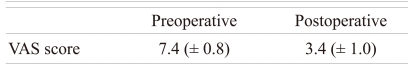Abstract
Study Design
This is a retrospective study.
Purpose
To evaluate the advantages and effects of posterior lumbar interbody fusion (PLIF) using allograft and posterior instrumentation in the lumbar pyogenic discitis, which are resistant to antibiotics.
Overview of Literature
To present preliminary results of PLIF using a compressive bone graft with allograft and pedicle screw fixation in the lumbar pyogenic discitis.
Methods
Fifteen patients who had lumbar pyogenic discitis were treated by posterior approach from May 2004 to July 2008. The mean follow-up duration was 27.2 ± 18.68 months. The standing radiographs of the lumbar spine and clinical results were compared and analyzed in order to assess the bony union, the changes in the distance between the two vertebral bodies and the changes in the lordotic angle formed between the fused bodies immediately after surgery and at the final follow-up.
Results
Fifteen solid unions at an average of 15.2 ± 3.5 weeks after operation. The mean preoperative lordotic angle of the affected segments was 14.3 ± 15.1°, compared to 20.3 ± 12.3° after surgery and 19.8 ± 15.2° at last follow-up. For the functional result according to the Kirkaldy-Willis criteria, the outcome was excellent in 9, good in 5, fair in 1, and there were no poor cases. The average visual analogue scale score was decreased from 7.4 before surgery to 3.4 at 2 weeks postoperative.
Conclusions
The main advantage in the procedure of PLIF using compressive bone graft with allograft and post instrumentation is early ambulation. We believe that this is another good procedure for patients with poor general condition because a further autograft bone harvest is not required.
Keywords: Lumbar spine, Discitis, Posterior lumbar interbody fusion, Homologous transplantation
Introduction
Pyogenic vertebral osteomyelitis among skeletal osteomyelitis has been reported to occur at a frequency of 2-7% [1]. The basic principle of the treatment of pyogenic spondylitis is to conduct a conservative treatment based on bed rest and administration of antibiotics. However, in the cases of the failure of antimicrobial therapy, clinically important abscesses such as large abscess, and neurologic defect, surgical treatments have been conducted.
In the surgical treatment of lumbar pyogenic spondylitis, debridement via the anterior approach and anterior lumbar interbody fusion have been used as general treatment methods since an infection source is located at the anterior vertebral region. Eysel et al. [2] reported good outcomes using the aforementioned methods in the treatment of pyogenic lumbar spondylitis. However, in a follow-up study on changes of the saggital angle, Rajasekaran and Shanmugasundaram [3] and Rajasekaran and Soundarapandian [4] reported that the grafted bone was stabilized in 41% of the cases that underwent anterior lumbar interbody fusion, and that the dislocation, fracture, absorption, and settlement of the grafted bone were observed in the remaining cases. Chen et al. [5] reported that kyphotic deformity deteriorated in patients who underwent anterior interbody fusion alone. In addition, the anterior approach has a potential risk of recurrence if metal is present in the infected area.
Due to the development of posterior lumbar interbody fusion, anterior vertebral abscess can be removed via the posterior approach. In particular, if an epidural abscess with concurrent spinal stenosis is present in the posterior area, it can be removed via the posterior approach. Furthermore, rigid internal fixation using a posterior pedicle screw has been believed not to only somewhat recover the normal lordotic angle, but also promote the spontaneous wound healing mechanism and to facilitate early ambulation and rehabilitation [6-8].
Compared to conventional treatments of pyogenic discitis focusing on autologous bone graft, a treatment method proactively considering an allogenic bone graft was used in this study along with the advantages of the posterior approach. The application of thorough drainage and compressive bone graft with allograft mixed with the allogenic bone and the autologous bone after removing inflammatory tissues was considered appropriate for patients with a poor general condition [9,10].
The authors conducted a posterior lumbar interbody fusion using compressive bone graft mixed with the allogenic bone and the autologous bone in patients with pyogenic lumbar discitis, and report its results herein.
