Abstract
Background
Newborn screening (NBS) for CF has become widespread, although there are multiple strategies. Little is known about outcomes such as age of diagnosis after different NBS methods.
Methods
We used the U.S. Cystic Fibrosis Foundation Patient Registry to identify infants with CF born between 2001 and 2008 in states that utilized NBS. We compared ages at diagnosis, genotyping, sweat test, and first visit to a CF Centre between states that used serial immunoreactive trypsinogen (IRT/IRT) levels and states that used IRT and DNA analysis (IRT/DNA).
Results
We identified 1,288 infants with CF. Compared to infants born in IRT/IRT states, infants born in IRT/DNA states were younger at the time of diagnosis (median 2.3 weeks versus 4.0 weeks in IRT/IRT states, p<0.001), genotyping (0.7 weeks versus 5.3 weeks, p<0.001), and initial CF Centre visit (5.9 weeks versus 7.7 weeks, p=0.008).
Conclusions
Although there is room to improve outcomes with both strategies, infants born in IRT/DNA states have treatment initiated at a younger age than infants born in IRT/IRT states.
INTRODUCTION
Newborn screening (NBS) is used to facilitate presymptomatic diagnosis and has been applied to cystic fibrosis (CF) since the early 1980s in Europe, Australia and New Zealand, and the U.S. (1, 2). The initial algorithm (2) demonstrating elevated values of immunoreactive trypsinogen on two blood specimens (IRT/IRT) is still used widely today. The U.S. CF Foundation recommends that the two blood specimens be obtained around day 2 and day 14 of life (3). Because of concerns (4) regarding suboptimal sensitivity of IRT/IRT, and the ability to test for CF transmembrane conductance regulator (CFTR) gene mutations (5), combining IRT with DNA analysis (IRT/DNA)for CFTR mutations on a single blood specimen was developed and is now used in many European countries and U.S. states (6–8). However, utilizing DNA analysis has several potential disadvantages that may be avoided using IRT/IRT: DNA analysis may increase the recognition of carriers, children with an equivocal diagnosis, and children with misidentified paternity.(9)
Studies of the benefits of NBS have revealed improvements in nutrition that are most apparent for those treated in the first two months of life (10, 11), and there is evidence of poor nutrition as early as 2 weeks of age in some infants with CF (12–14). More recently, analysis of the CF NBS programmes in France in 2002–2005 revealed that, excluding infants with meconium ileus, 52% of the infants were symptomatic at the initial CF Centre visit, which occurred at a median age of 34 days (15).
Because of the importance of prompt identification and treatment of infants with CF, and reports of possible delays using IRT/IRT methodology (2, 4, 16), we hypothesized that infants born in regions that use IRT/DNA would be diagnosed and treated at younger ages than infants with CF born and diagnosed after IRT/IRT screening. Additionally, our experience with NBS in Wisconsin led us to perform several quality improvement (QI) initiatives to improve our NBS programme (17). To assess the effectiveness of these QI projects, we compared our data to other states’ data using the U.S. Cystic Fibrosis Foundation Patient Registry (CFFPR). Our aim was to determine if infants with CF were diagnosed and treated earlier in states that used IRT/DNA, and whether QI initiatives implemented in Wisconsin led to improvements. Some of the results of these studies have been previously reported in the form of an abstract (18).
METHODS
Data from the CFFPR (19–21) from 2001–2008 was used to identify infants with CF born in states that had implemented NBS before 31/12/2008. The first year of NBS implementation was excluded from this analysis. Infants diagnosed through prenatal screening or with meconium ileus were excluded.
Because data was not normally distributed, we used the median score test to compare ages at relevant dates for children with CF diagnosed in IRT/DNA and IRT/IRT states. To evaluate trends over time, we compared relevant dates between 2001–2004 and 2005–2008. We repeated comparisons, using only infants with CF with an abnormal NBS result, as entered in the CFFPR. Results were compared against CF Foundation recommendations (6). Finally, we compared the age at relevant dates between children born in Wisconsin to other states to evaluate the effect of QI efforts (17).
