Abstract
Objectives
To evaluate the relationships between body composition and physical frailty in community-dwelling HIV-infected older adults (HOA).
Design
Cross-sectional.
Setting
Academic hospital-based infectious disease clinic in Rochester NY
Participants
Community-dwelling HIV-infected adults >50 years of age.
Measurements
Subjective and objective measures of functional status were evaluated by using the Physical Performance Test (PPT), graded treadmill test, knee strength, gait speed, balance and Functional Status Questionnaires (FSQ). Body composition was evaluated by using Dual Energy X-ray Absorptiometry (DXA) and Magnetic Resonance Imaging (MRI).
Results
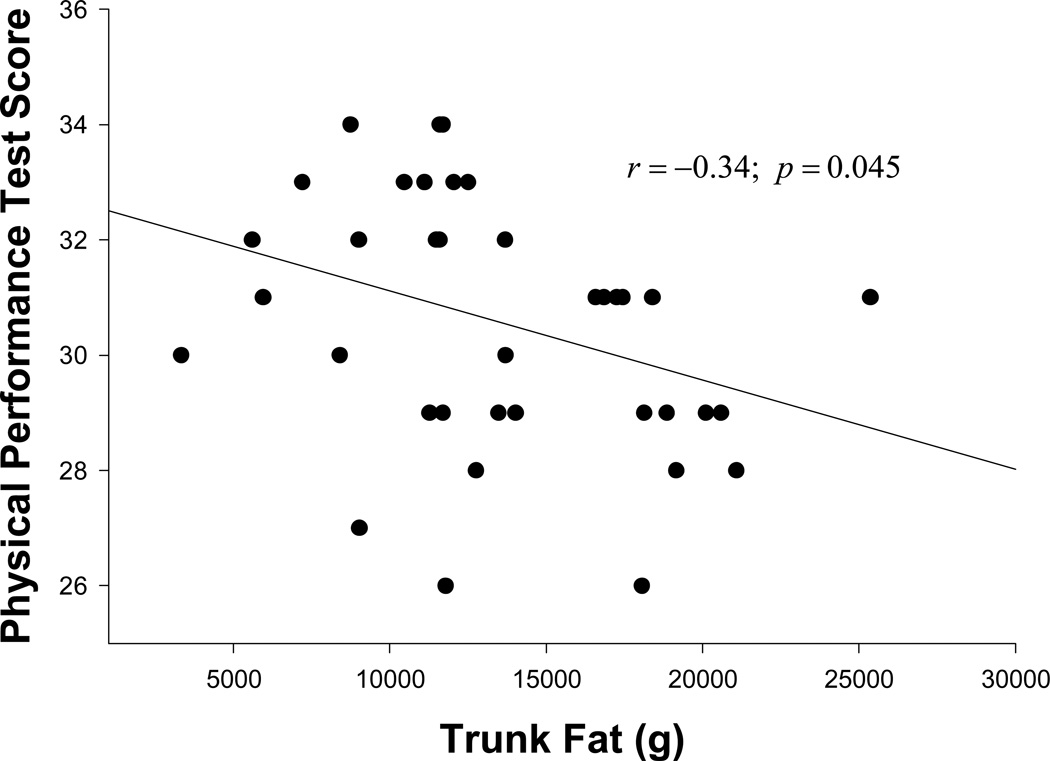
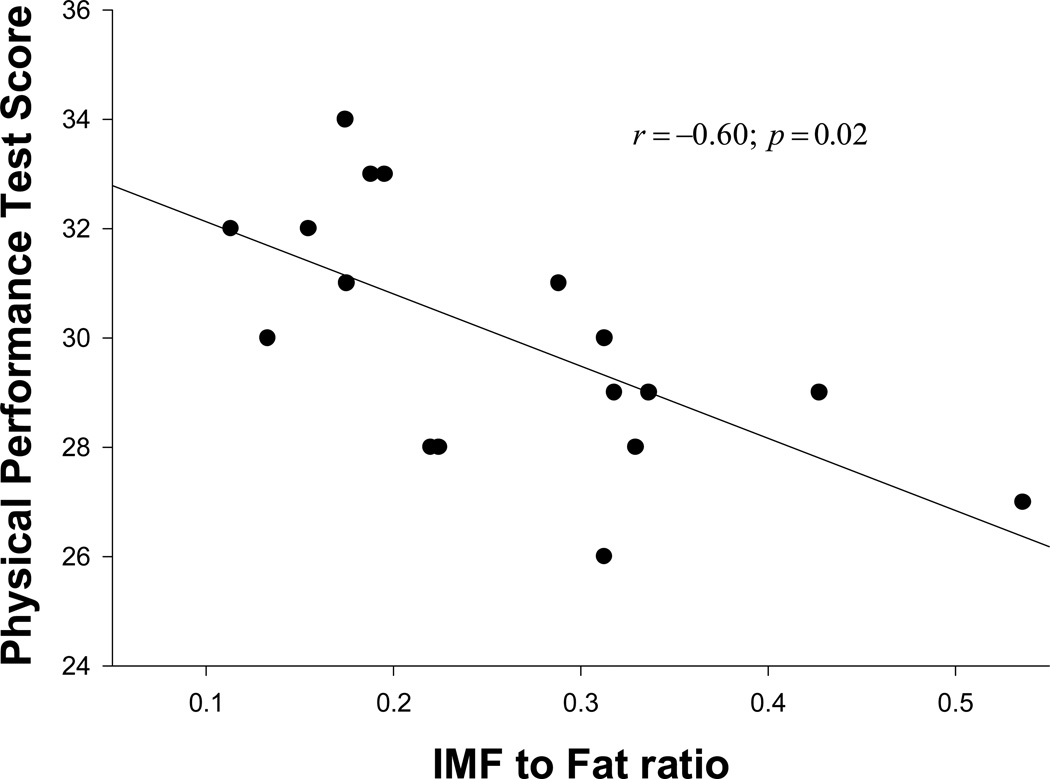
We studied 40 HOA on antiretroviral therapy (with mean: age 58 years, BMI 29, CD4 569 cells/ml, duration since HIV diagnosis 17 years; 28% female and 57% Caucasian) who were able to ambulate without assistive devices. Sixty percent (25/40) of the subjects met our standard criteria for physical frailty. Both frail (FR) and non-frail (NF) subjects were comparable in age, gender, CD4 count and viral load. Compared to NF HOA, FR HOA showed impairments in PPT, peak aerobic power (VO2peak), FSQ, walking speed, balance and muscle quality. Importantly, FR HOA had greater body mass index (BMI), fat mass and truncal fat with lipodystrophy. Moreover, PPT score was inversely related to both trunk fat (r=−0.34; p=0.045) and intermuscular fat (IMF) to total fat ratio (r=−60; p=0.02) after adjusting for covariates.
Conclusion
HOA represent an emerging cohort of older adults who frequently experience frailty at a much younger age compared to the general older population. Central obesity and fat redistribution are important predictors of frailty among community-dwelling HOA. These findings suggest that physical frailty in HOA may be amenable to lifestyle interventions, especially exercise and diet therapy.
Keywords: HIV, older adults, frailty, function, obesity, lipodystrophy
INTRODUCTION
There has been a significant increase in the number of older adults with HIV infection in the last 20 years, largely due to the success of antiretroviral therapy (ART)1;2. The CDC defines older adults with HIV infection at the relatively young age of 50 years. In 2006, over 25% of all adults with HIV infection in the US were age 50 and older, and this percentage is projected to increase to more than 50% by year 20153. That said, the clinical manifestations and physiology of this cohort will become increasingly important to geriatricians.
