Abstract
Nontypeable Haemophilus influenzae (NTHi) are human-adapted commensal bacteria that can cause a number of chronic mucosal infections, including otitis media and bronchitis. One way for these organisms to survive antibiotic therapy and cause recurrent disease is to stop replicating, as most antimicrobials target essential biosynthetic pathways. Toxin-antitoxin (TA) gene pairs have been shown to facilitate entry into a reversible bacteriostatic state. Characteristically, these operons encode a protein toxin and an antitoxin that associate following translation to form a nontoxic complex, which then binds to and regulates the cognate TA promoter. Under stressful conditions, the labile antitoxin is degraded and the complex disintegrates, freeing the stable toxin to facilitate growth arrest. How these events affected the regulation of the TA locus, as well as how the transcription of the operon was subsequently returned to its normal state upon resumption of growth, was not fully understood. Here we show that expression of the NTHi vapBC-1 TA locus is repressed by a complex of VapB-1 and VapC-1 under conditions favorable for growth, and activated by the global transactivator Factor for Inversion Stimulation (Fis) upon nutrient upshift from stationary phase. Further, we demonstrate for the first time that the VapC-1 toxin alone can bind to its cognate TA locus control region and that the presence of VapB-1 directs the binding of the VapBC-1 complex in the transcriptional regulation of vapBC-1.
Introduction
Unencapsulated (nontypeable) Haemophilus influenzae (NTHi) are Gram-negative obligate bacteria of the human upper respiratory tract that have the potential to cause mucosal diseases such as otitis media, bronchitis, and pneumonia [1]. These infections can be recurrent, and NTHi have evolved mechanisms to persist between episodes [2]. One successful survival strategy is the ability to undergo reversible growth arrest, mediated by toxin-antitoxin (TA) gene pairs [3]. TA loci have been found in nearly all bacterial genomes sequenced to date, and many code for a protein toxin and antitoxin that form a nontoxic complex upon translation which autorepresses the cognate promoter. Under stressful environmental conditions such as nutrient limitation, antibiotic therapy, or oxidative stress, the labile antitoxin is degraded and the more stable toxin is freed to facilitate growth arrest. Given that these events would destroy the autoregulatory protein complex, the means by which TA promoter regulation is returned to its normal state upon removal of stress and resumption of growth is not fully understood.
TA gene pairs have been divided into several families, of which the vapBC locus is by far the most numerous [4]. This remarkable conservation of vapBC is underscored by the fact that many microorganisms contain multiple vapBC loci. For example, one study found that 20 vapBC TA homologues identified in the Mycobacterium tuberculosis genome were active when expressed in Mycobacterium smegmatis [5]. NTHi strains maintain two vapBC alleles in their chromosomes (designated vapBC-1 and vapBC-2), making this organism an excellent model in which to study the regulation and function of these highly conserved gene pairs.
The VapC toxins are characterized by a 100 amino acid motif known as a PilT N-terminus (PIN) domain that contains highly conserved acidic residues shown to be essential for metal ion coordination in other PIN-domain containing proteins that are ribonucleases [6]. The PIN domains display similarity to the nuclease domains of Taq polymerase, T4 RNase H, and the 5′-3′ flap endonucleases [7]. The VapB antitoxins are thought to have evolved to block the ribonucleic activity of their associated VapC toxins. This is consistent with a study showing that the VapB antitoxins were specific for their cognate toxins in four separate vapBC loci in M. tuberculosis [5]. However, another study found that certain noncognate M. tuberculosis toxin and antitoxin homologues could associate, both in vivo and in vitro [8], so questions remain about the exact role of each antitoxin. VapB-1 is a member of the VagC superfamily of virulence-associated and related proteins, and contains a SpoVT/AbrB-like domain [9]. AbrB is an ambiactive transcriptional regulator important during the transition from vegetative to the sporulation state in Bacillus, and SpoVT is another member of this family with transcriptional regulation activity. Therefore, the VapB-1 antitoxin may serve to control genes involved in stress survival.
We have shown previously that NTHi vapBC-1 encodes a VapB-1 antitoxin that binds to and interferes with the activity of its cognate ribonuclease VapC-1 toxin. This toxin has a PIN domain and degrades RNA but not single- or double-stranded DNA in vitro [10]. However, we have yet to determine how the VapBC-1 complex may control its own expression and modulate the toxic VapC-1 ribonuclease function following stress.
In an earlier study, we also reported that the activity of vapBC-1 promoter::lacZ reporter gene fusions from two NTHi clinical isolates analyzed over the cell cycle in an Escherichia coli background displayed an inverse relationship to culture density [10]. This pattern of transcriptional activity is consistent with vapBC-1 locus regulation by the Factor for Inversion Stimulation (Fis), but Fis expression in NTHi had not been characterized, limiting our interpretation of this data [11]. Fis is a small nucleoid-associated protein that binds to sites throughout bacterial chromosomes and regulates a wide array of genes, both directly by promoter interaction and indirectly by altering DNA structure during various growth stages [12]–[16]. In Escherichia coli, the cellular levels of Fis are dramatically increased upon nutrient upshift from stationary phase in batch culture, conditions that would precede TA locus upregulation [11]. A high-affinity binding sequence (5′-GNNYAWWWWWTRNNC-3′, where W is A/T) has been characterized for E. coli Fis; however, the Fis binding sequence for E. coli and Salmonella is rather degenerate, suggesting significant variation in bacterial Fis recognition of DNA [14], [17]–[19]. We have identified a putative Fis binding site within the NTHi vapBC-1 locus control region that matches 13 of 15 residues of the E. coli sequence, suggesting its possible regulation by the NTHi Fis protein.
To gain understanding of the growth phase regulation of the vapBC-1 operon, we have conducted in vivo studies of the fis and vapBC-1 response to nutrient upshift in a NTHi clinical isolate, strain R2866, with the hypothesis that Fis and the VapBC-1 TA complex serve to regulate vapBC-1 expression. Additionally, the NTHi Fis and VapBC-1 TA proteins were purified and analyzed for their interaction with the vapBC-1 locus control region. The work presented herein elucidates a novel role for the VapC-1 toxin and supports a new model for vapBC-1 TA locus autoregulation.
Materials and Methods
Bacterial strains and culture conditions
The bacterial strains and plasmids used in these studies are listed in Table 1. E. coli strains were grown in LB broth or agar ±30 µg/ml kanamycin or 100 µg/ml ampicillin, as required. NTHi strains were grown in either brain-heart infusion (BHI) broth or agar supplemented with 10 µg/ml heme-histidine and β-NAD (sBHI), or on chocolate agar plates containing 5 kU bacitracin/L. Where required, the following antibiotics were added: 5 µg/ml gentamicin, 30 µg/ml kanamycin, or 2 µg/ml chloramphenicol.
Table 1. Bacteria and plasmids used in this study.