Materials and Methods
1. Study subjects
This study was conducted on 15 patients with pyogenic lumbar discitis who had undergone posterior lumbar interbody fusion using compressive bone graft mixed with the allogenic bone and the autologous bone from May 2004 to July 2008. The subjects included 7 male and 8 female patients. The mean age of the subjects was 59.8 ± 12.1 years, and the follow-up period was 10-50 months (the mean follow-up period was 2 years and 3 months). The underlying diseases were as follows: seven cases of diabetes mellitus, one case of oral glucose intolerance, two cases of liver cirrhosis, and four cases of previous surgery history. Most patients who had been diagnosed with pyogenic discitis suffered from severe back pain and gait disturbance, and in six patients with pyogenic discitis, this was accompanied with radiating pain of the lower extremity. Discitis distribution was as follows: nine cases of L4-5, two cases of L3-4, one case of L5-S1, and three cases of invading at level 2, which consisted of one case of L3-4-5 and two cases of L4-5-S1. The estimated reasons for the infection included four cases of postoperative infection and two cases of epidural injections, and there were nine cases of unknown reasons.
Indications determining the surgery included abnormal erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and persistent pain despite antibiotics administration for more than 3 weeks, and the occurrence of neurologic symptoms due to the compressed dura caused by abscess. Among the patients who showed the aforementioned indications, the following patients were selected as subjects for posterior lumbar interbody fusion: 1) those who had a relatively smaller amount of abscess present in the anterior vertebral body; 2) those with concurrent spinal stenosis; 3) those with posterior epidural abscess; or 4) those with instability of lumbar interbody. Accordingly, after thorough drainage and removal of inflammatory tissues, a compressive bone graft was conducted using either both the allogenic bone and the autologous bone or the allogenic bone alone.
2. Radiographic evaluation
In preoperative evaluation, the range of bone destruction and the change of lordotic angle were measured via lumbar plain radiographs, and the instability was assessed via flexion-extension view. In addition, a contrast enhanced magnetic resonance imaging (MRI) was conducted to measure spinal stenosis, soft tissue adhesion and the severity of the anterior abscess.
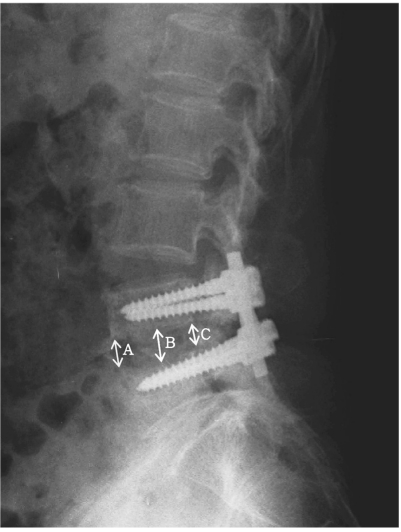
Postoperative bone union was assessed according to the criteria of the radiological bony union, as suggested by Lee et al. [11], including cases in which the bone bridge was forming in plain X-ray and cases with no movement of the bony region in flexion-extension view. The lumbar lordotic angle was assessed based on the epiphyseal plate of the uppermost and lowest vertebral body of the area to which internal fixation was applied. The height of intervertebral disc space was obtained from the mean value of the heights of the anterior, middle and posterior intervertebral discs by referring to the method suggested by Inoue et al. [12]. For relative comparison of the height of the intervertebral disc space, the height of the intervertebral disc, which was obtained from a surgery conducted according to the relative disc height as suggested by Inoue et al. [12], was divided by the height of the intervertebral disc between L2 and L3, on which surgery was not carried out, and had relatively less degenerative change among the lower lumbar. The value obtained from the aforementioned calculation was converted into a percentage value, and then used to compare the preoperative and postoperative changes of disc height (Fig. 1).
Fig. 1.
The radiographic measurements of the lumbar disc height. Anterior disc height (A), middle disc height (B), and posterior disc height (C). Disc height = (A+B+C)/3 (mm). Relative disc height = Disc height/L2-3 disc height × 100 (%).
3. Clinical evaluation
Laboratory examination, which assessed white blood cell (WBC) count, ESR, and CRP, was conducted twice a week during the visit, preoperative period and postoperative period until discharge. Bacterial culture and biopsy were conducted on the resected disc and subchondral bone tissues. Neurologic symptoms were assessed using the classification suggested by Frankel et al. [13]. Before the surgery, neurologic symptoms included three cases of grade B, six cases of grade C, and six cases of grade D. Functional evaluation was conducted using the Kirkaldy-Willis criteria [14]. In addition, the visual analogue scale (VAS) score was compared before the surgery and 2 weeks after the surgery.
