Abstract
OBJECTIVE
Coronary MDCT angiography has been shown to be an accurate noninvasive tool for the diagnosis of obstructive coronary artery disease (CAD). Its sensitivity and negative predictive value for diagnosing percentage of stenosis are unsurpassed compared with those of other noninvasive testing methods. However, in its current form, it provides no information regarding the physiologic impact of CAD and is a poor predictor of myocardial ischemia. CORE320 is a multicenter multinational diagnostic study with the primary objective to evaluate the diagnostic accuracy of 320-MDCT for detecting coronary artery luminal stenosis and corresponding myocardial perfusion deficits in patients with suspected CAD compared with the reference standard of conventional coronary angiography and SPECT myocardial perfusion imaging.
CONCLUSION
We aim to describe the CT acquisition, reconstruction, and analysis methods of the CORE320 study.
Keywords: coronary atherosclerosis, coronary CT angiography, myocardial CT perfusion imaging, myocardial ischemia, SPECT
Advances in MDCT have made noninvasive imaging of the coronary arterial lumen and wall feasible [1], making CT angiography (CTA) an appropriate clinical tool for the evaluation of suspected coronary artery disease (CAD) in patients with a low-to-intermediate likelihood of disease [2-4]. The main advantages of CTA are its noninvasive nature, high sensitivity, and negative predictive value compared with conventional coronary angiography, as found in several previous studies using 64 detector rows [5-10]. Although CTA is a sensitive method for detecting atherosclerosis, it is limited in its ability to predict the physiologic significance of coronary luminal stenosis [11, 12]. Multiple studies have shown that a 50% or greater coronary luminal stenosis identified by CTA is a poor predictor of reversible ischemia, with positive predictive values ranging from 29% to 31% [11-14]. Importantly, the detection of myocardial ischemia has significant implications in the diagnosis, prognosis, and treatment of patients with CAD [15-17].
Studies from Johns Hopkins University and others have found that MDCT alone, using 64-MDCT systems, is capable of accurately detecting the presence of obstructive atherosclerosis causing myocardial perfusion abnormalities [18-22]. Even though myocardial CT perfusion imaging is feasible with 64-MDCT systems, there are multiple potential advantages offered by wide-area detector systems, such as 320-MDCT, with full cardiac coverage in a single rotation [23]. The primary aim of this article is to describe the CTA and myocardial CT perfusion imaging acquisition, reconstruction, and analysis methods of the CORE320 multicenter multinational diagnostic study, with the overall goal of validating simultaneous CTA and CT perfusion imaging using 320-MDCT.
Study Methods
Objectives
The coprimary objectives of the CORE320 study are to test the diagnostic accuracy of 320-MDCT for identifying the combination of CTA-defined coronary artery stenosis 50% or greater and a corresponding myocardial perfusion defect on CT perfusion imaging in a patient with suspected CAD compared with the following reference standards: first, the combination of a quantitative coronary angiography–defined stenosis 50% or greater and a corresponding myocardial perfusion defect on SPECT and, second, a stenosis 50% or greater on quantitative coronary angiography alone. The primary diagnostic parameters will be the area under the receiver operating characteristic curve, sensitivity, and specificity.
Overall Study Design
CORE320 is a multicenter study involving 16 hospitals in eight countries (United States, Brazil, Canada, Singapore, Japan, Germany, Denmark, and The Netherlands). An internal committee comprising biostatisticians, radiologists, and cardiologists representing the CORE320 investigators designed the CORE320 study. All study procedures are approved by the central institutional review board (IRB) and each local IRB. Adverse events are tracked, reported, and reviewed by an independent data safety and monitoring board. All centers received approval of the informed consent and study protocol from their respective local ethics committees (IRBs).
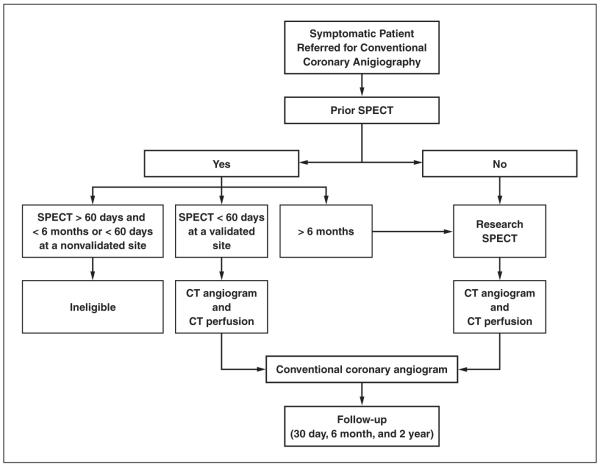
The overall enrollment strategy is summarized in Figure 1. In summary, the study will enroll 400 patients referred for conventional coronary angiography who underwent a clinical or research rest and stress SPECT study with a technetium-based tracer performed with a single-day protocol. All patients will undergo conventional coronary angiography, SPECT, CTA, and CT perfusion imaging, and imaging sessions will be required to occur within 60 days of each other. CT studies will always be performed before the clinically indicated conventional coronary angiography to avoid selection bias. Investigators, treating physicians, and patients will be blinded to the CT results, and all further clinical decisions will be based on the patient’s clinical standard of care. CTA, CT perfusion imaging, SPECT, and conventional coronary angiography studies will be forwarded to individual core laboratories and will be analyzed by separate teams blinded to clinical parameters and the other imaging tests. The study sponsor, Toshiba Medical Systems Corporation, is not involved in any stage of the study, such as design, data acquisition, data analysis, or manuscript preparation.