| Bacteria | Description | Source |
| H. influenzae | ||
| R2866 | Blood isolate from an immunocompetent child with meningitis immunized with the Hib vaccine. | A.L. Smith |
| R2866 ΔvapBC-1 | The vapBC-1 TA locus replaced with a kanamycin resistance cassette. | This work |
| R2866 Δfis | The fis gene replaced with a chloramphenicol resistance cassette. | This work |
| R2866 ΔfisΔvapBC-1 | fis and vapBC-1 double mutant. | This work |
| E. coli | ||
| BL21 | F− dcm ompT hsdS(rB − mB −) gal [malB +]K-12(λS) | EMD Bioscience |
| BL21(DE3) | F− ompT hsdS (rB −mB −) gal dcm λDE3 | EMD Bioscience |
| DD12 | MC4100 recA::RP4-2-Tc::Mu KmR | [23] |
| DH5α | F− endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG Φ80dlacZΔM15 Δ(lacZYA-argF)U169, hsdR17 | Lab collection |
Cloning of vapB-1, vapBC-1 and fis
Each wild-type vap gene was cloned into the expression vector pET24b as a C-terminal polyhistidine tag fusion either in single copy (vapB-1) or in tandem (vapBC-1) as described previously [10]. To determine whether the polyhistidine tag interfered with function, VapB-1 was also cloned with an N-terminal polyhistidine tag in the vector pTrcHisA, which was subsequently removed using the Enterokinase Cleavage Capture Kit (Novagen) according to the manufacturer's instructions. The crystal structure for E. coli Fis has been solved [20], and the first 23 amino acids of the homodimer are disordered, whereas the C-terminal domain is considered important for DNA binding. Therefore, we chose an N-terminal polyhistidine tag fusion to express and purify NTHi Fis using the vector pTrcHisA. This tag was subsequently removed using enterokinase cleavage as above. All PCR reactions were performed with Phusion FLASH high-fidelity DNA polymerase (New England Biolabs) using purified genomic DNA from NTHi strain R2866 as the template. Oligonucleotides used in this study were custom synthesized by Eurofins MWG Operon. Each pET24b-based construct was expressed in E. coli BL21(DE3). The fis gene was amplified from NTHi strain R2866 genomic DNA using high-fidelity PCR with the primers FisXhoIfor and FisKpnIrev (Table 2). The 315 bp product was cut with XhoI/KpnI and ligated to XhoI/KpnI-cut pTrcHisA, resulting in pDD715, the expression plasmid for Fis with an N-terminal polyhistidine tag. This construct was confirmed by DNA sequencing and expressed in E. coli BL21.
Table 2. Primers for fis and vapBC-1 cloning.a .
| FisXhoIfor | 5′-ATAACTCGAGATGTTAGAACAACAACG-3′ |
| FisKpnIrev | 5′-AAACGGTACCTTATCCCATACCG-3′ |
| D99NFor | 5′-[Phos]AATCTATGGATCGCTTGTCACG-3′ |
| D99NRev | 5′-[Phos]ATTATTTCCGATAGGTCGCC-3′ |
| VapCSacFor | 5′-CGAGGAGCTCTATGATTTATATGTTAGACACC-3′ |
| VapCXhoIRev | 5′-GAATCTCGAGTTTTGTCCAATCTTGCC-3′ |
| BCFor | 5′-GCTTTCTAGACAGGCTAAATATACCG-3′ |
| BCRev | 5′- GGTCTCTAGAGGCATTGTGCGCCAC -3′ |
| Fis28Sacfor | 5′-AAAAGAGCTCAATGTGCCAGTGACC-3′ |
| Fis28Xbarev | 5′-AATATCTAGACCGTTAAGGCATCAGCAGG-3′ |
| Fis28Xbafor | 5′-CGTGTCTAGAATATGCTTGGTATCAACCG-3′ |
| Fis28Pstrev | 5′-GCACCTGCAGTTTACTGGAAAAGTGC-3′ |
| CmNhefor | 5′-GCAGGCTAGCCCGACGCACTTTGC-3′ |
| CmNherev | 5′-CTTAGCTAGCTTACGCCCCGCCCTG-3′ |
| BC1403for | 5′-ACTAGAATTCATCATTTACTCATTGACTTGC-3′ |
| BC1403rev | 5′-GTTAGGATCCTGAAACACTTTAGTAAGC-3′ |
| 1403Xbafor | 5′-ATTTTCTAGAGTTATTGTCTCATGAGCGG-3′ |
| 1403Xbarev | 5′-TTCCTCTAGAGAAATACGGGCAGACATGG-3′ |
Restriction enzyme sites used for cloning are underlined.
Site-directed mutagenesis of VapC-1
The aspartate residue at position 99 in VapC-1 was changed to an asparagine by PCR mutagenesis of pDD686 using the following primers: D99NFor and D99NRev (Table 2). The PCR reaction was incubated with DpnI to digest the template DNA, and the column-purified product was used to transform competent DH5α cells. The resulting plasmid was sequenced on both strands to ensure the correct D99N mutation, and designated pDD758. To express the VapC-1 D99N mutant alone, pDD758 was amplified with the primers VapCSacFor and VapCXhoIRev and the 660 bp amplicon was cut with SacI/XhoI and ligated to SacI/XhoI-cut pET24b, creating pDD780 (Table 2). This plasmid was used to express the VapC-1 D99N protein with a C-terminal polyhistidine tag.
Protein purification
Proteins were purified from induced cultures using the Promega MagneHis protein purification system. Briefly, E. coli strains BL21 (for the pTrcHis vector-based constructs) or BL21(DE3) (for the pET24b vector-based constructs) carrying the various fusions were grown to logarithmic phase in LB broth with appropriate antibiotics and induced for 2 or 3 hours with 1 mM IPTG. The cells were pelleted, frozen at −80°C, and subjected to 3 freeze-thaw cycles prior to being processed using the manufacturer's protocol for native purification. Purified proteins were quantitated using the Bradford protein assay (Pierce). Aliquots of purified proteins were frozen at −80°C and thawed when needed. Where required, polyhistidine N-terminal tags were removed by enterokinase cleavage.
Deletion of vapBC-1 and fis
The vapBC-1 toxin-antitoxin locus was deleted from strain R2866 by allelic exchange as follows. A 2557 bp area around the vapBC-1 locus in the R2866 genome was amplified by Phusion high fidelity PCR using the primers BCFor and BCRev (Table 2). The amplicon was cut with XbaI and ligated to compatible ends in SpeI-cut pGEM5, creating pDD730. The pDD730 plasmid was then cut with BamHI and BglII and gel purified, which removed 564 of the 619 bp of the vapBC-1 operon. A 1275 bp Phusion PCR amplicon of the aphII gene from pUC4K with BamHI ends was ligated in the place of the vapBC-1 operon, creating pDD731. This plasmid was then amplified with primers that flanked the multiple cloning site, and the amplicon was used to replace the vapBC-1 operon from strain R2866. PCR of the flanking regions followed by DNA sequencing confirmed the vapBC-1 deletion.
The fis gene was deleted in the following manner. Attempts to clone the region surrounding the NTHi fis gene were unsuccessful, so a three-step cloning technique was used. For the first step, a 637 base pair fragment upstream from fis which included 40 base pairs into the fis coding region was amplified by PCR with the primers Fis28Sacfor and Fis28Xbarev (Table 2). This was cut with SacI/XbaI and ligated into the SacI/XbaI–digested vector pBluescript SK+, creating pDD729. For the second step, pDD729 was cut with XbaI/PstI and ligated to a XbaI/PstI-cut 317 base pair fragment representing the last 71 base pairs of fis plus flanking DNA amplified by PCR using the primers Fis28Xbafor and Fis28Pstrev, creating pDD732. Finally, pDD732 was cut with XbaI and ligated to a compatible NheI-cut 900 base pair fragment amplified with the primers CmNhefor and CmNherev (Table 2) that contained the chloramphenicol acetyltransferase (cat) resistance cassette from pACYC184, resulting in pDD733. This strategy deleted 188 bp of the 299 bp fis gene and inserted a cat cassette in its place. PCR of the flanking regions followed by DNA sequencing confirmed the fis deletion. Use of a high-fidelity PCR amplicon of this region in allelic exchange reactions successfully deleted fis from strains R2866 and R2866 vapBC-1.