4. Surgery method
Laminectomy was conducted on the patients in a prone position under general anesthesia. Nerve decompression was conducted, followed by discectomy. The infected laminar, disc, and granulation tissues were completely removed using pituitary forceps and a ring curette.
Compressive bone graft using both the allogenic bone and the autologous bone or the allogenic bone alone collected from the spinous process was conducted on the empty intervertebral disc space formed after debridement in a manner where the empty space was completely filled. The allogenic bone alone was used in eight patients with a poor spinous process. Then, the pedicle screws were fixed and pressure was applied using a compressor to firmly fix the grafted bone.
The drainage was removed if the drainage amount was 50 ml or lower. Ambulation was allowed starting from three days after the surgery, and a brace was installed for 3 months. After the surgery, appropriate antibiotics were intravenously administered to the patients who were positive to the bacterial culture, whereas empirical antibiotics were intravenously administered to the patients who were negative to the bacterial culture. After discharge, oral antibiotics were administered for 2-4 months until hematological indices (WBC count, ESR, CRP) returned to normal levels.
Results
1. Bony union and change of the lordotic angle
Bony union was assessed according to the criteria of radiologic bony union suggested by Lee et al. [11]. The rigid bony union was observed in all cases at a mean of 15.2 ± 3.5 weeks after the surgery (Fig. 2). The preoperative, postoperative, and last follow-up lordotic angle were a mean of 14.3 ± 15.1°, 20.3 ± 12.3°, and 19.8 ± 15.2°, respectively. When the postoperative disc height was compared using the relative disc height suggested by Inoue et al. [12], the mean reduction of 3.1 ± 3.2% was observed between after the surgery and the last follow up (Table 1).
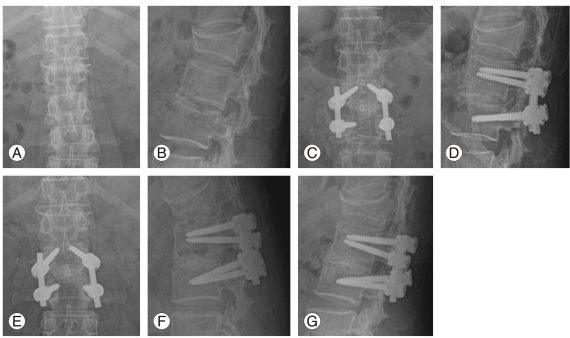
Fig. 2.
A 48-year-old female patient. Antero-posterior (AP) (A) and lateral (B) plain roentgenograms showed preoperative findings of L2-3 disc space widening and destruction. The operation was carried out with posterior laminectomy, discectomy, drainage, debridement, posterior lumbar interbody fusion using allogenous bone graft at L2-3, and posterior instrumentation at L2-3 level (C, D). At 15 months after surgery, AP (E), lateral flexion and extension (F, G) plain roentgenograms showed rigid fixation and fusion without any evidence of further infection.
Table 1.
Change of lordotic angle and intervertebral disc height
2. Clinical results
The mean operation time was 2 hours 50 minutes, and the mean period of bed rest was 4.9 ± 1.5 days. No particular complication was found. The mean administration period of IV antibiotics was 11.4 ± 5.4 days, and the mean administration period of oral antibiotics was 84 ± 35.4 days. The average hospital stay was 14.5 ± 4.5 days.
As a result of bacterial culture, six cases of Staphylococcus aureus and two cases of Pseudomonas aeroginosa were observed (Table 2). The result of biopsy conducted on the removed disc confirmed that all patients had been diagnosed with discitis. The mean ESR was 32.9 ± 26.5 mm/hr before the surgery and its normal level was set as 10 mm/hr. The ESR level was shown to decrease within six weeks after the surgery, and return to the normal level in 12 cases, and in three cases to return to the normal level three months after the surgery. The CRP level was 7.2 ± 5.6 mg/dl before the surgery and its normal level was set as 1.0 mg/dl. The CRP level was shown to significantly decrease to 0.9 ± 0.3 mg/dl 2 weeks after the surgery (p < 0.04), and return to the normal level in 13 cases within two months after the surgery, and in two cases return to the normal level three months after the surgery.
Table 2.
