Abstract
Study Design
Prospective, randomized, controlled human study.
Purpose
We checked the proportion of missed syrinx diagnoses among the examinees of the Korean military conscription.
Overview of Literature
A syrinx is a fluid-filled cavity within the spinal cord or brain stem and causes various neurological symptoms. A syrinx could easily be diagnosed by magnetic resonance image (MRI), but missed diagnoses seldom occur.
Methods
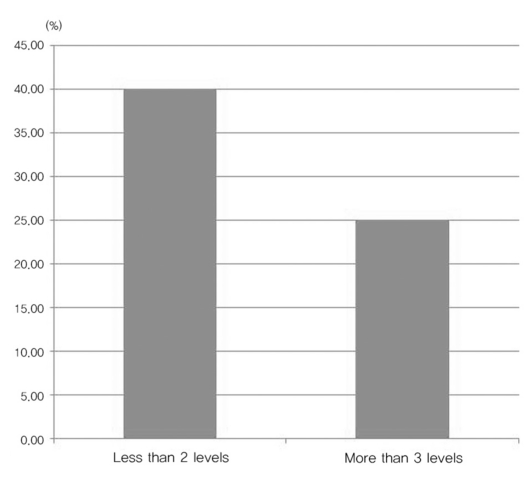
In this study, we reviewed 103 cases using cervical images, cervical MRI, or whole spine sagittal MRI, and syrinxes was observed in 18 of these cases. A review of medical certificates or interviews was conducted, and the proportion of syrinx diagnoses was calculated.
Results
The proportion of syrinx diagnoses was about 66.7% (12 cases among 18). Missed diagnoses were not the result of the length of the syrinx, but due to the type of image used for the initial diagnosis.
Conclusions
The missed diagnosis proportion of the syrinx is relatively high, therefore, a more careful imaging review is recommended.
Keywords: Syrinx, Diagnosis proportion, Conscription, Korea
Introduction
A syrinx is a fluid-filled cavity within the spinal cord or brain stem [1-3]. Predisposing factors include craniocervical junction abnormalities, spinal cord trauma, and spinal cord tumors [4,5]. Symptoms include flaccid weakness of the hands and arms and deficits in pain and temperature sensation in a capelike distribution over the back and neck; light touch and position and vibration sensation are not affected [1,6]. Diagnosis is by magnetic resonance image (MRI), but a missed diagnosis occasionally occurs. Thus, the authors reviewed the proportion of correct syrinx diagnoses among males in the conscription for the Korean military.
Materials and Methods
Korea has adopted the conscription system, for which all men must be medically examined. This survey was conducted at the Military Manpower Administration in Seoul from February 2008 to March 2011. In this period at a general hospital, 103 cases were checked with MRI, including whole spine sagittal scanning, and 18 cases were checked for the presence of a syrinx. These 18 examinees were included in this study with personal permission.
Each syrinx was reconfirmed by 1.5 T MRI at the Seoul Military Manpower Administration; all images were reviewed by one neurosurgeon (CHO) and one radiologist (JHL). All medical certificates submitted by the examinees were reviewed by the neurosurgeon. The authors classified the data by presence of syrinx diagnosis, by level of the syrinx, and by type of MRI initially performed. We defined the syrinx as a well-demarcated cavity with a low signal on T1-weighted image and increased signal on T2-weighted image on MRI within the spinal cord without considering expansion to the cord or abnormal adjacent cord signal. A missed diagnosis was defined as the presence of a syrinx observed during the conscription by an imaging study and no description of it on the medical certificate or no communication to the examinee about its presence.
Statistical analysis was performed using SAS ver. 9.1.3 (SAS Institute Inc., Cary, NC, USA) with a student t-test, and this study was conducted with the approval of the committee in the Military Manpower Administration in Seoul.
Results
A total of 103 cases were included in this study. Among them, cervical MRI in 71 cases, whole spine sagittal scanning with focusing cervical spine MRI in 12 cases, and whole spine sagittal scanning with focusing lumbar spine MRI in 20 cases were observed. Eighteen syrinx cases were among these during the conscription (Table 1). The agreement rate between two observers, one neurosurgeon and one radiologist, was 99.0% (1 case was ignored by the neurosurgeon).