Quantitative real time-polymerase chain reaction
The expression of fis in the wild type NTHi strain R2866 following nutrient upshift was analyzed by qRT-PCR. A 1.5 mL aliquot of a stationary phase culture of strain R2866 in sBHI was diluted into 25 mL of fresh, pre-warmed medium. Culture samples of 1 ml were obtained immediately prior to inoculation (time zero, stationary phase), 10, 30, 60, and 120 minutes following nutrient upshift. Each was immediately added to an equal volume of RNALater (Ambion) and held at 4°C until used (in all cases, less than 2 days). Total RNA from each sample was purified using the Charge Switch Total RNA kit (Invitrogen) according to the manufacturer's instructions, with a modification of changing the DNase I digestion step to 30 min at 37°C. Purified total RNA concentration was then quantified by NanoDrop spectrophotometry (Thermo Scientific), and each RNA sample was used as the template for PCR to ensure that there was no genomic DNA contamination prior to cDNA synthesis. 250 ng of total RNA from each sample was used for cDNA synthesis utilizing the Fermentas Maxima Universal First Strand cDNA Synthesis Kit with the included random hexamer primers. Each cDNA reaction was then diluted 1∶10 and used as template for PCR to ensure that the cDNA had successfully been synthesized. For real-time PCR, 25 µL reactions were prepared with 12.5 µl of 2× Power SYBR Green Master Mix (Applied Biosystems), 400 nM of each gene-specific primer set, and 5 µL of a 1∶10 dilution of cDNA. qPCR was performed using the Applied Biosystems 7300 Real-Time PCR System with the following cycling conditions: 10 min at 95°C, 40 cycles of 95°C for 15 sec and 60°C for 1 min. Relative expression of fis was calculated by the comparative Ct method (2–ΔΔCt) using time zero as the calibrator and the expression of a DNA gyrase subunit gene, gyrA, as the endogenous control. The gyrA gene was chosen because it had been used previously in qRT-PCR studies for this purpose in NTHi [21]. Primers for fis (forward: 5′-AGC AAA CCA TTA CGC GAT TC-3′, reverse: 5′-TTT GCT GCA CGA GTT TGA T-3′) and gyrA (forward: 5′-AGG TGT TCG CGG TAT CAA AC-3′, reverse: 5′-ATT GCA CCT TCA CCT TTT TGG-3′) were designed based on the genome sequence of R2866 using the Primer3 software (http://frodo.wi.mit.edu/primer3/). Three biological replicates and three technical replicates were analyzed for each time point.
Promoter::reporter gene fusions and activity assays
For the lacZ fusion, a 340 base pair sequence located upstream of the vapBC-1 locus in strain R2866 that included coding sequence for the first seven amino acids of vapB-1 was amplified by PCR with the primers BC1403for and BC1403rev, which included engineered EcoRI and BamHI sites, respectively (Table 2). This fragment was then ligated in-frame with a promoterless lacZ reporter gene in the vector pMC1403 [22], creating pDD693. To express this promoter fusion in NTHi, pDD693 was used as the template for PCR with the primers 1403Xbafor and 1403Xbarev. The amplicon was cut with XbaI and ligated to the compatible ends of the NheI-cut broad host-range conjugal plasmid pDD514 [23], resulting in pDD727. This plasmid was then conjugated into strain R2866 and the deletion mutants, and promoter activity was measured via β-galactosidase activity assays performed at least three times in triplicate at time zero (stationary phase) and every 30 minutes for the first 2 hours following nutrient upshift. The algorithm for determining β-galactosidase activity is: [OD420−(1.75*OD550)/t*v*OD600]*1000, where t = time of development of the reaction in minutes, v = volume of the sample in milliliters, and OD600 is the measure of the culture density [24]. This equation allows normalization of culture densities for comparison purposes.
DNase I protection analysis
A 153 bp substrate consisting of the vapB-1 translation initiation region and upstream sequence from the 340 bp of R2866 vapBC-1 locus control region above was produced by PCR amplification of pDD717 using high-fidelity Phusion FLASH DNA polymerase and the forward EcoRIBCpromfor, 5′- TTA GAA TTC GCT CGA TGA TTG CGG-3′ and reverse BC1403rev (see Table 2) primers at equimolar concentration. To enable the detection of DNase I products arising from the sense strand of the 153 bp vapBC-1 substrate, the forward primer was 5′-labeled with 32P using T4 polynucleotide kinase (New England Biolabs) and 3000 Ci/mmol [γ-32P]ATP (PerkinElmer). The length and purity of the PCR product was confirmed by electrophoresis on a 1% agarose gel in TAE buffer (40 mM Tris-acetate pH 8.3/2 mM EDTA).
All DNase I protection reactions contained 10 fmol/µL 32P-5′-labeled 153 bp substrate in total volume of 20 µL of DNase reaction buffer (25 mM Tris-HCl pH 7.4/70 mM KCl/7 mM Mg Cl2/3 mM CaCl2/1 mM EDTA/1 mM β-mercaptoethanol/50 µg/mL BSA/7% glycerol) and were incubated at 25°C for 30 min after the addition of the Fis or Vap proteins for DNA binding. Samples contained 20–800 fmol/µL protein as indicated by the molar ratio of protein to DNA given in the figure legend. For VapBC-1 reactions, VapBC-1 was reconstituted from VapB-1 and VapC-1 proteins at a 3∶1 molar ratio by incubation for 20 min at room temperature prior to addition to the samples. This VapB-1 to VapC-1 ratio was shown in a previous study to fully inhibit VapC-1 ribonuclease activity, suggesting that all VapC-1 molecules were sequestered in VapBC-1 complexes [10]. Therefore, the molar concentration of VapBC-1 complexes in the reconstituted system was estimated as the concentration of VapC-1, since the complexes could not be directly measured. Following the binding incubation, 0.02 U DNase I (New England Biolabs) was added, and the 25°C incubation continued for 2 min. Reactions were quenched with 75 µL of DNase I stop solution (90% ethanol/220 mM sodium acetate/70 ng/µL yeast tRNA), mixed, and placed immediately in a dry ice/ethanol bath for 30 min. The nucleic acid was precipitated by centrifugation at 13000× g for 30 min at 4°C, the pellets were washed with cold 70% ethanol, then dried. The pellets were resuspended in 95% formamide/0.01% bromophenol blue/0.01% xylene cyanol and resolved on a 5% denaturing 19∶1 polyacrylamide gel in TBE (89 mM Tris-borate/1 mM EDTA). DNase I fragments of the 32P-5′-labeled strand were detected using a GE Healthcare Storm 845 phosphorimaging system.
Electrophoretic mobility shift assays
A set of 32P-5′-labeled, 50 bp substrates were prepared for analysis of protein interaction with the vapBC-1 locus control region. Complementary single-stranded oligonucleotides containing vapB-1 translation initiation region (TIR) sequence or an upstream (US) sequence, shown to lack DNase I protection sites for Fis and the VapBC-1 complex, were obtained from Eurofins MWG Operon (Huntsville, AL). The sense strand of each 50 bp sequence, shown in Table 3, was 32P labeled, as described above, and annealed at a 1∶1.2 molar ratio with the unlabeled, complementary oligonucleotide by heating at 90°C for 3 min and cooling slowly to 4°C.
Table 3. Gel shift substrates.a .
| 50TIR | 5′-AAT GAT TAG TAT ATA CTT ATT AAA TAC ATA GTA TAT ACG AGA GGG TAA AT-3′ |
| TG50TIR | 5′-AAT GAT TAG TAT ATA CTT ATT AAA TAC ATA GTA GAT ACG AGA GGG TAA AT-3′ |
| GC50TIR | 5′-AAT GAT TAC TAT ATA CTT ATT AAA TAC ATA GTA TAT ACG AGA GGG TAA AT-3′ |
| 2M50TIR | 5′-AAT GAT TAC TAT ATA CTT ATT AAA TAC ATA GTA GAT ACG AGA GGG TAA AT-3′ |
| 50US | 5′-AAC AAC GGT AAT TTG ATC TTC TTA CTT GCA TAC AGC AAT TGA AAT GAT TA-3′ |
Sense strand sequence of the 50 bp substrate shown with substitutions underlined.