Fig. 1.
Overall enrollment strategy of CORE320 Multicenter study. All patients enrolled with clinical indication for invasive coronary angiography will undergo either clinical or research SPECT study at validated site, MDCT angiography and perfusion study, and invasive coronary angiography within 60 days of each other.
Clinical follow-up of patients enrolled in the study will occur at 30 days, 6 months, and 2 years. Clinical status and interval events are reviewed via telephone with the participant, review of medical records, and written communication. Clinical outcomes to be followed are the occurrence of death, myocardial infarction, stroke, coronary revascularization, or hospitalization. Study participant safety will be monitored by an independent data safety monitoring board and by central and local IRBs.
Patient Population
CORE320 is designed to prospectively include patients 45–85 years old who are referred for clinically indicated conventional coronary angiography for suspected or known CAD in the next 60 days and who are willing and able to provide written informed consent. Women of childbearing potential will require a negative pregnancy test within 24 hours of the CT study. Exclusion criteria are listed in Table 1. If a clinical SPECT was performed within the previous 6 months but more than 60 days before screening or in a nonvalidated center within 60 days before screening, the patient will be excluded. Dual-isotope studies, thallium studies, or sestamibi studies that included rest and stress performed using a 2-day protocol will also be excluded. The study will enroll and analyze all patients, myocardial territories, and coronary segments, regardless of Agatston calcium score and presence of stents.
TABLE 1.
Exclusion Criteria
| Atrial fibrillation or uncontrolled tachyarrhythmia |
| Advanced atrioventricular block (second- or third-degree heart block) |
| Evidence of acute coronary syndrome with thrombolysis in myocardial infarction risk score ≥ 5 or elevated cardiac enzymes in the past 72 hours |
| Known or suspected moderate or severe aortic stenosis |
| Evidence of severe symptomatic heart failure (New York Heart Association class III or IV) |
| Previous coronary artery bypass, cardiac surgery, or coronary artery intervention within the last 6 months |
| History of allergic reaction to iodinated contrast media or a history of contrast-induced nephropathy |
| Multiple myeloma |
| Previous organ transplantation |
| Elevated serum creatinine level (> 1.5 mg/dL) or calculated creatinine clearance of < 60 mL/min (using the Cockcroft-Gault formula) |
| Contraindications to vasodilator stress (systolic blood pressure < 90 mm Hg) |
| Recent use of dipyridamole and dipyridamole-containing medications |
| Profound sinus bradycardia of < 40 beats/min |
| Known or suspected intolerance or contraindication to β-blockers (including known allergy to β-blockers, history of moderate-to-severe bronchospastic lung disease, including moderate-to-severe asthma, and severe pulmonary disease with the use of inhaled bronchodilator over the past year) |
| Body mass index > 40 kg/m2 |
| Presence of intracardiac devices or metallic implants within the imaging field of view |
| History of high radiation exposure (≥ 2 nuclear or CT studies or ≥ 5.0 rem) in the 18 months before consent |
| Presence of any other history or condition that the investigator judged to be a significant reason for exclusion |
Quality Assurance and Safety: CT Accreditation
Before certification, all technologists participate in a four-phase CORE320-specific training process that includes didactic lectures, hands-on training using a water phantom, the performance of three clinical CTAs using the CORE320 coronary artery calcium score (CACS) and rest CTA scan protocol, and the performance of the entire CORE320 protocol performed under the IRB approval.
Step 1
Each site is required to provide at least three clinical CTA studies using the CORE320 rest scan protocol to the CT Core Laboratory, which reviews these studies for protocol compliance and data quality. Step-1 scans are also used to test the study protocol for providing an optimal balance between image noise and radiation dose to subjects. Sites receive step-1 accreditation once three scans free of protocol deviations are received by the CT Core Laboratory.
Step 2
Each site is mandated to acquire one step-2 study documenting strict IRB protocol adherence, including a CACS, rest CTA and CT perfusion imaging, and adenosine stress CT perfusion imaging, followed by SPECT and conventional coronary angiography. All images are sent to the respective core laboratories for protocol compliance and data quality. Upon step-1 and step-2 accreditation, sites are able to start patient enrollment.
CT Acquisition Protocol
Prescan Preparation for CT
Patients are required to be fasting for at least 4 hours and abstain from caffeine at least 12 hours before their CT examination. Patients receive two 18–20-gauge IV lines, one preferably in the right antecubital vein for contrast agent administration and a second in the left antecubital vein for adenosine administration. A small sample of blood is tested for serum creatinine level. Patients receive IV hydration using normal saline (250–500 mL) to minimize the risk of contrast nephropathy and to avoid hypovolemia before vasodilator stressor administration. Baseline ECG, heart rate, and blood pressure are recorded and reviewed by one of the study investigators.