RESULTS
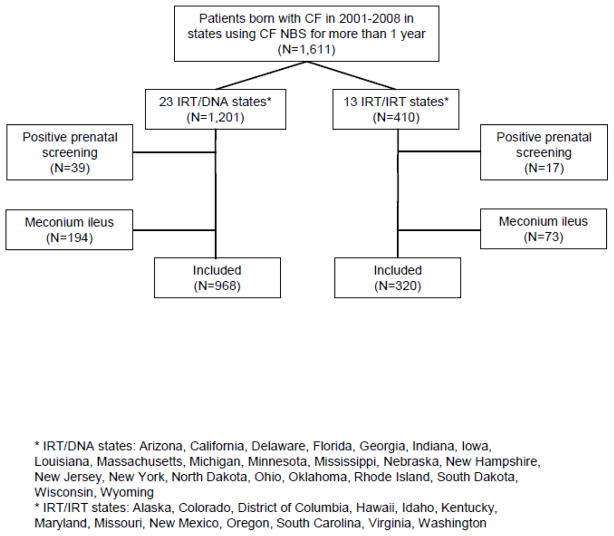
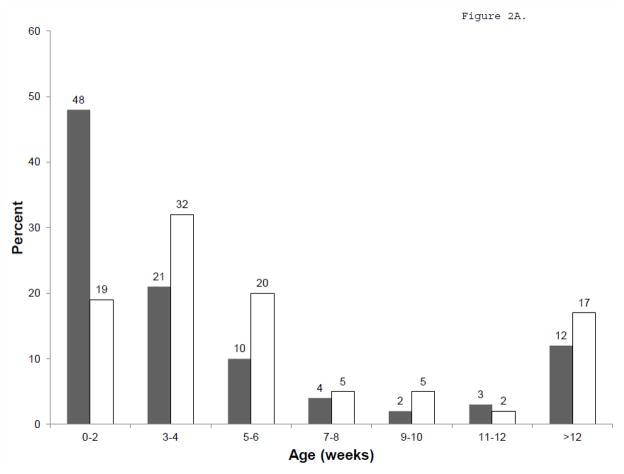
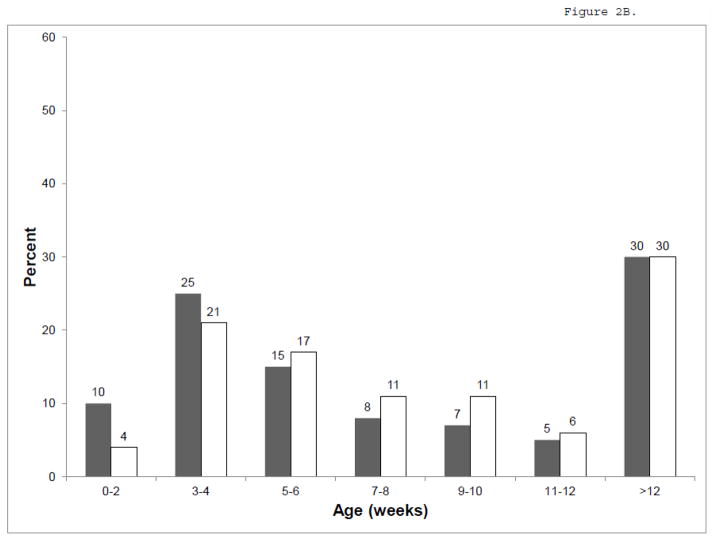
There were 1,611 infants from 23 IRT/DNA and 13 IRT/IRT states that used NBS for at least one year during 2001–2008 (Figure 1). Compared to infants born in IRT/IRT states (Table 1), infants born in IRT/DNA states were younger at the time of diagnosis (median 2.3 weeks versus 4.0 weeks in IRT/IRT states, p<0.001, Figure 2A), genotyping (0.7 weeks versus 5.3 weeks, p<0.001), and initial CF Centre visit (5.9 weeks versus 7.7 weeks, p=0.008, Figure 2B). A higher proportion of infants had an entry in the CFFPR for a positive newborn screening result in IRT/DNA states (82%) than in IRT/IRT states (62%), p<0.001. Results were similar when restricted to infants who had an entry for a positive newborn screening result.
Figure 1.
Flowchart of study cohort.
Table 1.