For DNA binding reactions, all samples contained 1 fmol/µL 32P-labeled 50 bp substrate in total volume of 10 µL of EMSA reaction buffer (20 mM HEPES pH 7.5/100 mM NaCl/0.1 mM EDTA/1 mM dithiothreitol/50 µg/mL BSA/5% glycerol). Proteins were added at the protein∶DNA ratios indicated in the figure legends and incubated at 25°C for 30 min. VapBC-1 was reconstituted in a 3∶1 VapB-1 to VapC-1 molar ratio as described above. For the VapB-1 titration, VapC-1 was incubated with substrate at 25°C for 20 min, VapB-1 was added, and incubation was continued for 20 min prior to gel loading. Immediately following addition of 2.5 µL 50% glycerol buffer (50% glycerol/10 mM Tris-HCl pH 7.4/0.01% bromophenol blue/0.01% xylene cyanol) samples were loaded onto an 18 cm×16 cm×1.5 mm 5% native 29∶1 polyacrylamide gel containing 1% glycerol and 0.5× TBE buffer (44 mM Tris-borate/0.5 mM EDTA) that had been pre-electrophoresed for 30 min in 0.5× TBE/1% glycerol at 4°C. DNA species were resolved by electrophoresis at 20 mA for 45–55 min at 4°C and detected by phosphorimaging as above.
Results
Fis is subject to growth-phase regulation in NTHi
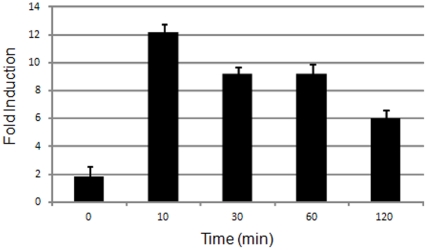
If Fis functions as an activator of vapBC-1 expression, the cellular levels of fis transcript should correlate with growth phase increases in mRNA expression from the vapBC-1 promoter. Although the regulation of Fis has been studied in other organisms, no data was available for the NTHi Fis. Therefore, quantitative real time-PCR was conducted to determine whether transcription of fis mRNA was induced upon nutrient upshift of NTHi. Using the gyrA gene as an endogenous control, relative expression of fis was examined at zero, 10, 30, 60, and 120 minutes following stationary phase culture dilution into fresh sBHI media (nutrient upshift) (Fig. 1). A dramatic increase in fis mRNA levels following nutrient upshift was observed. At ten minutes, fis expression was more than 10-fold that of the stationary phase culture, with a decrease at 120 minutes post-dilution, corresponding to the onset of exponential phase growth in NTHi. These results for NTHi fis expression were consistent with the pattern of growth phase regulation previously established for Fis in E. coli [11].
Figure 1. Expression of NTHi fis following nutrient upshift.
Quantitative real-time PCR was performed for fis mRNA following nutrient upshift of NTHi wild type strain R2866. The zero time point is fis expression in the stationary phase culture prior to dilution into fresh media, while samples at 10, 30, 60, and 120 minutes indicate fis mRNA levels after nutrient upshift. Each time point included three biological replicates and three technical replicates. Error bars are standard deviations.
Fis enhances gene expression from the vapBC-1 locus control region upon nutrient upshift in vivo
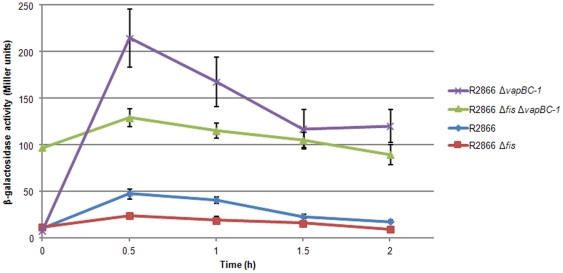
To determine the effect of Fis and VapBC-1 proteins on vapBC-1 expression, NTHi ΔvapBC-1 and Δfis mutants were constructed, along with a ΔvapBC-1 Δfis double mutant. The broad host-range plasmid pDD727, containing the vapBC-1 promoter::lacZ fusion, was then conjugated into each of the wild type parent, ΔvapBC-1, Δfis, and Δfis ΔvapBC-1 NTHi strains. Although designated the “vapBC-1 promoter” for ease of reference, the pDD727 construct contains both the promoter of R2866 vapBC-1 and the translation initiation region (TIR) of vapB-1, cloned as described above. Therefore, this protocol analyzes the genetic effects of Fis and Vap proteins on both the transcription and translation of the TA operon. Expression of lacZ from the vapBC-1 promoter was monitored by β-galactosidase activity assays every 30 minutes for the first two hours following nutrient upshift (Fig. 2). The vapBC-1 mutant expresses the activator Fis upon nutrient upshift, but not the VapBC-1 repressor complex, therefore transcription induction was the highest in this strain of the four strains analyzed. The wild type strain has both Fis and the VapBC-1 repressor complex, which in combination led to a regulated and lower expression of the vapBC-1 operon. The Fis deletion strain has only the VapBC-1 repressor present, and resulted in the lowest transcription level. Finally, the Fis and VapBC-1 double deletion mutant has neither the activator (Fis) nor the repressor (VapBC-1), which led to unregulated constitutive transcriptional activity.
Figure 2. Expression from the NTHi vapBC-1 promoter.
β-galactosidase activity indicating lacZ expression under vapBC-1 promoter control in wild type R2866 (diamonds), and R2866 mutants, Δfis (squares), ΔvapBC-1 (crosses), and Δfis ΔvapBC-1 (triangles), was measured every 30 minutes for 2 hours after nutrient upshift. The zero time point indicates expression in the stationary phase culture prior to dilution into fresh media. Each data point is the average of three independent assays performed in triplicate. Error bars are standard deviations.
The presence of Fis in either the wild type or the ΔvapBC-1 strain activated lacZ expression from the vapBC-1 promoter upon nutrient upshift. The highest level of β-galactosidase activity was observed in the ΔvapBC-1 mutant (which contained a functional fis gene), while activity was suppressed below wild-type levels in the Δfis mutant (which contained a functional vapBC-1 operon), supporting the hypothesis that Fis enhances vapBC-1 expression during growth phase and the VapBC-1 protein complex autorepresses the vapBC-1 locus control region. Interestingly, the Δfis ΔvapBC-1 double mutant showed little relative change in β-galactosidase activity upon nutrient upshift and maintained lacZ expression during stationary phase, suggesting that vapBC-1 expression is constitutive in the absence of both VapBC-1 and Fis and that the locus is subjected to little or no growth phase-related transcriptional or translational regulation in the double mutant. These data also argue against other regulatory proteins interacting with the vapBC-1 locus control region under the conditions of this assay.
Fis and Vap proteins bind distinct sequences in the vapBC-1 locus control region
The R2866 NTHi vapB-1 TIR contains a putative Fis binding site that matches 13 of 15 residues of the E. coli Fis high-affinity consensus sequence [18], [19]. An inverted repeat sequence, which also may be utilized by regulatory proteins, overlaps a portion of the Fis site (Fig. 3A). The NTHi Fis protein shares 81% identity with the 97 amino acid E. coli Fis, but is divergent in the N-terminal region, where a proline insertion results in a 98 amino acid protein. Therefore, NTHi Fis is expected to display DNA binding properties similar to the E. coli protein and may utilize the putative site to mediate nutrient-dependent regulation of vapBC-1.
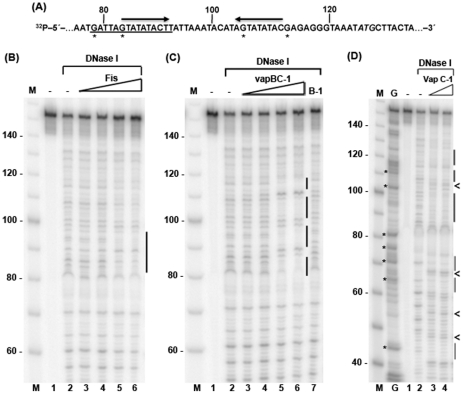
Figure 3. DNase I protection of the vapBC-1 locus control region by Fis and Vap proteins.