Beta-Blocker Protocol
Patients may receive oral or IV metoprolol before CT. If the body mass index is less than 30 kg/m2 and the heart rate is greater than 60 beats/min, 75 mg of oral metoprolol will be given. If the body mass index is 30 or higher and heart rate is greater than 60 beats/min, 150 mg of oral metoprolol will be given. If the heart rate remains greater than 60 beats/min, IV metoprolol (2.5–5.0 mg every 5 minutes, until a maximum dose of 15 mg) will be administered.
CACS
The patient will lie supine in a 320-MDCT scanner (Aquilion ONE, Toshiba Medical Systems) and will be attached to a rhythm monitor and automated blood pressure monitor. After anteroposterior and lateral scanograms, CACS will be performed using prospective ECG triggering over a single heartbeat with a gantry rotation and x-ray exposure time of 0.35 second, 0.5-mm slice collimation, tube voltage of 120 kV, and tube current of 140 mA.
Rest CTA and CT Perfusion Imaging Scan Protocol
The rest CT will simultaneously acquire the CTA and rest CT perfusion imaging data. The start and end positions will be determined using the CACS. To minimize radiation exposure scan, the range will be reduced as much as possible. In most cases, z-axis coverage will be 140 mm but may range from 120 to 160 mm depending on the z-axis length of the heart. The rest CTA protocol will be performed using 240–320 detectors with a 0.5-mm detector width, a peak tube voltage of 120 kV, a gantry rotation time of 0.350–0.375 second, and prospective ECG triggering. The rest CT angiogram acquisition parameters are detailed in Table 2.
TABLE 2.
320-MDCT Rest Angiography Parameters
| Heart Rate (beats/min |
Body Mass Index (kg/m2) |
No. of Detector Rows |
Slice Thickness (mm) |
Tube Voltage (kV) |
Tube Current (mA) |
Target Cardiac Phase (%) |
Window (%) | No. of Beats | |
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||
| ≤ 65 | ≤ 19.9 | 240–320 | 0.5 | 120 | 300 | 350 | 75 | 10 | 1 |
| ≤ 65 | 20.0–24.9 | 240–320 | 0.5 | 120 | 370 | 400 | 75 | 10 | 1 |
| ≤ 65 | 25.0–29.9 | 240–320 | 0.5 | 120 | 400 | 450 | 75 | 10 | 1 |
| ≤ 65 | 30.0–34.9 | 240–320 | 0.5 | 120 | 450 | 520 | 75 | 10 | 1 |
| ≤ 65 | 35.0–39.9 | 240–320 | 0.5 | 120 | 460 | 550 | 75 | 10 | 1 |
| > 65 | ≤ 19.9 | 240–320 | 0.5 | 120 | 300 | 350 | 60 | 40 | 2 |
| > 65 | 20.0–24.9 | 240–320 | 0.5 | 120 | 340 | 400 | 60 | 40 | 2 |
| > 65 | 25.0–29.9 | 240–320 | 0.5 | 120 | 340 | 440 | 60 | 40 | 2 |
| > 65 | 30.0–34.9 | 240–320 | 0.5 | 120 | 450 | 520 | 60 | 40 | 1 |
| > 65 | 35.0–39.9 | 240–320 | 0.5 | 120 | 460 | 550 | 60 | 40 | 1 |
Patients with systolic blood pressure 110 mm Hg or higher will receive sublingual fast-acting nitrates. Iopamidol (Isovue 370, Bracco Diagnostics) will be injected using a triphasic contrast injection protocol: 100% contrast, followed by 30% contrast and 70% saline mix, followed by 100% saline chaser. Contrast dose will be 50–70 mL, adjusted according to patient weight (Table 3). Real-time bolus tracking will be performed in the descending aorta starting 5 seconds after the beginning of contrast injection. A breath-hold command is given 14 seconds into the contrast bolus. Once the target threshold of 300 HU is reached in the descending aorta, the rest of the CTA will be initiated in the next one to two heartbeats.
TABLE 3.
Contrast Dose and Flow Rate by Patient Weight
| Weight |
|||||
|---|---|---|---|---|---|
| Kilograms | Pounds | Contrast (mL) | 30% Contrast and 70% Saline Mix (mL) |
Saline (mL) | Injection Rate (mL/s) |
| < 60 | < 131 | 44 | 20 | 50 | 4 |
| 60–70 | 131–154 | 54 | 20 | 50 | 4.5 |
| 71–100 | 155–220 | 54 | 20 | 50 | 5 |
| > 100 | > 220 | 64 | 20 | 50 | 5 |
Adenosine Stress CT Perfusion Imaging
Twenty minutes after the administration of sublingual nitrates for the rest CTA, IV adenosine (0.14 mg/kg/min) will be administered during continuous ECG monitoring. Three minutes into the adenosine infusion, the scanner will be instructed to acquire the heart rate and adjust the gantry rotation time. A breath-hold exercise will not be performed during adenosine infusion because of our previously observed lack of heart rate changes during breath-holding performed during adenosine infusion. Furthermore, breath-holding during an adenosine infusion causes marked fluctuations in heart rate immediately after expiration. A physician will be present at all times during adenosine infusion to monitor the patient for the development of advanced atrioventricular block, bronchospasm, or any other concerning adverse effects.