Frailty is defined as a syndrome of decreased physiological reserve which increases vulnerability to negative outcomes such as loss of independence, increased nursing home admissions, and increased morbidity and mortality4;5. Clinically, frailty states are characterized by low endurance, decreased strength, impaired balance and low physical activity. Given the demographic shift and the rapid increase in the aging population, frailty has been recognized as a major challenge facing the world today. Recent studies suggest HIV-infected individuals have reduced physical function and are at increased risk of frailty6;7. In fact, some HIV-infected patients manifest frailty characteristics at a much younger age compared to HIV-uninfected patients with frailty7, thereby suggesting that the HIV-infected population may experience a form of accelerated aging8;9. Hence, the growing number of HOA is an important public health concern with significant implications with respect to functional status and quality of life (QOL).
Little is known with respect to body composition changes which may contribute to physical frailty in HOA. This knowledge is important because it could lead to new insight on prevention and the treatment of frailty and extension of QOL in HOA. While HOA experience normal physiologic age-associated changes such as loss in skeletal muscle mass with gains in body fat10, their changes may be compounded by decades of HIV infection and long-term ART toxicity8.
Therefore, the aim of this study was to investigate the interrelationship of body composition and physical frailty in community-dwelling HOA on ART.
METHODS
Subjects
HIV-infected subjects were recruited from our urban hospital-based infectious disease clinic, which provides services to over 1,000 HIV-infected patients. Forty subjects were recruited. Eligibility criteria included age >50 years, stable ART regimen for 3 months prior to enrollment, able to ambulate without assistive devices, and free of any AIDS defining illness (ADI) for 6 months prior to enrollment. Subjects with severe cardiopulmonary illness, severe anemia, significant orthopedic or neuromuscular impairments, renal failure, cirrhosis, significant cognitive or sensory impairments, untreated depression, unstable manic or psychotic disorder, and active malignancy were excluded. The following subjects were initially considered, but later excluded: 1 subject was suspected of active substance abuse, 1 subject was incarcerated, 1 subject was likely non-compliant with ART, 1 subject had undiagnosed psychiatric disorder, and 3 subjects expressed no interest in the study as they had time constraints. All subjects received a baseline medical evaluation and EKG. Medical history of subjects was obtained primarily from self-completed questionnaires regarding the subjects past medical history and secondarily from electronic medical records. Subjects who did not have CD4 cell count and HIV viral load documented 3 months prior to enrollment provided blood samples as part of the research protocol. Informed consent was obtained from all subjects prior to enrollment and the study was approved by the Research Subjects Review Board and Clinical Research Center Scientific Advisory Committee of the University of Rochester Medical Center.