(A) The 32P-labeled sense strand of 153 bp DNA substrate containing vapB-1 TIR and upstream sequence in the vapBC-1 locus control region, is shown with numbers indicating the distance from the 5′-labeled end. The putative Fis site (underline), inverted repeat regions (arrows), vapB-1 translation start ATG (italics), and G cleavage products (*) seen in (D, lane G) are noted. On each gel shown, a 10 bp DNA ladder (lane M), 153 bp substrate without protein (lane 1), and DNase I digest of the substrate (lane 2) are indicated. Gels show DNase I cleavage products from samples containing: (B) a Fis∶DNA molar ratio of 2∶1, 7.5∶1, 15∶1, or 30∶1 (lanes 3–6), (C) a VapBC-1∶DNA molar ratio of 2∶1, 7.5∶1, 15∶1, or 30∶1 (lanes 3–6) or 40∶1 VapB-1∶DNA (lane 7), and (D) VapC-1∶DNA molar ratio of 40∶1 or 80∶1 (lanes 3 and 4). Vertical bars indicate the DNase I footprint from protein binding. Arrows (<) in panel D indicate DNase I hypersensitive sites. The gels each represent one of two independent experiments.
To analyze Fis and Vap protein interaction with the vapBC-1 locus control region, we performed DNase I protection assays with a 153 bp substrate containing the vapB-1 TIR and upstream locus control region sequence of R2866 vapBC-1 used in the pDD727 construct for the in vivo assays. A single DNase I protection footprint for Fis was noted in the 80–100 base region on the 5′-labeled strand of the substrate (Fig. 3B). The footprint corresponds to the location of the putative Fis site in the TIR. These findings are consistent with a role for Fis in growth phase activation of vapBC-1 expression.
The overexpression of wild-type VapC-1 is toxic to E. coli, and the toxin must be co-expressed with inhibitory VapB-1, which can co-purify in complexes with the toxin and limit the analysis of VapC-1 properties. To facilitate in vitro studies of VapC-1 in the absence of VapB-1, the ribonuclease function of the toxin was eliminated by an active site aspartate to asparagine substitution, forming D99N VapC-1. The purified D99N protein (henceforth referred to as VapC-1) retained its ability to associate with purified VapB-1, and VapBC-1 complexes were reconstituted by mixing of the antitoxin and toxin at a 3∶1 molar ratio. This preparation of VapC-1 allowed for the investigation of its role in the DNA binding activity of the TA pair.
DNase I protection footprints for VapBC-1 were observed in nucleotide regions 82–87, 90–98, 102–109, and 112–117 in the substrate, all of which lie within 50 bases of the ATG translation start site for vapB-1, which is at position 125 from the labeled end of the substrate (Fig. 3C). The footprints correspond to the inverted repeat region of the substrate, and the 82–98 site overlaps with the single Fis footprint, indicating that VapBC-1 and Fis may compete for binding of the vapB-1 TIR in the regulation of vapBC-1 expression. VapB-1 alone showed no DNA interaction when present at high concentration, suggesting that it must be bound to VapC-1 in order to interact with DNA (lane 7, Fig. 3C). Interestingly, VapC-1 alone exhibited several sites of DNase I protection on the substrate, with footprints overlapping those of VapBC-1 but also appearing at unique sites within the 40–70 nucleotide region, upstream of the TIR (Fig. 3D). Unlike VapBC-1, VapC-1 binding also induced DNase I hypersensitive sites, suggesting that several VapC-1 molecules may interact with each substrate molecule, leaving short stretches of DNA exposed to the nuclease. Higher VapC-1 concentrations were required than in the VapBC-1 samples to observe DNase I protection, likely because VapC-1 occupied more sites than the TA complex. A Maxam-Gilbert chemical digestion of the 153 bp substrate was performed to obtain a banding pattern for strong cleavage at G and weak cleavage at A [25] (lane G, Fig. 3D). The digest aided the location of the VapC-1 within the substrate and revealed that the substrate DNase I fragments migrated slightly faster in the gel than the corresponding marker fragments (comparing lanes M and G where asterisks indicate substrate positions 48, 68, 79, 84, 106, and 114). These results indicate that VapC-1 possesses DNA binding activity, and the toxin may be responsible for VapBC-1 interaction with the TIR of vapB-1.
Fis, VapC-1 and VapBC-1 interact with the translation initiation region of vapB-1
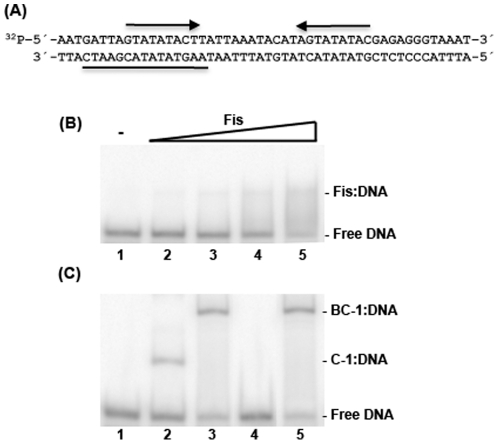
Since Fis, VapBC-1 and VapC-1 share footprints in the vapB-1 TIR of the vapBC-1 locus control region, 50 base pair DNA substrates containing the TIR (50TIR), the TIR with a T to G mutation in the inverted repeat (TG50TIR), the TIR with a G to C mutation at the −7 position in the putative Fis site (GC50TIR), or sequence upstream of the TIR that lacked Fis and VapBC-1 footprints (50US) were studied by gel shift analysis for Fis and Vap protein binding (Table 2). Initial experiments were performed to monitor the binding of Fis, VapBC-1 or individual Vap proteins with the 50TIR substrate (Fig. 4A). Fis decreased the mobility of 50TIR in a concentration-dependent manner; however, the Fis-bound DNA did not migrate as a distinct band, suggesting that the Fis∶DNA complexes were labile under the gel conditions (Fig. 4B). The VapC-1 toxin and VapBC-1 complex bound stably to 50TIR, and VapC-1∶DNA complexes were shifted to a DNA mobility corresponding to that of VapBC-1∶DNA complexes when VapB-1 was added following VapC-1 pre-incubation with the substrate (Fig. 4C). The VapB-1 antitoxin displayed no DNA binding activity, likewise no DNA interaction was observed with a purified VapB-1 protein lacking the N-terminal polyhistidine tag, confirming that the His tag was not interfering with VapB-1 association with DNA (data not shown). These observations were consistent with the DNase I protection seen for each protein, but the Fis findings indicate that the polyhistidine tag on the purified Fis compromised DNA binding or that Fis may not interact specifically with the vapB-1 TIR.
Figure 4. Fis, VapC-1 and VapBC-1 bind the vapB-1 TIR.
(A) The sequence of the 50TIR indicating the putative Fis binding site (underline) and the inverted repeat regions (arrows). (B) Gel shift products from samples containing 50TIR alone (lane 1) and 10, 30, 150 or 600 molar ratios of Fis to DNA. (C) Products from samples containing 50TIR alone (lane 1), VapC-1 (lane 2), VapC-1 followed by VapB-1 (lane 3), VapB-1 (lane 4), or the reconstituted VapBC-1 complex (lane 5). VapB-1 and VapC-1 are present in a 150∶1 molar ratio to 50TIR. In VapBC-1 samples, VapB-1 and VapC-1 are at a 3∶1 molar ratio, with a VapC-1∶DNA molar ratio of 150∶1. This ratio is reported since VapC-1 is the DNA binding protein and the actual amount of VapBC-1 complexes cannot be determined. The identity of each band is noted at the right of each gel. Each gel represents one of two independent experiments.
