry show no change between control and IL-1β hemispheres in the APP/PS1-IL-1βXAT mice at any timepoint measured (Fig. 5A). Two-way ANOVA demonstrates no significant difference of AChE mean gray value between hemispheres (p =0.8096) or time points (p =0.3254) (Fig. 5B). Neither short term nor long term IL-1β upregulation caused depletion of cholinergic fibers in this AD mouse model.
Figure 5.
AChE axon staining following IL-1β induction in the hippocampus. A, Representative images of AChE staining at each timepoint in APP/PS1-XAT mice following 1 or 5 months of unilateral IL-1β overexpression compared to FIV-LacZ injected control hemisphere. Bar = 100 µm. B, Quantification of AChE staining was performed using three representative fields in the molecular layer per section (boxed example in A). n = 5–8 animals per timepoint, 2–3 sections per animal analyzed. Data presented as average mean gray value ± SEM. C=control, I=IL-1β, m=months of IL-1β overexpression.
DISCUSSION
IL-1β has long been implicated in the pathogenesis of AD. In an earlier study, we found that sustained elevation of IL-1β leads to profound reduction in plaque pathology in APPswe/PS1dE9 mice on a mixed B6C3F1/J background (Shaftel et al., 2007a). In the current study, we aimed to reproduce these findings in a congenic C57BL/6 model at different stages of plaque formation and following short and long term periods of IL-1β overexpression. We discovered that IL-1β reduces histochemical plaque indices at all timepoints measured; however, Congo red staining showed trends of greater reduction in the two groups with five-month IL-1β overexpression, indicating that longer periods of neuroinflammation may result in greater plaque reduction. This conclusion is supported by Aβ ELISA data showing greatest reductions of insoluble Aβ1–40 peptides within the 3–8 month and 7–12 month groups. Significant reductions were also found at the later timepoints (7–12 month and 11–12 month) for insoluble Aβ1–42, with the 3–8 month group approaching significance. Taken together, these data suggest that the age at induction and length of induction may contribute differently to the balance of amyloid deposition and clearance in this mouse model of AD. However, plaque clearance occurs with IL-1β upregulation regardless of age or duration. Although research suggests a role for IL-1β in APP expression and processing (Goldgaber et al., 1989; Heneka et al., 2005; Paris et al., 2010), Western and ELISA data for full length APP, BACE-1, and soluble Aβ1–40 & 1–42 revealed no significant changes in APP production or processing following IL-1β overexpression. This suggests that amyloid burden is reduced via clearance mechanisms rather than by reduced deposition in this model.
Several studies have shown that different species of Aβ induce varied toxic effects in the CNS. For example, protofibrils and oligomers of Aβ1–40 & 1–42, rather than fibrillar Aβ plaques contribute to early dendritic and synaptic injury in AD models (Heneka and O'Banion, 2007). As already stated, we did not detect quantitative changes in soluble Aβ concentrations following IL-1β overexpression. We also did not observe neuronal apoptosis or loss of AChE staining, indicating that sustained IL-1 does not cause overt neurotoxicity in this AD mouse model. However, further studies with additional markers are required to fully establish the impact of IL-1β in the context of transgenic Aβ overexpression.
We do not yet know how our observed changes in Aβ load will translate to changes in AD-related phenotypic impairments. One caveat to many AD mouse models is the lack of neuronal death as a disease endpoint; however, behavioral impairments exist in many APP transgenic mouse models (Reiserer et al., 2007). Future experiments will include behavioral assessments of APP/PS1 × IL-1βXAT mice to determine how changes in plaque load coupled with chronic neuroinflammation correlate with behavior. However, such studies may be challenging to interpret, since we previously demonstrated that IL-1 overexpression impairs hippocampal dependent memory as assessed by Morris water maze and by contextual fear conditioning (Moore et al., 2009; Hein et al., 2010, Matousek et al., 2010). Interestingly, the behavioral deficit observed with IL-1β overexpression did not occur in mice lacking cyclooxygenase-1 and corresponding increased levels of hippocampal prostaglandin E2 (Matousek et al., 2010). Additional studies are underway to understand the biological basis for these observations in the IL-1βXAT mice.