Four minutes into the adenosine infusion, iopamidol will be administered in three phases: 100% contrast agent, followed by 30% contrast agent and 70% saline mix, then 100% saline. Contrast dose will be 50–70 mL at a flow rate of 4–5 mL/s and will be adjusted according to patient weight (Table 3). Real-time bolus tracking will be performed in the descending aorta and will begin 5 seconds after contrast administration begins. A breath-hold command will be given 14 seconds into the contrast bolus. When a threshold of 300 HU is achieved in the descending aorta, the stress CT perfusion imaging study will be acquired in the next one to two heart-beats. The stress CT perfusion imaging acquisition parameters are detailed in Table 4. Immediately after adenosine stress CT acquisition, a 12-lead ECG and blood pressure measurement will be repeated after discontinuation of adenosine and will be reviewed by a physician. IV hydration will be continued during recovery, not to exceed a total of 500 mL, if deemed appropriate by the super-vising physician.
TABLE 4.
320-MDCT Stress Myocardial CT Perfusion Parameters
| Heart Rate (beats/min) |
Body Mass Index (kg/m2) |
No. of Detector Rows |
Detector Width (mm) |
Tube Voltage (kV) |
Tube Current (mA) |
Target Cardiac Phase (%) |
Window (%) | No. of Beats |
|
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||
| ≤ 65 | ≤ 19.9 | 240–320 | 0.5 | 120 | 270 | 350 | 85 | 20 | 1 |
| ≤ 65 | 20–24.9 | 240–320 | 0.5 | 120 | 300 | 370 | 85 | 20 | 1 |
| ≤ 65 | 25–29.9 | 240–320 | 0.5 | 120 | 350 | 400 | 85 | 20 | 1 |
| ≤ 65 | 30–34.9 | 240–320 | 0.5 | 120 | 370 | 450 | 85 | 20 | 1 |
| ≤ 65 | 35–39.9 | 240–320 | 0.5 | 120 | 400 | 470 | 85 | 20 | 1 |
| > 65 | ≤ 19.9 | 240–320 | 0.5 | 120 | 270 | 300 | 85 | 20 | 2 |
| > 65 | 20–39.9 | 240–320 | 0.5 | 120 | 300 | 350 | 85 | 20 | 2 |
Using the dose-length product reported on the scanner and converted to effective dose using a standard method outlined elsewhere [24], the mean radiation dose is estimated as follows: total mean effective dose is 9–13 mSv (CACS, 1.0 mSv; rest CTA and CT perfusion imaging, 3–5 mSv; and adenosine stress CT perfusion imaging, 5–7 mSv). The overall radiation dose for the study is not to exceed 25.5 mSv.
Data Handling and Analysis
Image Reconstruction and Transfer
Deidentified image raw data will be transferred to the CT Core Laboratories for reconstruction and blinded analysis by separate CTA and CT perfusion imaging Core Laboratories that are blinded to each other’s results.
CACS
Unenhanced CT images for CACS will be reconstructed with a 3-mm slice thickness using a convolution kernel (FC12) and will be analyzed as described elsewhere [25, 26].
CTA
CTA raw data will be reconstructed using both a standard (FC43) and a sharp (FC05) convolution kernel, 0.5-mm slice thickness with a 0.25-mm increment, and a motion-free phase chosen using automated phase selection software (PhaseXact, Toshiba Medical Systems). Optimal reconstruction will be identified on a per-patient and a per-vessel basis. Images from at least three different phases will be provided for image analysis.
Myocardial CT perfusion imaging
Rest and stress dynamic bolus-tracking images will be reconstructed every 0.5 second to determine the arterial input function before CT perfusion imaging. Additionally, rest and stress images will be reconstructed every 1% of the R-R interval inclusive of all available phases (Tables 2 and 4) with a 0.5 mm slice thickness. Images will be reconstructed using a myocardial perfusion kernel (FC03) that has no edge enhancement and uses a previously validated beam hardening correction algorithm [27]. A central laboratory processing technician will visually inspect all reconstructed phases and select the phase with least cardiac motion for myocardial perfusion analysis.
Noncardiac Findings
The CTA study will be reviewed for noncardiac findings by a locally qualified institutionally approved radiologist and will be reported back to the site investigator within 3 weeks of the acquisition.
CTA Coronary Analysis
Coronary artery segmentation
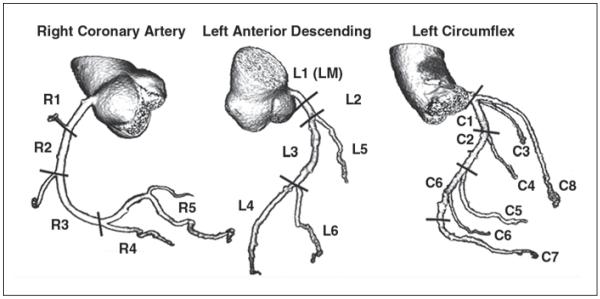
Image data will be transferred to a workstation (Vitrea fX version 3.0, Vital Images) for analysis. All arterial segments of at least 1.0 mm in diameter will be segmented according to a modified 29-segment American College of Cardiology and American Heart Association model nomenclature condensed to 19 segments, developed in the CORE-64 study [25, 28] (Fig. 2). Segments not visualized will be recorded as such.
Fig. 2.