NTHi Fis exhibits non-specific binding with the vapBC-1 locus control region
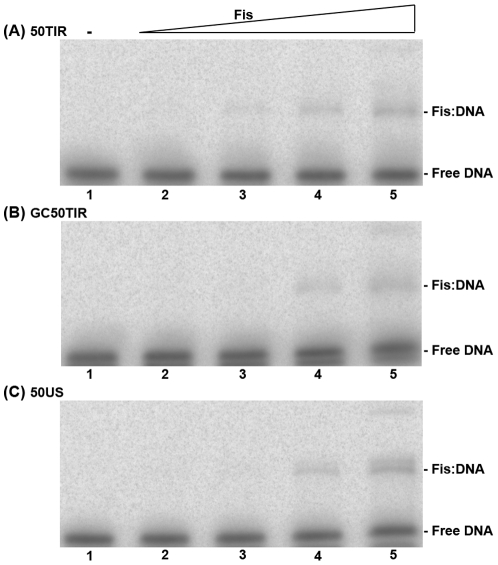
The presence of a single DNase I protection site for Fis suggested that Fis binding to the vapB-1 TIR was specific; however, the results above indicate a low affinity of Fis for this sequence. To more carefully examine Fis interaction with the vapBC-1 locus control region, purified Fis lacking the N-terminal polyhistidine tag was prepared to study Fis binding to 50TIR, as well as the GC50TIR and 50US substrates, to which Fis binding was expected to be diminished or absent. While a polyhistidine tag did not alter VapB-1 binding properties, the N-terminal His tag on Fis appeared to be partly responsible for the instability of Fis complexes with 50TIR observed in Fig. 4, as a distinct Fis∶DNA band was resolved for Fis without the tag (Fig. 5). Surprisingly, Fis displayed similar affinity to all three substrates. These results indicate that, while Fis obviously upregulates vapBC-1 expression upon nutrient upshift in vivo, the protein may do so indirectly through its effects on DNA structure and not by high-affinity binding to specific sequences in the locus control region.
Figure 5. Fis interacts non-specifically with the vapB-1 TIR.
Gel shift products from a titration of Fis, lacking a polyhistidine tag, with (A) 50TIR, (B) GC50TIR or (C) 50US are shown. The gels show the products of the following samples: DNA only (lane 1) and the addition of Fis at 10, 30, 90 or 300 molar ratios with the DNA substrate (lanes 2–5). The identity of each band is noted at the right of each gel. Each gel represents one of two independent experiments.
VapBC-1 specifically interacts with the vapB-1 translation initiation region
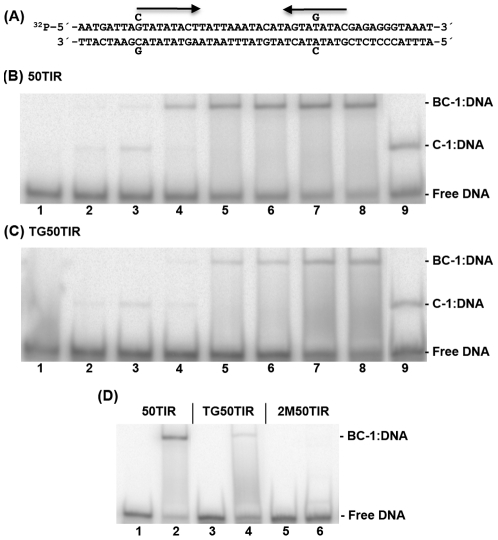
The VapBC-1 complex protected four areas of the vapB-1 TIR from DNase I digestion. The footprints were within a 40-base region that contained both the putative Fis site and an inverted repeat. As the inverted repeat may serve as a recognition sequence for the complex, a T to G transversion was introduced in one half of the repeat, generating TG50TIR (Fig. 6A). The substitution diminished binding of VapBC-1 by an average of 5 to 10-fold over a concentration range of VapBC-1, implicating the inverted repeat in VapBC-1 recognition and possible autoregulation of the vapBC-1 operon (Fig. 6B). Interestingly, a band corresponding to VapC-1∶DNA complexes was observed for both 50TIR and TG50TIR at the low concentrations of VapBC-1. The band shifted to the BC-1∶DNA mobility as the concentration of protein increased. This observation suggests that VapBC-1 formation is not required prior to DNA binding, and that VapB-1 can associate with VapC-1∶DNA complexes. A double-mutant TIR substrate, 2M50TIR, was created by including a G to C transversion in the TG50TIR substrate, which altered the upstream half of the inverted repeat. Nucleotide changes in both sides of the inverted repeat completely abolished VapBC-1 interaction with the TIR sequence (Fig. 6D). VapC-1 binding to the 2M50TIR substrate was reduced only by half compared to that observed with 50TIR (data not shown). This result shows that optimal binding of VapBC-1 to the vapB-1 TIR depends on the inverted repeat. VapBC-1 utilization of both sides of the repeat suggests that the complex may bind as a dimer, and the requirement for VapB-1 in conferring complex specificity indicates that both VapB-1 and VapC-1 contribute to the DNA binding surface of the complex.
Figure 6. VapBC-1 specifically interacts with the vapB-1 TIR.
(A) The sequence of the 50TIR is shown with arrows indicating the inverted repeat regions and bases above and below the sequence indicating the position of the G to C and T to G substitutions in the TG50TIR and 2M50TIR substrates (see Table 3). Gel shift products from a titration of VapBC-1 with (B) 50TIR or (C) TG50TIR are shown. The lanes in panels B and C contain the following samples: DNA only (lane 1), the addition of VapBC-1 at a VapC-1∶DNA ratio of 10, 25, 50, 100, 200, 400 or 800 molar ratio with the DNA substrate (lanes 2–8), and VapC-1 only at an 800∶1 protein to DNA ratio (lane 9). (D) A comparison of VapBC-1 binding at a 400∶1 molar ratio with each DNA substrate (lanes 2, 4, and 6). Lanes 1, 3, and 5 contain only DNA. The identity of each band is noted at the right of each gel. Each gel represents one of three independent experiments.
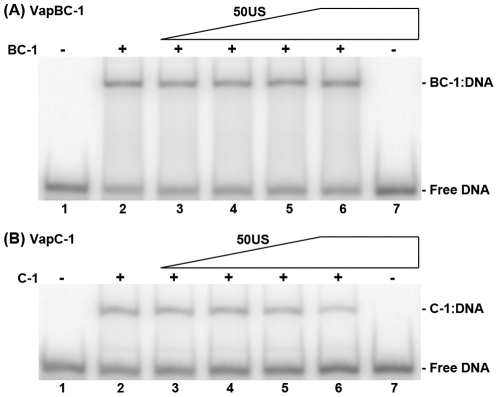
To assess whether VapBC-1 binding was specific to the vapB-1 TIR, the 50US sequence, which contained no detectable VapBC-1 DNase I footprints, was analyzed for VapBC-1 and VapC-1 binding competition with the 50TIR substrate. A titration of unlabeled 50US substrate into samples with constant amounts of VapBC-1 and 50TIR failed to diminish the BC-1∶DNA band (Fig. 7A). Indeed, no interaction of VapBC-1 with a labeled 50US was observed up to a 300 to 1 molar ratio of protein to DNA (data not shown). On the other hand, 50US did disrupt binding of VapC-1 to the 50TIR substrate, indicating that VapC-1 alone does not have high affinity for the vapB-1 TIR (Fig. 7B). These results confirmed that VapBC-1 interacts specifically with the vapB-1 TIR and that VapB-1 may target the complex to the inverted repeat as a recognition site in suppressing vapBC-1 expression.
Figure 7. Competition binding of 50TIR and 50US by Vap proteins.
Gel shift products from addition of 50US substrate into samples containing 50TIR substrate and either (A) VapBC-1 or (B) VapC-1 at a 150∶1 molar ratio of VapC-1 protein to DNA. VapB-1 and VapC-1 are at a 3∶1 molar ratio in the VapBC-1 samples, but VapC-1∶DNA molar ratios are reported since VapC-1 is the DNA binding protein and the actual amount of VapBC-1 complexes cannot be determined. The gels show the products of the following samples: 50TIR without protein (lane 1), protein with only 50TIR (lane 2), the addition of a 1∶1, 5∶1, 10∶1 or 50∶1 molar ratio of cold 50US:50TIR (lanes 3–6), and DNA only at 50∶1 molar ratio of 50US:50TIR (lane 6). The identity of each band is noted at the right of each gel. Each gel represents one of two independent experiments.