Bacterial culture
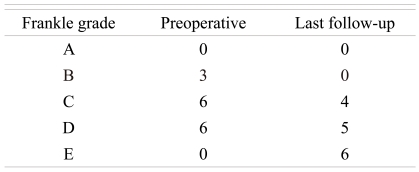
In the last follow-up, the classification of neurologic deficit showed that the Frankel grade was improved from grade D to E in four cases, from grade C to D in three cases, from grade C to E in two cases, and from grade B to C in three cases (Table 3). In addition, the result of functional assessment using the Kirkaldy-Willis criteria [10] showed that nine cases were excellent, five cases were good, and one case was fair. The mean VAS score was 7.4 ± 0.8 before the surgery and decreased to 3.4 ± 1.0 2 weeks after the surgery (p < 0.03) (Tables 4 and 5).
Table 3.
Change of Frankel neurological classification
Table 4.
The clinical results assessed by the Kirkaldy-Willis
Table 5.
Change of visual analogue scale (VAS) score
p < 0.03, Willcoxon rank-sum test.
Discussion
In general, it is not easy to treat pyogenic lumbar discitis. The basic principle of the treatment is to conduct a non-surgical treatment where a conservative treatment based on the appropriate administration of antibiotics is conducted. However, based on the fact that providing stability to osteomyelitis (which occurred in the long bone), using metal was effective in inflammation control [15], studies have been conducted to provide stability to the treatment of pyogenic spondylitis, and to achieve early ambulation via a posterior approach. As a result, some studies [16,17] have reported good outcomes.
As the direct insertion of a metal implant into the infected site may cause deterioration or recurrence of the infection in the treatment of musculoskeletal infectious disease, it has been contraindicated to date. Bacteria have been known to attach to the surface of an artificial implant located at the inflammatory tissues, and form a biofilm [18]. Such biofilm formation has been known to cause the persistence of the infection by blocking the antibiotic approach. However, in recent years, direct fixation following the complete debridement of the lesions of tuberculous spondylitis and pyogenic discitis has been conducted and this method was reported to be effective and did not increase the risk of infection recurrence in the treatment of vertebral inflammation [2,19]. As internal fixation is located at the cancellous bone with abundant blood flow, bacterial growth is expected to be inhibited if the drainage of common bacteria including Staphylococcus aureus via complete debridement prior to biofilm formation is performed together with an antibiotic approach.
In general, pyogenic and tuberculous spinal infection invade the anterior vertebral region [20]. Accordingly, if a conservative treatment does not work, anterior fusion using the tricortical autograft following anterior drainage of the infected site and necrotic tissue debridement has been used as a common surgical treatment [21,22]. Safran et al. [19] and Krödel et al. [23] reported that anterior debridement and posterior fixation were effective in the treatment of lumbar osteomyelitis. Park et al. [21] reported the result of the treatment of tuberculous spondylitis via anterior debridement, anterior fusion and anterior fixation.
If debridement without internal fixation is conducted in an anterior approach, long-term bed rest or a body cast, or a surgery via an additional posterior approach are required. If anterior metal fixation is conducted via an anterior approach, it has a risk of recurrence due to the metal present in the lesion [6]. In addition, the anterior approach may cause complications such as injuries of gastrointestinal or urinary tissues due to neurogenic injury, hernia, and the adhesion of infected perispinal tissues [6]. Furthermore, it is difficult to access the lower lumbar inflammation that occurred in L5-S1 via the anterior approach. If an abscess or spinal deformity is observed in the posterior dura, a posterior surgery is additionally required.
Posterior pedicle screw fixation has advantages of early ambulation and early rehabilitation due to rigid fixation, and the partial correction of lumbar lordotic angle loss caused by bony destruction due to inflammation into the normal lordotic angle [6,16,17,24]. In addition, as pyogenic discitis frequently occurs in elderly patients with decreased immunity, concurrent spinal stenosis frequently occurs. In such cases, spinal stenosis can be surgically treated via posterior debridement [6]. Although relatively fewer studies on lumbar interbody fusion and pedicle screw fixation in the treatment of lumbar osteomyelitis have been conducted, Przybylski and Sharan [16], Rath et al. [17], and Park et al. [6] conducted autologous bone graft following posterior debridement via posterior approach alone, and then conducted posterior pedicle screw fixation in the treatment of pyogenci discitis via posterior approach alone, and reported that good outcomes were obtained.