Comparison of infants born in IRT/DNA and IRT/IRT states in 2001–2008
| Median (25th, 75th percentiles) age in weeks | |||
|---|---|---|---|
| IRT/DNA (N=968) | IRT/IRT (N=320) | p-value | |
| Diagnosis age | 2.3 (0.3, 5.1) | 4.0 (2.7, 7.4) | <0.001 |
| Sweat test age* | 4.4 (2.7, 9.3) | 4.3 (3.0, 8.3) | 0.65 |
| Genotype age** | 0.7 (0.1, 4.4) | 5.3 (3.4, 10.1) | <0.001 |
| First CF Centre visit age | 5.9 (3.0, 15.1) | 7.7 (4.0, 13.9) | 0.008 |
Sweat tests were not reported for 136 (14%) in IRT/DNA states and 86 (27%) in IRT/IRT states, p<0.001
Genotype was not reported for 40 (4%) in IRT/DNA states and 38 (12%) in IRT/IRT states, p<0.001
Figure 2.
Age at Diagnosis (A) and initial CF Centre visit (B). IRT/DNA in gray, IRT/IRT in white.
The median age at genotyping decreased in IRT/DNA states between 2001–2004 (1.9 weeks) and 2005–2008 (0.4 weeks, p <0.001), but there were no other differences in age at sweat test, genotype, or first CF Centre visit between 2001–2004 and 2005–2008.
There were 120 infants with CF born in Wisconsin during 2001–2008. These infants were younger at their first CF Centre visit (median 4.3 weeks, as compared to 6.1 weeks for other IRT/DNA states, p = 0.1, and 7.7 weeks for IRT/IRT states, p = 0.02), and at the time of the sweat test: median 2.6 weeks in Wisconsin, 4.9 weeks in other IRT/DNA states, and 4.3 weeks in IRT/IRT states, p<0.001.
DISCUSSION
We found that infants born in states that utilized IRT/DNA methodology were diagnosed, genotyped, and seen for an initial CF Centre visit at younger ages than infants born in states that utilized IRT/IRT methodology. Similar results were reported in a survey of CF NBS programmes in Europe that used more heterogeneous NBS strategies (22). Nineteen of the 26 NBS programmes in Europe used DNA analysis as part of their NBS; these 19 programmes reported a significantly younger median age at diagnosis (approximately 5 weeks, compared to approximately 8 weeks for programmes that did not use DNA in their NBS programmes), although there is no data on age of sweat test or first CF Centre visit (22). It is apparent that the IRT/IRT protocol leads to a longer time to diagnosis, although IRT/DNA protocols are likely to increase the recognition of carriers, children with an equivocal diagnosis, and children with misidentified paternity (9).
The U.S. CF Foundation has recommended that a sweat test be performed following a positive NBS by 2–4 weeks of age, including results with 2 mutations detected by DNA analysis (6). At the time of a positive sweat test, a CF clinician should evaluate the patient, develop short-term treatment plans with the parents, and schedule an initial CF Centre visit by 1–2 months of age (3, 6). The European CF Society recommends that a sweat test preferably be performed in the first month of life (24). These goals were formulated with the intention of maximizing potential benefits to patients with CF. Our findings indicate that these goals were not being met for a large proportion of infants: at least half of patients in our study were sweat tested after the first month of life. The median age at the first CF Centre visit did fall within the recommended range for both IRT/DNA and IRT/IRT states, but 37% of infants with CF in IRT/DNA and 40% in IRT/IRT states were not seen for their initial visit until after 8 weeks of age.
While all NBS programmes have the potential for delayed care, or even missed cases, QI can minimize negative outcomes and maximize positive ones. After our experience with the Wisconsin Randomized Control Trial of CF Newborn Screening, the Wisconsin NBS programme made several changes (17) designed to increase the efficiency of the screening process and minimize the time to sweat testing (Table 2). After making those changes, we report in the current study that infants in Wisconsin were younger at their sweat test and first CF Centre visit than infants born in other IRT/DNA or IRT/IRT states.
Table 2.