VapB-1 targets VapC-1 to the vapB-1 translation initiation region
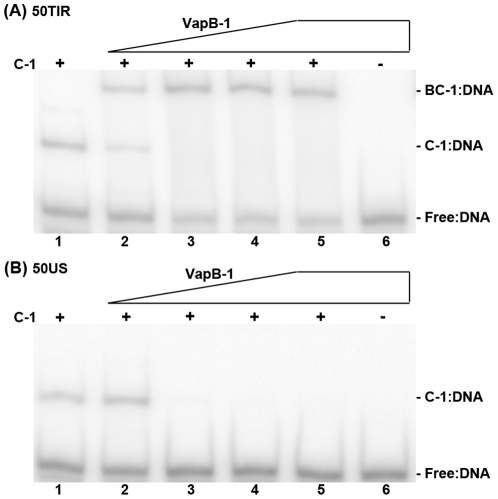
Both the DNase I results and initial experiments with 50TIR indicate that VapC-1 alone binds the locus control region of vapBC-1; however, the role of VapC-1 binding relative to that of the VapBC-1 complex is unclear, especially since the toxin is thought to act primarily as a ribonuclease that is inhibited by VapB-1. Since VapB-1 has no DNA binding property of its own, this raises the possibility that VapC-1 facilitates DNA binding by the VapBC-1 complex, and that VapB-1 binds to VapC-1 for DNA interaction. The observations above indicate that VapBC-1 formed in the cytosol may specifically bind the vapB-1 TIR or that VapB-1 may bind to a VapC-1:TIR complex to enhance DNA affinity. To elucidate whether VapB-1 interacts with VapC-1 on DNA and how this interaction may affect VapBC-1 specificity, VapB-1 was added to samples following VapC-1 preincubation with either the 50TIR or 50US substrates (Fig. 8). VapC-1 alone forms stable complexes with both substrates, revealing that VapC-1 lacks specificity for the vapB-1 TIR. As observed in the previous experiments, VapB-1 shifts the 50TIR substrate from the C-1∶DNA mobility to a slower BC-1∶DNA complex band (Fig. 8A), but interestingly, it causes the dissociation of VapC-1 from 50US (Fig. 8B). VapB-1 alone did not associate with either substrate, even at its highest concentration. These findings indicate that VapB-1 alters the affinity of VapC-1 for DNA and confers specificity for the vapB-1 TIR to the VapBC-1 complex.
Figure 8. VapB-1 targets VapC-1 to the vapB-1 translation initiation region.
Gel shift products from a titration of VapB-1 into samples following VapC-1 binding to (A) 50TIR or (B) 50US at a 100∶1 molar ratio of protein to DNA. The gels show the products of the following samples: VapC-1 and DNA without VapB-1 (lane 1), the addition of Vap B-1 at a 50, 100, 200 or 300 molar ratio with the DNA substrate (lanes 2–5), and VapB-1 only at a 300∶1 protein to DNA ratio (lane 6). The identity of each band is noted at the right of each gel. Each gel represents one of two independent experiments.
Discussion
Bacteria often encounter microenvironments in the host that include suboptimal conditions, such as nutrient limitation, oxidative or antibiotic stress. Organisms that cannot respond to these challenges in a constructive fashion may not survive until conditions improve. One straightforward way for a nonsporulating species to endure poor environments is to enter a reversible bacteriostatic state. Since most drugs target essential biosynthetic pathways that are not active during periods of dormancy, growth arrest confers many benefits upon a bacterium, including very low metabolic energy requirements as well as nonspecific antibiotic tolerance. The key to utilizing such a state effectively, however, lies in the ability of the organism to transition back to replication and growth when stress is relieved. This novel mechanism of growth modulation has been attributed to the action of TA loci, and the vapBC locus has been shown to regulate growth arrest by inhibiting translation in a number of bacteria [26]–[28]. Although vapBC loci from various organisms share little nucleotide homology, many show conservation of function in their ribonuclease activity [7], [29], [30].
The current work focused on understanding the regulation of the vapBC-1 TA locus in NTHi, a bacterium responsible for a number of chronic mucosal infections in humans. Given the growth phase regulation of its operon and its role in gene regulation in E. coli, NTHi fis expression and its effect on expression the vapBC-1 locus were studied. We have shown that the NTHi fis gene, like its homolog in E. coli, is dramatically upregulated by nutrient upshift, suggesting a role for NTHi Fis in growth phase dependent gene regulation [11]. Through β-galactosidase activity studies of vapBC-1 promoter activity in ΔvapBC-1, Δfis, and Δfis ΔvapBC-1 NTHi strains, we have found that fis is necessary for nutrient upshift activation of the vapBC-1 promoter driving lacZ and that the genomic vapBC-1 attenuates promoter activity. In fact, the highest level of vapBC-1 promoter activity was observed in the ΔvapBC-1 strain. While a DNA sequence homologous to the E. coli Fis consensus site was identified in the translation initiation region (TIR) of vapB-1, Fis binding to this sequence was not specific, indicating that Fis upregulates vapBC-1 expression indirectly by altering DNA structure [13]. The autoregulatory effects of VapBC-1 implied by the β-galactosidase assay appear to be mediated by recognition of the vapBC-1 operon by the TA pair. We demonstrated that the VapBC-1 complex binds specifically to the vapB-1 TIR of the vapBC-1 locus control region by utilizing an inverted repeat sequence. Interestingly, we discovered that the VapC-1 toxin possesses DNA binding activity, which appears to facilitate interaction of the VapBC-1 complex with the vapBC-1 locus. VapC-1 can associate independently with DNA, while VapB-1 does not bind DNA under any of our experimental conditions. Some antitoxins do not bind individually to their cognate promoters in the absence of their toxins. For example, no DNA binding activity could be detected for two RelB-like antitoxin proteins in M. tuberculosis [31]. We have previously shown that VapC-1 is a ribonuclease [10], and its ability to independently bind double-stranded nucleic acid suggests that it may act upon RNA secondary structure. The finding that VapC-1 binds DNA is remarkable considering that many TA loci are autoregulated by the antitoxin product, with the toxin acting as a co-repressor [32]. Finally, the VapB-1 antitoxin conferred binding specificity to VapC-1, as it caused VapC-1 to dissociate from sites outside of the vapB-1 TIR but formed a stable complex with VapC-1 on the vapB-1 TIR. Taken together, these results suggest novel roles for the vapBC-1 TA pair in regulation of growth arrest with Fis serving to indirectly enhance vapBC-1 expression after growth stimulation.
Previously, the role of VapB-1 in NTHi survival was thought to be the binding and inactivation of VapC-1 ribonuclease activity. However, our findings suggest a new paradigm for TA locus autoregulation and for antitoxin activity in bacterial survival of nutrient stress (Fig. 9). In NTHi, Fis appears to stimulate vapBC-1 expression on resumption of growth, as is observed for other bacterial species. We have confirmed that VapBC-1 suppresses its own operon when fis expression drops in early log phase growth (Fig. 9A). However, VapBC-1 autoregulation of the TA operon is facilitated through the DNA binding property of the VapC-1 toxin, with the antitoxin serving to target the TA complex to the vapB-1 TIR. VapC-1 may associate with VapB-1 in the cytosol prior to DNA interaction or bind to DNA alone, suggesting that DNA binding by the toxin may also play a role in attenuating its ribonuclease activity. When nutrient deprivation or environmental stress arrests NTHi growth, VapB-1 would be degraded by Lon and Clp proteases, freeing VapC-1 from VapBC-1 complexes and releasing the inhibition on the VapC-1 ribonuclease (Fig. 9B) [33]. The toxin could remain on the vapB-1 TIR or dissociate to degrade the cellular RNA pool to inhibit translation (Fig. 9C). Since the VapC-1 interaction with vapB-1 TIR appears less stable in the absence of VapB-1, it may be readily displaced by Fis-induced structural changes in the promoter when conditions favor the resumption of NTHi growth (Fig. 9D). This model is consistent with another study that demonstrated reversible growth inhibition in the absence of the VapB antitoxin when the transcription of the VapC toxin was first induced, then subsequently blocked, in a M. smegmatis strain containing a deletion in its single vapBC homologue [28]. Indeed, if this model serves to explain vapBC-1 regulation in NTHi, drugs that stabilize the interaction of VapB-1 and VapC-1 or inhibit the ribonuclease function of VapC-1 should provide a new and effective approach for the treatment of chronic NTHi infections.