Among the patients with pyogenic lumbar discitis who were not treated with 3-week or more antibiotic administration, 15 patients who had a relatively smaller amount of abscess present in the anterior vertebral body, who had concurrent spinal stenosis, or who had a posterior epidural abscess, were selected as subjects for posterior lumbar interbody fusion.
In conventional pyogenic and tuberculous discitis, surgery using the autologous bone has been mainly conducted. In the case of fusion using the autologous bone, it has an advantage of an increased ratio of bony fusion, but has disadvantages of the occurrence of pain and hemorrhage of the donor site after harvesting the autologous bone [25,26]. Accordingly, unlike conventional treatments of pyogenic discitis that focus on autologous bone graft, in this study, allograft transplantation alone or bone graft with both the allogenic bone and the autologous bone were conducted on the intervertebral disc space formed after debridement. Previous studies have reported that the allogenic bone was used instead of the autologous bone in the treatment of osteomyelitis that occurred in the long bone [27]. O'Brien et al. reported that a good outcome was obtained when the femoral cortical allogenic bone was used with the autologous bone in lumbar interbody fusion, and that it could be an ideal method for bone graft [9,10]. Bendo et al. [28] conducted anterior lumbar interbody fusion where the femoral head was used as the allogenic bone, and reported that easy revascularization promoted creeping substitution, a straightforward change of the intervertebral disc type, and the maintenance of the height of the disc interval.
Raut et al. [29] reported that a high treatment rate of 86% was obtained from artificial joint replacement conducted again after the complete removal of the infected soft and bony tissues, following the complete removal of internal structures such as metal and polyethylene via a single re-surgery in artificial joint replacement conducted on the deeply infected tissues. They emphasized that the complete removal of the infected tissues rather than the number of surgeries was critical for the successful treatment of infection. Thus, this factor should be considered in the treatment of pyogenic discitis.
As allograft transplantation does not require an additional surgery to harvest the autograft in elderly patients with decreased level of immunity, particularly those with poor general conditions, it can help to shorten operation time, reduce the amount of bleeding, and avoid complications occurring in the donor site. Despite the superiority of the autograft in inflammation treatment, the complete removal of dead tissues via debridement is considered a more important factor determining prognosis.
Conclusions
In the treatment of pyogenic lumbar discitis, posterior lumbar interbody fusion using compressive bone graft with the allograft and the autograft or with allograft alone can be usefully applied to patients with less bone destruction of the spine and a small amount of anterior abscess, or with concurrent spinal stenosis, or with lower lumbar discitis where the anterior approach is difficult, or particularly to patients with poor general condition or elderly patients. In particular, it has advantages of early ambulation and early rehabilitation after surgery.
Acknowledgements
This work was supported by the 2010 Inje University reserch grant.
References
- 1.Liebergall M, Chaimsky G, Lowe J, Robin GC, Floman Y. Pyogenic vertebral osteomyelitis with paralysis: prognosis and treatment. Clin Orthop Relat Res. 1991;(269):142–150. [PubMed] [Google Scholar]
- 2.Eysel P, Hopf C, Vogel I, Rompe JD. Primary stable anterior instrumentation or dorsoventral spondylodesis in spondylodiscitis? Results of a comparative study. Eur Spine J. 1997;6:152–157. doi: 10.1007/BF01301428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am. 1987;69:503–509. [PubMed] [Google Scholar]
- 4.Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treated by anterior arthrodesis. J Bone Joint Surg Am. 1989;71:1314–1323. [PubMed] [Google Scholar]
- 5.Chen WJ, Chen CH, Shih CH. Surgical treatment of tuberculous spondylitis: 50 patients followed for 2-8 years. Acta Orthop Scand. 1995;66:137–142. doi: 10.3109/17453679508995507. [DOI] [PubMed] [Google Scholar]
- 6.Park WW, Park YS, Cheon SJ, Jung JY. Posterior lumbar interbody fusion in the pyogenic discitis. J Korean Soc Spine Surg. 2001;8:39–45. [Google Scholar]