Table 1.
Total 18 cases which diagnosed during conscription
MRI: Magnetic resolution image, WSSS: Whole spine sagittal scanning.
The age distribution of the examinees was from 19 to 32 years old, and all examinees were male, as the data were derived from military conscription. The location of the syrinx was distributed from C2 to T9, and the length was variable. All 18 cases were checked for multiple degenerative disc changes with MRI. Two pieces of block vertebrae in C4/5 and C6/7 were observed in case No. 3, and scoliosis with about 23° of Cobb's angle was seen in case No. 16. In two cases (No. 9 and 13), Chiari malformation type I was observed.
In the cases of syrinx presence, the presenting symptoms were cervical pain (94.4%), stiffness in the back, shoulders, arms, or legs (61.1%), arm weakness (27.7%), and leg weakness (16.7%). Other symptoms included headaches (72.2%), and a loss of the ability to feel extremes of hot or cold (11.1%). Each examinee experienced a different combination of symptoms. These symptoms might vary depending on the extent and the location of the syrinx within the spinal cord; the correlation between a particular symptom and these variations were not examined in this study.
The diagnosis of syrinx was given on 12 cases among the 18 cases (66.7%). Missed diagnoses of the syrinx included 1 case with cervical spine MRI, 1 case with whole spine sagittal scanning with focusing cervical spine MRI, and 4 cases with whole spine sagittal scanning with focusing lumbar spine MRI. The missed diagnosis proportion was largest in whole spine sagittal scanning with focusing lumbar spine MRI.
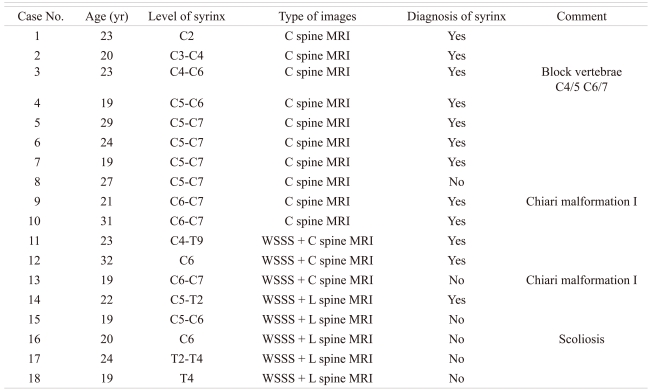
The missed diagnosis proportion according to the type of MRI or the length of lesion is shown in Figs. 1 and 2. In Fig. 1, the proportion of missed diagnoses according to MRI type was 20% (4 cases among 20) in whole spine sagittal scanning with focusing lumbar spine MRI, but 2.41% (2 cases among 83) in cervical or whole spine sagittal scanning with focusing lumbar spine MRI. A statistically significant difference between the group was noted (p < 0.001).
Fig. 1.
The missed diagnosis rate of syrinx according to type of magnetic resolution image (MRI). WSSS: Whole spine sagittal scanning (p < 0.001).
Fig. 2.
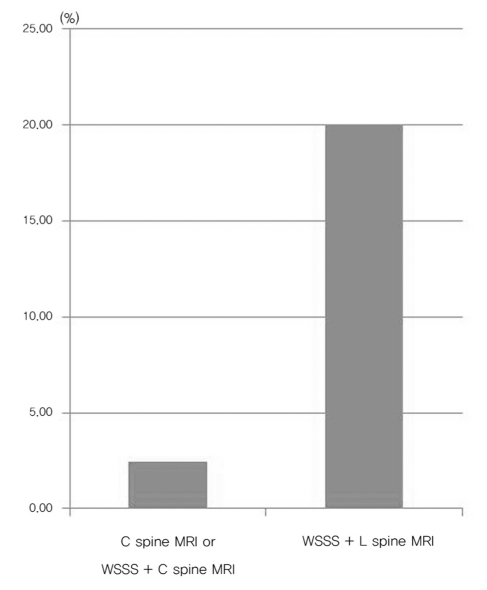
The missed diagnosis rate of syrinx according to length of lesion (p = 0.246).