Coronary artery segmentation model used for CORE-64 study with 19 segments: five in right coronary artery, six in left anterior descending coronary artery (including left main), seven in left circumflex coronary artery, and one in intermediate branch. Entire coronary artery tree is included in model. All segments ≥ 1.0 mm will be included in data analysis. C1 = proximal left circumflex coronary artery, C2 = midleft circumflex coronary artery, C3 = first obtuse marginal, C4 = second obtuse marginal, C5 = third obtuse marginal, C6 = grouped distal left circumflex coronary artery (first left posterolateral, second left posterolateral, and third left posterolateral; segments 19.1, 23, 24, 25, and 26 of Coronary Artery Surgery Study model [28]), C7 = left posterior descending, C8 = intermediate branch, L1 = left main (LM), L2 = proximal left anterior descending, L3 = midleft anterior descending, L4 = distal left anterior descending, L5 = first diagonal branch, L6 = second diagonal branch, R1 = proximal right coronary artery, R2 = midright coronary artery, R3 = distal right coronary artery, R4 = right posterior descending, R5 = grouped right posterolateral (first right posterolateral, second right posterolateral, and third right posterolateral; segments 5, 6, 7, 8 of Coronary Artery Surgery Study model). Adapted with permission from [25].
CTA coronary analysis
All studies will be analyzed by two blinded investigators. All studies will be assessed for image quality, calcium burden, and arterial lumen stenosis independently for all coronary arterial segments. Images will be viewed using volume-rendered images, curved multiplanar reformations, maximum-intensity projections, and cross-sectional images in systolic (if available) or diastolic phases as well as from standard and sharp kernels.
Visual CTA coronary arterial analysis
Visual assessment of arterial segment lumen diameter stenosis will be performed using a categoric scale: no stenosis, 1–29%, 30–49%, 50–69%, 70–99%, and 100%. For this purpose, the minimum lumen diameter is identified for each arterial segment and then compared with an appropriate reference site, such as a disease-free site in closest proximity to the lesion site. Disagreements between the two readers will be resolved by consensus. Visual assessment scores will not be modified after the quantitative assessment, and both datasets will be kept separate and processed individually. Image quality will be scored on a 4-point scale, as described elsewhere [29]: 1, optimal quality (absence of motion artifact and optimal contrast opacification); 2, adequate quality (minor imaging artifacts); 3, poor (significant motion artifact, calcification artifact, or poor contrast opacification); or 4, nonassessable (absence of contrast opacification or incomplete scan).
Quantitative CTA coronary arterial analysis
Quantitative assessment of the degree of diameter stenosis will be performed for all stenoses visually assessed as 30% or greater. Quantitative measurements will be performed using a semiautomatic contour detection algorithm or by manual measurements of reference or lesion diameters using electronic calipers provided by the commercial software. Cross-sectional and longitudinal projections will be considered. Contour editing of lumen borders will be performed at the discretion of the readers. The reference diameter will be calculated as the average of the proximal and distal diameter within a segment at disease-free sites. The minimum lumen diameter will be determined for each arterial segment, and a percentage of diameter stenosis will be calculated. Differences between the readers will be resolved by consensus.
CTA reader qualification
Reader 1 is level 3 trained in CTA with more than 500 study interpretations. Reader 2 is a level 3–trained Certification Board of Cardiovascular CT board-certified expert in cardiac CT with over 1000 study interpretations.
Myocardial CT Perfusion Analysis
Myocardium segmentation
A technologist will load the rest and stress CT perfusion imaging datasets into the software package (Myocardial Perfusion, Toshiba Medical Systems). The arterial input function will be analyzed using bolus-tracking image data by measuring the change in attenuation over time in the descending aorta. Axial volumetric image data will be arranged in the short axis, vertical long axis, and horizontal long axis with a slice thickness of 3 mm. An automatic border detection algorithm will define the endocardial and epicardial borders, with manual input as needed by the technologist and CT perfusion imaging readers. The software will then divide the myocardium using a modified 17-segment model [30] that is condensed into 13 segments (Fig. 3).
Fig. 3.
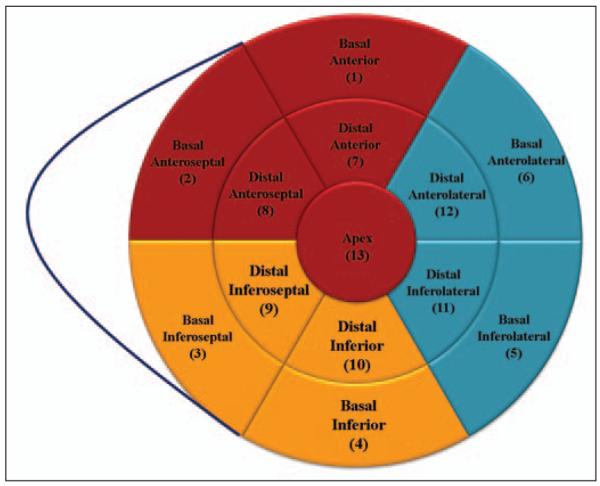
Thirteen-segment myocardial model. Red denotes left anterior descending artery territory, blue denotes left circumflex territory, and yellow denotes right coronary artery territory.