- 7.Güven O, Kumano K, Yalçin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine (Phila Pa 1976) 1994;19:1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sundararaj GD, Behera S, Ravi V, Venkatesh K, Cherian VM, Lee V. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85:100–106. doi: 10.1302/0301-620x.85b1.13300. [DOI] [PubMed] [Google Scholar]
- 9.Kozak JA, Heilman AE, O'Brien JP. Anterior lumbar fusion options: technique and graft materials. Clin Orthop Relat Res. 1994;(300):45–51. [PubMed] [Google Scholar]
- 10.Liljenqvist U, O'Brien JP, Renton P. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J. 1998;7:125–131. doi: 10.1007/s005860050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements: the results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:356–361. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Ohmori K, Miyasaka K, Hosoe H. Radiographic evaluation of the lumbosacral disc height. Skeletal Radiol. 1999;28:638–643. doi: 10.1007/s002560050566. [DOI] [PubMed] [Google Scholar]
- 13.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 14.Kirkaldy-Willis WH, Paine KW, Cauchoix J, McIvor G. Lumbar spinal stenosis. Clin Orthop Relat Res. 1974;(99):30–50. doi: 10.1097/00003086-197403000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Meyer S, Weiland AJ, Willenegger H. The treatment of infected non-union of fractures of long bones: study of sixty-four cases with a five to twenty-one-year follow-up. J Bone Joint Surg Am. 1975;57:836–842. [PubMed] [Google Scholar]
- 16.Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 2001;94(1 Suppl):1–7. doi: 10.3171/spi.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 17.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Costerton JW, Irvin RT, Cheng KJ. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- 19.Safran O, Rand N, Kaplan L, Sagiv S, Floman Y. Sequential or simultaneous, same-day anterior decompression and posterior stabilization in the management of vertebral osteomyelitis of the lumbar spine. Spine (Phila Pa 1976) 1998;23:1885–1890. doi: 10.1097/00007632-199809010-00018. [DOI] [PubMed] [Google Scholar]
- 20.Buyukbebeci O, Karakurum G, Guleç A, Erbagci A. Tuberculous osteomyelitis of the lumbosacral region: a spinal epidural abscess with presacral extension. Arch Orthop Trauma Surg. 2004;124:346–348. doi: 10.1007/s00402-004-0660-y. [DOI] [PubMed] [Google Scholar]
- 21.Park JT, Ahn GY, Kim HG, Seong YW. The results of anterior fusion with anterior instrumentation in spinal tuberculosis (preliminary study) J Korean Soc Spine Surg. 1996;3:217–224. [Google Scholar]
- 22.Chen WJ, Wu CC, Jung CH, Chen LH, Niu CC, Lai PL. Combined anterior and posterior surgeries in the treatment of spinal tuberculous spondylitis. Clin Orthop Relat Res. 2002;(398):50–59. doi: 10.1097/00003086-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Krödel A, Krüger A, Lohscheidt K, Pfahler M, Refior HJ. Anterior debridement, fusion, and extrafocal stabilization in the treatment of osteomyelitis of the spine. J Spinal Disord. 1999;12:17–26. [PubMed] [Google Scholar]
- 24.Lee JS, Suh KT. Posterior lumbar interbody fusion with an autogenous iliac crest bone graft in the treatment of pyogenic spondylodiscitis. J Bone Joint Surg Br. 2006;88:765–770. doi: 10.1302/0301-620X.88B6.17270. [DOI] [PubMed] [Google Scholar]
- 25.Wetzel FT, LaRocca H. The failed posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1991;16:839–845. doi: 10.1097/00007632-199107000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Wimmer C, Krismer M, Gluch H, Ogon M, Stöckl B. Autogenic versus allogenic bone grafts in anterior lumbar interbody fusion. Clin Orthop Relat Res. 1999;(360):122–126. doi: 10.1097/00003086-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Park IH, Kyung HS, Kim DH. One-staged saucerization and cancellous chip allograft for treatment of chronic localized osteomyelitis. J Korean Orthop Assoc. 1998;33:606–613. [Google Scholar]
- 28.Bendo JA, Spivak JM, Neuwirth MG, Chung P. Use of the anterior interbody fresh-frozen femoral head allograft in circumferential lumbar fusions. J Spinal Disord. 2000;13:144–149. doi: 10.1097/00002517-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Raut VV, Siney PD, Wroblewski BM. One-stage revision of infected total hip replacements with discharging sinuses. J Bone Joint Surg Br. 1994;76:721–724. [PubMed] [Google Scholar]