The missed diagnosis proportion according to lesion length is shown in Fig. 2. Among 18 cases, 10 cases with syrinx lengths less than 2 spinal levels and 8 cases with more than 3 spinal levels were observed. The missed diagnosis proportion with the length less than 2 spinal levels was 40% and with the length more than 3 spinal levels was 25%. This difference was not statistically significant (p = 0.246).
Discussion
Syrinxes usually result from lesions that partially obstruct cerebrospinal fluid flow [7-9]. At least half of syrinxes occur in patients with congenital abnormalities of the craniocervical junction (e.g., herniation of cerebellar tissue into the spinal canal, called Chiari malformation), brain (e.g., encephalocele), or spinal cord (e.g., myelomeningocele) [6,10]. For unknown reasons, these congenital abnormalities often expand during the teen or young adult years. A syrinx can also develop in patients who have a spinal cord tumor, scarring due to previous spinal trauma, or no known predisposing factors [11-15]. About 30% of people with a spinal cord tumor eventually develop a syrinx [16].
Syringomyelia is a paramedian, usually irregular, longitudinal cavity. It commonly begins in the cervical area but may extend downward along the entire length of the spinal cord. Syringobulbia, which is rare, usually occurs as a slit-like gap within the lower brain stem and may disrupt or compress the lower cranial nerves or ascending sensory or descending motor pathways [4,17].
Symptoms usually begin insidiously between adolescence and age of 45 [16]. Syringomyelia develops in the center of the spinal cord, causing a central cord syndrome. Pain and temperature sensory deficits occur early but may not be recognized for years. The first abnormality recognized may be a painless burn or cut. Syringomyelia typically causes weakness, atrophy, and often fasciculations and hyporeflexia of the hands and arms; a deficit in pain and temperature sensation in a capelike distribution over the shoulders, arms and back is also characteristic [4]. Light touch and position and vibration sensation are not affected. Later, spastic leg weakness develops. In this study, the presenting symptoms were cervical pain, headache, and stiffness in the back, shoulders, arms, or legs. Additionally, arm or leg weakness and a loss of the ability to feel extremes of hot or cold were observed, but most symptoms were ambiguous. Thus, it is very difficult to diagnose the presence of a syrinx by the presenting symptoms, although this diagnosis should be kept in mind.
A syrinx is suggested by an unexplained central cord syndrome or other characteristic neurologic deficits, particularly pain and temperature sensory deficits in a capelike distribution [4,16,17]. MRI of the entire spinal cord and brain should be performed. Gadolinium enhancement is useful for detecting any associated tumor [16].
Underlying problems (e.g., craniocervical junction abnormalities, postoperative scarring, or spinal tumors) are corrected when possible [6,10]. Surgical decompression of the foramen magnum and upper cervical cord is the only useful treatment, but surgery usually cannot reverse severe neurologic deterioration.
The golden standard diagnostic tool for the syrinx is MRI of the spinal cord and brain with gadolinium [16]. Currently, imaging techniques for MRI have greatly improved, including techniques for whole spine sagittal scanning. The overall diagnostic proportion of the syrinx was only 66.7%; this proportion was unaffected by the length of the syrinx. It means that low detection of the syrinx was not related with unintended fault. This was more reasonable by the significant association between the detection proportion and the type of MRI. In cervical MRI or whole spine sagittal scanning with focusing cervical spine MRI, cervical lesions were not main concerned lesion. But, in the whole spine sagittal scanning with focusing lumbar spine MRI, the cervical lesion is not the main concern of the examiners or reporters. So, cervical lesions such as a syrinx in whole spine sagittal scanning with focusing lumbar spine MRI were easily dismissed.
In conclusion, a syrinx is an easily detectable disease by MRI, but the missed diagnosis proportion of the syrinx was relatively high. Thus, we suggest that the physician should consider syrinx as a possible diagnosis, as the symptoms are vague. Furthermore, physicians should more carefully review whole spine sagittal scanning MR images, so as to not miss the syrinx diagnosis.