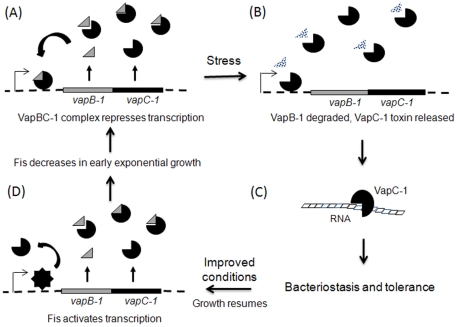
Figure 9. Model for the regulation of the vapBC-1 locus.
(A) During colonization under favourable conditions, the VapBC-1 complex binds to and autorepresses TA operon transcription. (B) Stress induces Lon and Clp proteases that degrade VapB-1, releasing active VapC-1 toxin. (C) The ribonuclease activity of VapC-1 facilitates a state of bacteriostasis, resulting in nonspecific antibiotic tolerance. (D) Upon improved conditions, Fis activates vapBC-1 operon transcription, displacing any bound VapC-1. Fis levels decrease in early exponential growth, allowing the VapBC-1 complex to bind and restore transcriptional equilibrium.
Acknowledgments
We thank Arnold L. Smith for NTHi strain R2866.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by National Institutes of Health grant DC010187 from the National Institute on Deafness and Other Communication Disorders to DAD. Website: www.nidcd.nih.gov/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Murphy TF, Apicella MA. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:266–272. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 3.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 4.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucl Acid Res. 2005;33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage HR, Connolly LE, Cox JS. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution. PLoS Genet. 2009;5:e10000767. doi: 10.1371/journal.pgen.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatica A, Tollervey D, Dlakic M. PIN domain of Nob1p is required for D-site cleavage in 20S pre-rRNA. RNA. 2004;10:1698–1701. doi: 10.1261/rna.7123504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arcus VL, McKenzie JL, Robson J, Cook GM. The PIN-domain ribonucleases and the prokaryotic VapBC toxin-antitoxin array. Protein Eng, Design & Select. 2011;24:33–40. doi: 10.1093/protein/gzq081. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Sharp JD, Kobayashi H, Woychik NA, Inouye M. Noncognate Mycobacterium tuberculosis toxin-antitoxins can physically and functionally interact. J Biol Chem. 2010;285:39732–39738. doi: 10.1074/jbc.M110.163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: specific functional annotation with the Conserved Domain Database. Nucl Acids Res. 2009;37:D205–210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daines DA, Wu MH, Yuan SY. VapC-1 of nontypeable Haemophilus influenzae is a ribonuclease. J Bacteriol. 2007;189:5041–5048. doi: 10.1128/JB.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ball CA, Osuna R, Ferguson KC, Johnson RC. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider R, Lurz R, Luder G, Tolksdorf C, Traver A, et al. An architectural role of the Escherichia coli chromatin protein FIS in organising DNA. Nucl Acids Res. 2001;29:5107–5114. doi: 10.1093/nar/29.24.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opel ML, Aeling KA, Holmes WM, Johnson RC, Benham CJ, et al. Activation of transcription initiation from a stable RNA promoter by a Fis protein-mediated DNA structural transmission mechanism. Mol Microbiol. 2004;53:665–674. doi: 10.1111/j.1365-2958.2004.04147.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch M, Elliott T. Fis regulates transcriptional induction of RpoS in Salmonella enterica. J Bacteriol. 2005;187:1568–1580. doi: 10.1128/JB.187.5.1568-1580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grainger DC, Hurd D, Goldberg MD, Busby SJ. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 2006;34:4642–4652. doi: 10.1093/nar/gkl542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoko D, Yoo D, Bai H, Schnurr B, Yan J, et al. Mechanism of chromosome compaction and looping by the Escherichia coli nucleoid protein Fis. J Mol Biol. 2006;364:777–798. doi: 10.1016/j.jmb.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengen PN, Bartram SL, Stewart LE, Schneider TD. Information analysis of Fis binding sites. Nucl Acids Res. 1997;25:4994–5002. doi: 10.1093/nar/25.24.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Feldman-Cohen LS, Osuna R. Functional characterization of the Escherichia coli Fis-DNA binding sequence. J Mol Biol. 2008;376:771–785. doi: 10.1016/j.jmb.2007.11.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman-Cohen LS, Shao Y, Meinhold D, Miller C, Colon W, et al. Common and variable contributions of Fis residues to high-affinity binding at different DNA sequences. J Bacteriol. 2006;188:2081–2095. doi: 10.1128/JB.188.6.2081-2095.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostrewa D, Granzin J, Stock D, Choe H-W, Labahn J, et al. Crystal structure of the factor for inversion stimulation FIS at 2.0 Å resolution. J Mol Biol. 1992;226:209–226. doi: 10.1016/0022-2836(92)90134-6. [DOI] [PubMed] [Google Scholar]
- 21.Mason KM, Munson RS, Jr, Bakaletz LO. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect Immun. 2003;71:3454–3462. doi: 10.1128/IAI.71.6.3454-3462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casadaban MJ, Chou J, Cohen SN. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980;143:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daines DA, Smith AL. Design and construction of a Haemophilus influenzae conjugal expression system. Gene. 2001;281:95–102. doi: 10.1016/s0378-1119(01)00788-0. [DOI] [PubMed] [Google Scholar]
- 24.Miller JH. Experiments in molecular genetics. 1972. Cold Spring Harbor Laboratory Press: Cold Spring Harbor.
- 25.Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977;74:560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winther KS, Gerdes K. Ectopic production of VapCs from Enterobacteria inhibits translation and trans-activates YoeB mRNA interferase. Mol Microbiol. 2009;72:918–930. doi: 10.1111/j.1365-2958.2009.06694.x. [DOI] [PubMed] [Google Scholar]
- 27.Buts L, Lah J, Dao-Thi M-H, Wyns L, Loris M. Toxin-antitoxin modules as bacterial metabolic stress managers. TIBS. 2005;30:672–679. doi: 10.1016/j.tibs.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Robson J, McKenzie JL, Cursons R, Cook GM, Arcus VL. The vapBC operon from Mycobacterium smegmatis is an autoregulated toxin-antitoxin module that controls growth via inhibition of translation. J Mol Biol. 2009 doi: 10.1016/j.jmb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Ahidjo BA, Kuhnert D, McKenzie JL, Machowski EE, Gordhan BG, et al. VapC toxins from Mycobacterium tuberculosis are ribonucleases that differentially inhibit growth and are neutralized by cognate VapB antitoxins. PLOS One. 2011;6:e21738. doi: 10.1371/journal.pone.0021738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miallau L, Faller M, Chiang J, Arbing M, Guo F, et al. Structure and proposed activity of a member of the VapBC family of toxin-antitoxin systems: VapBC-5 from Mycobacterium tuberculosis. J Biol Chem. 2009;284:276–283. doi: 10.1074/jbc.M805061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Gao C, Wang Y, Zhang H, He ZG. Characterization of the interaction and cross-regulation of three Mycobacterium tuberculosis RelBE modules. PLOS One. 2010;5:e10672. doi: 10.1371/journal.pone.0010672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey SES, Hayes F. Influence of operator site geometry on transcriptional control by the YefM-YoeB toxin-antitoxin complex. J Bacteriol. 2009;191:762–772. doi: 10.1128/JB.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gronlund H, Gerdes K. Toxin-antitoxin systems homologous with relBE of Escherichia coli plasmid P307 are ubiquitous in prokaryotes. J Mol Biol. 1999;285:1401–1415. doi: 10.1006/jmbi.1998.2416. [DOI] [PubMed] [Google Scholar]