Quality improvement procedures undertaken in Wisconsin to improve CF newborn screening
| The IRT assay was changed from a radiometric to a fluorescent dissociation enhanced lanthanide fluorescence immunoassay method |
| A “floating” IRT referral level for DNA testing was introduced to minimize seasonal effects on measured IRT levels |
| The IRT referral level for DNA testing was decreased from the 98.5th to the 94th percentile; this was later revised up to the 96th percentile to minimize carrier detection |
| DNA testing was performed 3 days per week (increased from 1 day per week previously) |
| DNA detection changed from F508del only to routine screening for the American College of Medical Genetics (ACMG) 25 CF transmembrane conductance regulator (CFTR) mutations using a strip detection system (CF-Gold LAp, Roche Molecular Biochemicals) in March, 2002. When R117H is detected, re ex testing for the polythymidine tract in intron 8 (5T, 7T, and 9T) is performed. In July 2008, the screening changed to the ACMG 23 CFTR mutations using the Invader® Assay (Hologic Inc.) |
| Infants with IRT levels higher than the 99.9th percentile but without detectable CFTR mutations are reported as “possible” abnormal. Sweat testing is recommended when there are symptoms or a positive family history. |
| As a quality control measure, the Wisconsin State Newborn Screening lab routinely analyzes specimens with abnormal results in a blinded fashion to ensure repeatability of results |
| The Wisconsin State Newborn Screening lab implemented a formal process to follow up on all abnormal results to maximize the likelihood that a follow up sweat test would be performed |
| Primary care providers are notified by phone by the Wisconsin State Newborn Screening lab when two disease-causing mutations are identified |
| Sweat tests are scheduled as soon as possible, as long as the infant weighs at least 2.95 kilograms. |
| In 2005, sweat tests were made available at an affiliate CF Centre to decrease the distance some patients had to travel |
Our study has several limitations. First, while the CFFPR has been used in many studies of patients with CF (19–21), data entry on diagnosis-related variables is not standardized. This is demonstrated by the distribution of diagnosis dates, where the diagnosis date is listed as the birth date for 11% of infants born in IRT/IRT states and 14% of infants born in IRT/DNA states. While this limits our ability to interpret differences in the diagnosis date, we expect that the sweat test and initial CF Centre visit dates are reliable, as these dates are clearly recorded in the patients’ medical records. Additionally, a surprisingly high number of infants were reported to not have had a positive newborn screening result (18–28%). This is most likely due to this box not being checked during entry of data into the CFFPR, since our results were similar when we restricted our analysis to infants with a positive newborn screening result. Second, while we excluded the first year of data in states that began CF NBS programmes during 2001–2008, one year may not be sufficient for NBS programmes to establish best practices. Third, we do not have any information on false positive NBS results and potential negative outcomes (e.g., carrier recognition, equivocal diagnosis, or misidentified paternity). Finally, we can only speculate on the possible reasons for delayed dates of diagnosis and initial CF Centre visits in IRT/IRT states, although it is known that the IRT/IRT method has a higher rate of false negative tests (2), is more difficult to apply to premature infants (23), depends on the timing of blood sampling (16), and requires successful procurement of a second specimen (which fails in 5–20% (2)). It is not surprising that genotyping occurs earlier in IRT/DNA states, since DNA analysis is integral to the protocol.
In conclusion, during the years 2001–2008, IRT/DNA states diagnosed and treated infants with CF at younger ages than IRT/IRT states. In both IRT/DNA and IRT/IRT states, there is need for quality improvements that can shorten the time to diagnosis and treatment. Additionally, there is need for quality improvements in data entry into CF registries if the CF community is to use these valuable resources to continue to improve our newborn screening processes.
Acknowledgments
We thank Dr. Bruce Marshall from the Cystic Fibrosis Foundation for providing the Patient Registry data and Dr. Zhumin Zhang for assistance with the data analysis. Dr. Farrell is supported by NIH R01 DK34108 and Dr. Farrell and Dr. Sanders are supported by NIH R01 DK34108-23S1 Revised.