Quantitative CT perfusion imaging analysis
The software will automatically generate CT attenuation measurements throughout the myocardium in the subendocardial, midmyocardial, and subepicardial layers from the rest and stress images. These CT attenuation numbers will be automatically transmitted to the database for quantitative analysis. Several CT perfusion imaging metrics will be calculated from the data in conjunction with the arterial input function data, including a measurement of the transmural perfusion ratio (i.e., subendocardial attenuation divided by mean subepicardial attenuation), mean myocardial attenuation, and myocardial attenuation normalized to the arterial input function for each myocardial segment and displayed in a 13-segment polar plot (Fig. 4), as described elsewhere [19, 20, 22].
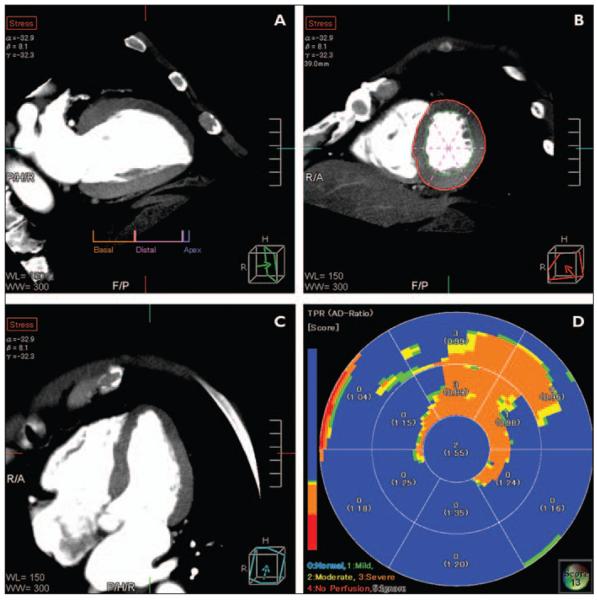
Fig. 4.
Adenosine stress CT myocardial perfusion analysis using combination of visual and quantitative data to assess myocardium for perfusion abnormalities.
A, B, and C, Multiplanar reconstructions show vertical long-axis (A), short-axis (B), and horizontal long-axis (C) views.
D, Thirteen-segment polar plot displays color-coded map of transmural perfusion ratio (i.e., subendocardial attenuation divided by subepicardial attenuation). Perfusion deficit is confirmed both visually and quantitatively to be present in anterior and anteroseptal walls corresponding to obstructive atherosclerosis in left anterior descending artery and first obtuse marginal artery.
Visual CT perfusion imaging analysis
Visual assessments will be performed by two independent readers blinded to all other data. Quantitative transmural perfusion data will be available for interpretation (Fig. 4). For each myocardial segment, rest and stress perfusion images will be visually assessed using a categoric scale: 0, normal; 1, mild perfusion deficit; 2, moderate perfusion deficit; 3, severe perfusion deficit; 4, infarct with myocardial thinning; and 5, artifact. Any disagreements by the two readers will be resolved by a third reader using a consensus process. Overall image quality for the rest and stress images will be scored as excellent, good, fair, or poor with the following definitions: 1, excellent (study is of diagnostic image quality and is devoid of artifacts that would interfere with interpretation); 2, good (study is of diagnostic quality with minimal artifacts, and any artifacts present are subtle and do not interfere with the interpretation of the study); 3, fair (study still of diagnostic quality, but there are significant artifacts limiting the interpretation of portions of the study); and 4, poor (image quality is severely limited making study uninterpretable). In addition, when the interpretation of individual segments is compromised by artifacts, the source of the artifact (motion, beam-hardening, or reconstruction artifact) will be noted.
CT Perfusion Imaging Reader Qualification
Consensus reader
The consensus reader is level 3 trained in coronary CTA and has 7 years of experience reading rest and stress CT perfusion images in both preclinical and clinical studies.
Reader 1 and 2
Readers 1 and 2 are level 2 trained in coronary CTA and have 1.5 and 1.0 years of experience, respectively, reading CT perfusion images. A training set of 80 cases was used to train the readers in CT perfusion image interpretation. In addition, we have prespecified that the first 50 cases will be read by consensus of all three readers to minimize variation among the readers.
Adjudication: Matching Coronary Vessels With Myocardial Territories
The independent CTA and CT perfusion imaging core laboratories will complete their blinded analyses before the adjudication process. Because the primary aim of the CORE320 study is to detect obstructive atherosclerosis causing corresponding myocardial perfusion abnormalities, abnormal myocardial segments will require anatomic coregistration with the culprit vessel. Matching coronary segments to myocardial territories can be very challenging because of variation in coronary anatomy. In summary, the 13 myocardial segments will be assigned to a vessel using standard methods (Fig. 3). The anteroseptal, anterior, and apical walls will be assigned to the left anterior descending artery, the lateral segments to the left circumflex, and the inferoseptal and inferior walls to the right coronary artery. After completion and locking of all qualitative and quantitative measurements, CTA and CT perfusion imaging data will be aligned to ensure that a coronary segment with a greater than 50% stenosis detected by CTA supplies an ischemic myocardial territory. If there is alignment, a match between angiography and perfusion will be confirmed, and there is no need for adjudication. On the other hand, if there is a mismatch, an adjudication process will be triggered to evaluate the possibility of a different alignment based on an anatomic variance. The adjudication committee will be composed of one member from the CT perfusion imaging core laboratory, one from the CTA core laboratory, and a third independent member. A final decision will be reached by consensus. No visual assessment or quantitative assessment scores by either modality will be changed during the adjudication process. Changes in the alignment will also follow prespecified rules according to different coronary anatomy patterns and will be recorded in the database.