Conclusions
The missed diagnosis proportion of the syrinx is relatively high, therefore, a more careful imaging review is recommended.
References
- 1.Barnett HJ, Botterell EH, Jousse AT, Wynn-Jones M. Progressive myelopathy as a sequel to traumatic paraplegia. Brain. 1966;89:159–174. doi: 10.1093/brain/89.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Bastian HC. On a case of concussion-lesion, with extensive secondary degenerations of the spinal cord, followed by general muscular atrophy. Med Chir Trans. 1867;50:499–542.1. doi: 10.1177/095952876705000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips WA, Hensinger RN, Kling TF., Jr Management of scoliosis due to syringomyelia in childhood and adolescence. J Pediatr Orthop. 1990;10:351–354. doi: 10.1097/01241398-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Williams B. Pathogenesis of post-traumatic syringomyelia. Br J Neurosurg. 1992;6:517–520. doi: 10.3109/02688699209002367. [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Terry AF, Jones F, McSweeney T. Syringomyelia as a sequel to traumatic paraplegia. Paraplegia. 1981;19:67–80. doi: 10.1038/sc.1981.18. [DOI] [PubMed] [Google Scholar]
- 6.Umbach I, Heilporn A. Review article: post-spinal cord injury syringomyelia. Paraplegia. 1991;29:219–221. doi: 10.1038/sc.1991.32. [DOI] [PubMed] [Google Scholar]
- 7.Cho KH, Iwasaki Y, Imamura H, Hida K, Abe H. Experimental model of posttraumatic syringomyelia: the role of adhesive arachnoiditis in syrinx formation. J Neurosurg. 1994;80:133–139. doi: 10.3171/jns.1994.80.1.0133. [DOI] [PubMed] [Google Scholar]
- 8.Koyanagi I, Iwasaki Y, Hida K, Houkin K. Clinical features and pathomechanisms of syringomyelia associated with spinal arachnoiditis. Surg Neurol. 2005;63:350–355. doi: 10.1016/j.surneu.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Byun MS, Shin JJ, Hwang YS, Park SK. Decompressive surgery in a patient with posttraumatic syringomyelia. J Korean Neurosurg Soc. 2010;47:228–231. doi: 10.3340/jkns.2010.47.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossier AB, Foo D, Shillito J, Dyro FM. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108(Pt 2):439–461. doi: 10.1093/brain/108.2.439. [DOI] [PubMed] [Google Scholar]
- 11.Alexander MA, Bunch WH, Ebbesson SO. Can experimental dorsal rhizotomy produce scoliosis? J Bone Joint Surg Am. 1972;54:1509–1513. [PubMed] [Google Scholar]
- 12.Alvisi C, Cerisoli M. Long-term results of the surgical treatment of syringohydromyelia. Acta Neurochir (Wien) 1984;71:133–140. doi: 10.1007/BF01401158. [DOI] [PubMed] [Google Scholar]
- 13.Chuma A, Kitahara H, Minami S, Goto S, Takaso M, Moriya H. Structural scoliosis model in dogs with experimentally induced syringomyelia. Spine (Phila Pa 1976) 1997;22:589–594. doi: 10.1097/00007632-199703150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Gardner JW, Collis JS. Skeletal anomalies associated with syringomyelia, diastematomyelia, and myelomeningocele. J Bone Joint Surg Am. 1960;42:1265. [Google Scholar]
- 15.Gurr KR, Taylor TK, Stobo P. Syringomyelia and scoliosis in childhood and adolescence. J Bone Joint Surg Br. 1988;70:159. [Google Scholar]
- 16.Merck manual for healthcare professional. Syrinx [Internet] Whitehouse Station (NJ): Merck Sharp & Dohme Corp; c2007. [Cited 2007 Jan 20]. Available from: http://www.merckmanuals.com/professional. [Google Scholar]
- 17.Tomlinson RJ, Jr, Wolfe MW, Nadall JM, Bennett JT, MacEwen GD. Syringomyelia and developmental scoliosis. J Pediatr Orthop. 1994;14:580–585. doi: 10.1097/01241398-199409000-00005. [DOI] [PubMed] [Google Scholar]