Footnotes
Data from this manuscript was presented at the North American Cystic Fibrosis Conference in October, 2010, in Baltimore, Maryland, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilcken B, Wiley V, Sherry G, Bayliss U. Neonatal screening for cystic fibrosis: a comparison of two strategies for case detection in 1.2 million babies. J Pediatr. 1995;127(6):965–70. doi: 10.1016/s0022-3476(95)70040-4. [DOI] [PubMed] [Google Scholar]
- 2.Hammond KB, Abman SH, Sokol RJ, Accurso FJ. Efficacy of statewide neonatal screening for cystic fibrosis by assay of trypsinogen concentrations. N Engl J Med. 1991;325(11):769–74. doi: 10.1056/NEJM199109123251104. [DOI] [PubMed] [Google Scholar]
- 3.Comeau AM, Accurso FJ, White TB, Campbell PW, Hoffman G, Parad RB, et al. Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation workshop report. Pediatrics. 2007;119(2):e495–518. doi: 10.1542/peds.2006-1993. [DOI] [PubMed] [Google Scholar]
- 4.Neonatal screening for cystic fibrosis: position paper. Pediatrics. 1983;72(5):741–5. [PubMed] [Google Scholar]
- 5.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 6.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balfour-Lynn IM. Newborn screening for cystic fibrosis: evidence for benefit. Arch Dis Child. 2008;93(1):7–10. doi: 10.1136/adc.2007.115832. [DOI] [PubMed] [Google Scholar]
- 8.Southern KW, Mérelle MM, Dankert-Roelse JE, Nagelkerke AD. Newborn screening for cystic fibrosis. Cochrane Database Syst Rev. 2009;(1):CD001402. doi: 10.1002/14651858.CD001402.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilfond BS, Fost N. The cystic fibrosis gene: medical and social implications for heterozygote detection. JAMA. 1990;263(10):2777–83. [PubMed] [Google Scholar]
- 10.Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A, et al. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics. 2007;119(1):19–28. doi: 10.1542/peds.2006-1498. [DOI] [PubMed] [Google Scholar]
- 11.Farrell PM. The meaning of “early” diagnosis in a new era of cystic fibrosis care. Pediatrics. 2007;119(1):156–7. doi: 10.1542/peds.2006-3074. [DOI] [PubMed] [Google Scholar]
- 12.Marcus M, Sondel S, Farrell P, Laxova A, Carey P, Langhough R, et al. Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. Am J Clin Nutr. 1991;54(3):578–85. doi: 10.1093/ajcn/54.3.578. [DOI] [PubMed] [Google Scholar]
- 13.Farrell P, Kosorok M, Rock M, Laxova A, Zeng L, Lai H, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics. 2001;107(1):1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Koscik R, Farrell P, Kosorok M, Zaremba K, Laxova A, Lai H, et al. Cognitive function of children with cystic fibrosis: deleterious effect of early malnutrition. Pediatrics. 2004;113(6):1549–58. doi: 10.1542/peds.113.6.1549. [DOI] [PubMed] [Google Scholar]
- 15.Munck A, Dhondt JL, Sahler C, Roussey M. Implementation of the French nationwide cystic fibrosis newborn screening program. J Pediatr. 2008;153(2):228–33. 33.e1. doi: 10.1016/j.jpeds.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Rock MJ, Mischler EH, Farrell PM, Wei LJ, Bruns WT, Hassemer DJ, et al. Newborn screening for cystic fibrosis is complicated by age-related decline in immunoreactive trypsinogen levels. Pediatrics. 1990;85(6):1001–7. [PubMed] [Google Scholar]
- 17.Rock MJ, Hoffman G, Laessig RH, Kopish GJ, Litsheim TJ, Farrell PM. Newborn screening for cystic fibrosis in Wisconsin: nine-year experience with routine trypsinogen/DNA testing. J Pediatr. 2005;147(3 Suppl):S73–7. doi: 10.1016/j.jpeds.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Sanders D, Lai H, Rock M, Farrell P. Infants with CF diagnosed in IRT/DNA states are treated earlier than those diagnosed in IRT/IRT states. Pediatric Pulmonology. 2010;45(S33):A389. [Google Scholar]
- 19.Dasenbrook E, Merlo C, Diener-West M, Lechtzin N, Boyle M. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(8):814–21. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 20.Lai H, Kosorok M, Sondel S, Chen S, FitzSimmons S, Green C, et al. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: evaluation of various criteria used to identify malnutrition. J Pediatr. 1998;132(3 Pt 1):478–85. doi: 10.1016/s0022-3476(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 21.Goss CH, Mayer-Hamblett N, Aitken ML, Rubenfeld GD, Ramsey BW. Association between Stenotrophomonas maltophilia and lung function in cystic fibrosis. Thorax. 2004;59(11):955–9. doi: 10.1136/thx.2003.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6(1):57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Kloosterboer M, Hoffman G, Rock M, Gershan W, Laxova A, Li Z, et al. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics. 2009;123(2):e338–46. doi: 10.1542/peds.2008-1681. [DOI] [PubMed] [Google Scholar]
- 24.Castellani C, Southern K, Brownlee K, Dankert Roelse J, Duff A, Farrell M, et al. European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros. 2009;8(3):153–73. doi: 10.1016/j.jcf.2009.01.004. [DOI] [PubMed] [Google Scholar]