Discussion
Coronary CTA has been one of the most important advances in the noninvasive diagnosis of CAD in the past decade. It is capable of excluding CAD with unsurpassed negative predictive value [5-8]. In the setting of non-obstructive and obstructive CAD, it is capable of determining the percentage of luminal stenosis and determining the constituents of plaque. However, in its current form, it does not provide important information on the physiologic significance of coronary stenoses.
Adenosine stress CT perfusion imaging has been well validated in preclinical and clinical studies [18-22, 31]. Those previous studies were performed with mostly single- and dual-source 64-MDCT systems. In contrast, CORE320 will use a wide-area 320-MDCT system. This scanner is capable of full cardiac coverage over a single heartbeat without the need for table movement, providing several key advantages derived from the temporal uniformity of the acquisition, including the elimination of variations in contrast enhancement and the ability to target a specific portion of the contrast bolus and optimize the timing of CT perfusion imaging [23]. In addition, very short scan acquisition times allow reductions in contrast dose and enable arterial phase imaging before contrast transit to the venous side of the coronary circulation. Finally, prospective ECG triggering can be performed using half-scan acquisition and reconstruction for slower heart rates and segmented acquisition and reconstruction for higher heart rates commonly seen during the infusion of adenosine.
There are several components of the CORE320 acquisition, reconstruction, and analysis protocol that should be discussed. The first is the order of the CTA followed by CT perfusion imaging. Although all patients in the CORE320 study will undergo CTA and CT perfusion imaging, we think that the clinical use of CTA and CT perfusion imaging will use CTA first, followed by CT perfusion imaging in patients with moderate-to-severe stenoses. Using this sequence, a future diagnostic algorithm will take advantage of the high negative predictive value of CTA and will exclude disease in most patients. Only those patients with moderate-to-severe stenoses will then require a CT perfusion imaging study. This strategy also allows us to maintain a lower heart rate during the CTA. This improves image quality and reduces radiation dose [32-34]. In a pilot study using a 256-MDCT scanner, we found in 19 patients that performance of a stress CT perfusion imaging study first results in higher heart rates during the subsequent CTA. That study showed that heart rates are 7 beats/min higher, compared with baseline, when a CTA follows a recent adenosine stress CT perfusion imaging study [19]. These higher heart rates during CTA are undesirable because they could result in motion artifacts and eliminate the ability to use single-heartbeat prospective ECG triggering and, thus, increase radiation dose.
The CORE320 CT perfusion imaging protocol targets the upslope to peak of the contrast bolus in mid-to-late diastole measured in the descending aorta. This target is supported by preclinical and clinical data from Johns Hopkins University. An analysis in preclinical models of coronary ischemia that underwent dynamic adenosine stress CT perfusion imaging revealed that, on average, the maximum attenuation density differences in ischemic versus remote territories (56.7 ± 20.2 HU) occur in the upslope of the contrast bolus 3.8 ± 2.6 seconds before peak arterial enhancement. These differences in myocardial attenuation are less marked at peak enhancement (40.4 ± 18.9 HU) and quickly disappear in the down slope of the contrast bolus [21]. These differences were confirmed in a similar preclinical model that underwent adenosine stress helical CT perfusion imaging during the upslope of the contrast bolus [22]. The selection of targeting mid-to-late diastole is supported by adenosine stress CT perfusion imaging studies in 75 patients. The first group of patients underwent retrospective ECG-gated adenosine stress CT perfusion imaging (n = 43). Image datasets were reconstructed throughout systole and diastole and were examined for the best motion-free phase. The best motion-free images were noted in mid-to-end diastole in 79% of cases. When examining diastole only, the best phase, on average, was 86% of the R-R interval (range, 75–100%) [19]. This finding was confirmed in 32 patients who underwent adenosine stress 320-MDCT perfusion imaging that used prospective ECG triggering in mid-to-late diastole. In this group of patients, the best phase was 80% on average, with a range of 56–99% [35].
The CORE320 study will use a previously validated myocardial perfusion-specific reconstruction kernel that includes a beam-hardening correction algorithm described elsewhere [27]. That study used myocardial phantoms and animal models of coronary ischemia and found that beam-hardening artifacts can be adequately corrected for and that their correction improves the measurement of myocardial perfusion [27]. In addition, the reconstruction kernel for CORE320 CT perfusion imaging studies lacks edge enhancement. This fact is extremely important in the assessment for subendocardial perfusion deficits, because edge enhancement can artificially lower the measured attenuation in the subendocardium as it interfaces with the contrast-enhanced left ventricular blood pool and can artificially increase the measured attenuation in the subepicardium as it interfaces with the air-filled lungs. Together, these effects of edge enhancement can give the false appearance of subendocardial perfusion deficits in normal territories.
The limitations of the CORE320 study are additional iodinated contrast and radiation given for the perfusion study. In this study, we use prospective ECG triggering and have reduced x-ray exposure times to the minimum time required to maintain image quality while reducing effective radiation dose. Although, the 320-MDCT scanner allows prospective ECG triggering with segmental acquisition and reconstruction to improve temporal resolution, β-blockers are still required to maintain optimal image quality, avoid motion artifacts, and reduce radiation dose. Studies have shown that β-blockers can blunt differences between ischemic and nonischemic myocardium, and this could affect our sensitivity for myocardial ischemia [36].