Increased numbers of plaque-associated microglial and astrogliosis were observed at all timepoints in APP/PS1 mice following IL-1β overexpression, suggesting that the innate immune response contributes to plaque clearance during sustained IL-1β expression. Indeed, it is well known that microglia are capable of phagocytosing Aβ in vitro (Majumdar et al., 2007), and recent work has revealed that astrocytes can take up Aβ (Pihlaja et al., 2011). Furthermore, a growing body of literature suggests that microglial phagocytosis can play a beneficial role in AD (Wilcock et al., 2004; Barger, 2005; Takata et al., 2007; Chakrabarty et al., 2010). For example, methods used to alleviate amyloid pathology via immune activation include passive or active Aβ immunotherapy (Lemere and Masliah, 2010), acute LPS administration (DiCarlo et al., 2001; Malm et al., 2005), viral infection (Stahl et al., 2006), administration of a nitric-oxide releasing NSAID (Jantzen et al., 2002), and overexpression of the proinflammatory cytokine IL-6 (Chakrabarty et al., 2010). Microglia in neurodegenerative settings have been implicated in mediating the harmful effects of neuroinflammation (Morgan et al., 2005; Town et al., 2005; Wyss-Coray, 2006). However, the absence of any overt neuronal death or cholinergic axonal degeneration in our study indicates that the inflammatory environment generated by sustained IL-1β is not overtly neurotoxic, as one might expect in a state of chronic gliosis. Aging studies have indicated that microglia become more inflamed yet less capable of phagocytosis and proliferation with age in a process termed ‘inflammaging’ (Giunta et al., 2008) or cellular senescence (Miller and Streit, 2007). Indeed, the persistence of plaques in AD brains despite evidence that microglia are capable of phagocytosis indicates that their phagocytic abilities may become limited over time. Based on the current work, it appears that IL-1 may be able to reactivate these microglia. Alternatively, IL-1 overexpression may increase recruitment of peripheral myeloid cells, which have been implicated in plaque clearance (Simard et al., 2006; El Khoury et al., 2007). Additional experiments utilizing bone marrow chimeric mice are currently underway to address this possibility. Regardless of the mechanism, it appears that the innate immune system may be harnessed as a tool for increased amyloid removal.
Acknowledgments
This work was supported by National Institute of Health Grants RO1 AG030149 and F31 AG031667 to MKO and SBM, respectively.
Footnotes
Conflict of Interest Disclosure: No potential conflicts of interest exist.
REFERENCES
- Barger SW. Vascular consequences of passive Abeta immunization for Alzheimer's disease. Is avoidance of "malactivation" of microglia enough? J Neuroinflammation. 2005;2:2. doi: 10.1186/1742-2094-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb J. 2010;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22:1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Cortical cholinergic fibers in aging and Alzheimer's disease: a morphometric study. Neuroscience. 1989;33:469–481. doi: 10.1016/0306-4522(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J. Inflammaging as a prodrome to Alzheimer's disease. J Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, Bacon SJ, Price DL. A modified histochemical technique to visualize acetylcholinesterase-containing axons. J Histochem Cytochem. 1985;33:134–140. doi: 10.1177/33.2.2578498. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YC, Shaftel SS, Miller JN, Tallents RH, Chang Y, Pinkert CA, Olschowka JA, Dickerson IM, Puzas JE, O'Banion MK, Kyrkanides S. Intraarticular induction of interleukin-1beta expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 2006;54:1184–1197. doi: 10.1002/art.21771. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WS. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Matousek SB, Hein AM, Shaftel SS, Olschowka JA, Kyrkanides S, O'Banion MK. Cyclooxygenase-1 mediates prostaglandin E(2) elevation and contextual memory impairment in a model of sustained hippocampal interleukin-1beta expression. J Neurochem. 2010;114:247–258. doi: 10.1111/j.1471-4159.2010.06759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McGuinness MC, Powers JM, Bias WB, Schmeckpeper BJ, Segal AH, Gowda VC, Wesselingh SL, Berger J, Griffin DE, Smith KD. Human leukocyte antigens and cytokine expression in cerebral inflammatory demyelinative lesions of X-linked adrenoleukodystrophy and multiple sclerosis. J Neuroimmunol. 1997;75:174–182. doi: 10.1016/s0165-5728(97)00020-9. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O'Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, March A, Ait-Ghezala G, Mullan MJ. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M. Multiple cellular and molecular mechanisms are involved in human Aβ clearance by transplanted astrocytes. Glia. 2011;59:1643–1657. doi: 10.1002/glia.21212. [DOI] [PubMed] [Google Scholar]
- Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007a;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007b;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer's disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94:1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Stahl T, Reimers C, Johne R, Schliebs R, Seeger J. Viral-induced inflammation is accompanied by beta-amyloid plaque reduction in brains of amyloid precursor protein transgenic Tg2576 mice. Eur J Neurosci. 2006;24:1923–1934. doi: 10.1111/j.1460-9568.2006.05069.x. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer's disease analyzed in the APP23 transgenic mouse model. Ann N Y Acad Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Tago H, McGeer PL, McGeer EG. Acetylcholinesterase fibers and the development of senile plaques. Brain Res. 1987;406:363–369. doi: 10.1016/0006-8993(87)90808-0. [DOI] [PubMed] [Google Scholar]
- Takata K, Kitamura Y, Yanagisawa D, Morikawa S, Morita M, Inubushi T, Tsuchiya D, Chishiro S, Saeki M, Taniguchi T, Shimohama S, Tooyama I. Microglial transplantation increases amyloid-beta clearance in Alzheimer model rats. FEBS Lett. 2007;581:475–478. doi: 10.1016/j.febslet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial "activation" continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]