In summary, the CORE320 multicenter multinational study will rigorously evaluate the accuracy of CTA combined with CT perfusion imaging to diagnose obstructive atherosclerosis causing myocardial perfusion abnormalities using 320-MDCT.
Acknowledgments
This study was supported by a research grant from Toshiba Medical Systems.
Footnotes
FOR YOUR INFORMATION
The comprehensive book based on the ARRS 2011 annual meeting categorical course on Imaging of the Active Lifestyle: From the Weekend Warrior to the Pro Athlete is now available! For more information or to purchase a copy, see www.arrs.org.
References
- 1.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–557. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P. Who is a candidate for noninvasive coronary angiography? Ann Intern Med. 2006;145:466–467. doi: 10.7326/0003-4819-145-6-200609190-00012. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JE, Boxt LM, Desjardins B, Fishman EK, Larson PA, Schoepf J. ACR practice guideline for the performance and interpretation of cardiac computed tomography (CT) J Am Coll Radiol. 2006;3:677–685. doi: 10.1016/j.jacr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 5.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Garcia MJ, Lessick J, Hoffmann MH. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–411. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 7.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 9.Mowatt G, Cook JA, Hillis GS, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–1393. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 10.Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152:167–177. doi: 10.7326/0003-4819-152-3-201002020-00008. [DOI] [PubMed] [Google Scholar]
- 11.Di Carli MF, Dorbala S, Curillova Z, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol. 2007;14:799–809. doi: 10.1016/j.nuclcard.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Schuijf JD, Wijns W, Jukema JW, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48:2508–2514. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 13.Hacker M, Jakobs T, Matthiesen F, et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experiences. J Nucl Med. 2005;46:1294–1300. [PubMed] [Google Scholar]
- 14.Rispler S, Keidar Z, Ghersin E, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059–1067. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 16.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 17.Iskandrian AS, Chae SC, Heo J, Stanberry CD, Wasserleben V, Cave V. Independent and incremental prognostic value of exercise single-photon emission computed tomographic (SPECT) thallium imaging in coronary artery disease. J Am Coll Cardiol. 1993;22:665–670. doi: 10.1016/0735-1097(93)90174-y. [DOI] [PubMed] [Google Scholar]
- 18.Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009;54:1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 19.George RT, Arbab-Zadeh A, Miller JM, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2:174–182. doi: 10.1161/CIRCIMAGING.108.813766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George RT, Ichihara T, Lima JA, Lardo AC. A method for reconstructing the arterial input function during helical CT: implications for myocardial perfusion distribution imaging. Radiology. 2010;255:396–404. doi: 10.1148/radiol.10081121. [DOI] [PubMed] [Google Scholar]
- 21.George RT, Jerosch-Herold M, Silva C, et al. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007;42:815–822. doi: 10.1097/RLI.0b013e318124a884. [DOI] [PubMed] [Google Scholar]
- 22.George RT, Silva C, Cordeiro MA, et al. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48:153–160. doi: 10.1016/j.jacc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Dewey M, Zimmermann E, Deissenrieder F, et al. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009;120:867–875. doi: 10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 24.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006;79:968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 25.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009;19:816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa K, George RT, Arbab-Zadeh A, Lima JA, Lardo AC. Characterization and correction of beam-hardening artifacts during dynamic volume CT assessment of myocardial perfusion. Radiology. 2010;256:111–118. doi: 10.1148/radiol.10091399. [DOI] [PubMed] [Google Scholar]
- 28.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography)—developed in collaboration with the Society for Cardiac Angiography and Interventions J Am Coll Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 29.Dewey M, Vavere AL, Arbab-Zadeh A, et al. Patient characteristics as predictors of image quality and diagnostic accuracy of MDCT compared with conventional coronary angiography for detecting coronary artery stenoses: CORE-64 Multicenter International Trial. AJR. 2010;194:93–102. doi: 10.2214/AJR.09.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 31.Daghini E, Primak AN, Chade AR, et al. Evaluation of porcine myocardial microvascular permeability and fractional vascular volume using 64-slice helical computed tomography (CT) Invest Radiol. 2007;42:274–282. doi: 10.1097/01.rli.0000258086.78179.90. [DOI] [PubMed] [Google Scholar]
- 32.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 33.Steigner ML, Otero HJ, Cai T, et al. Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 2009;25:85–90. doi: 10.1007/s10554-008-9347-8. [DOI] [PubMed] [Google Scholar]
- 34.Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008;246:742–753. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 35.George RT, Kitagawa K, Laws K, Lardo AC, Lima JA. Combined adenosine stress perfusion and coronary angiography using 320-row detector dynamic volume computed tomography in patients with suspected coronary artery disease. Circulation. 2008;118:S_936. [Google Scholar]
- 36.Koepfli P, Wyss CA, Namdar M, et al. Beta-adrenergic blockade and myocardial perfusion in coronary artery disease: differential effects in stenotic versus remote myocardial segments. J Nucl Med. 2004;45:1626–1631. [PubMed] [Google Scholar]