Study Measurements
Assessment of Body Composition. Fat mass (FM) and lean mass (LM) were measured using Dual Energy X-ray Absorptiometry, Hologic Discovery (DXA). Appendicular fat was calculated as the total fat in the extremities. In the upper extremities, the appendicular fat included subcutaneous adipose tissue from the shoulder to the wrist. In the lower extremities, appendicular fat included subcutaneous adipose tissue from the hip to the ankle. Trunk fat was the amount of fat measured by the DXA from below the neck to the pelvis. The bone mineral-free portion of the appendicular lean mass represents skeletal muscle in the extremities11. Magnetic Resonance Imaging (MRI) was used to quantify thigh intermuscular fat (IMF) and total fat volumes on a subset of subjects (n=18). Eight transverse images, 10-mm thick, were acquired just superior to the patella with a 1.5-T superconducting MRI instrument and a T1-weighted pulse sequence. Analyze Direct software (version 9.0; Mayo Clinic, Rochester, MN) was used for the analysis 12. The MRI data from one subject was excluded because of incorrect field of view.
Assessment of Frailty. Measures that have established validity for disability and mortality in the older population were used to assess physical frailty in this study. Accordingly, and as in our previous studies12–15, subjects were considered to be mild to moderately frail if they met at least two of the following operational criteria: a PPT score of 18–32, VO2peak of 11–18 mL/kg per minute, and report of difficulty or need of assistance with two or more instrumental activities of daily living (ADL) or one basic ADL.
Assessment of Physical Function. The PPT is a global measure of physical function that evaluates the ability to perform usual daily activities 12;16–18. The PPT includes seven standardized tasks (50-foot walk, putting on and removing a coat, picking up a penny, standing up from a chair, lifting a book, climbing one flight of stairs, and progressive Romberg test) and two additional tasks (climbing four flights of stairs and performing a 360° turn). The score for each item ranges between 0 and 4; a perfect score is 3612;16–18. VO2peak was assessed during a graded treadmill walking test as described12. During a 5-minute warm-up at 0% grade, the speed was adjusted to identify the fastest comfortable walking speed. Speed was held constant and treadmill incline was increased by 3% every 2 minutes. Participants were allowed to lightly hold on to a handrail to maintain their balance during the test. Blood pressure was measured by auscultation every 2 minutes. Cardiorespiratory data were collected at 30-second intervals by using a computerized system. The test was terminated when the participant becomes too fatigued to continue12. Information regarding the ability to perform ADLs was collected using the FSQ with a score range of 0–3612. Assessment of specific physical functions (strength, balance and gait) was performed. Knee flexor strength was determined using the Biodex isokinetic dynamometer while subjects were seated with their back supported and hips placed at 120° of flexion. Test was performed at an angular velocity of 60°/s11. Static balance was assessed using the single-limb leg stance time whereby subjects were asked to stand on each leg individually (eyes open) for up to 30s12. Dynamic balance was assessed as the time needed to complete an obstacle course whereby subjects from a standard 18-in-high chair, walk forward ~6 ft, step over a 2 × 2-in obstacle, walk forward another ~6 ft, ascend a 6-in-high curb, turn around, step down off the curb, and return to the chair as quickly and safely as possible12. Fast gait speed was measured as the time needed to walk 50 ft12. Muscle quality was defined by the relationship between muscle strength and muscle mass (force per cross-sectional area of muscle). Muscle quality was assessed by calculating the ratio of isokinetic torque at the knee (in Newton-meter) to appendicular LM (in kilograms), determined by DXA11. All frailty and functional measures were performed by a single experienced physical therapist.
Statistical Analysis
Descriptive data are presented as means and standard deviations (SD). Comparison between categorical groups was performed using Chi-square tests. Student’s t-test was used for normally distributed, continuous variables and Mann-Whitney U test for non-normally distributed, continuous variables. Correlations of PPT with IMF to fat ratio and trunk fat were analyzed using partial correlation after adjusting for age, and duration of HIV. All analyses were done by using SPSS for Windows software version 17.0 (SPSS Inc, Chicago, Ill). Statistical significance was set at P≤0.05.
RESULTS
We studied 40 HOA (mean±SD: age 58±5 years, body mass index (BMI) 29±5, CD4 569±254 cells/ml, duration since HIV diagnosis 17±5 years; 28% female and 57% Caucasian). Clinical, physical function and body composition characteristics of the frail (FR) and non-frail (NF) groups are shown in Table 1. Of the HOA subjects, 25/40 (60%) met our standard criteria for physical frailty. We observed that 1) 24/25 frail subjects had PPT scores <32, 2) 18/25 frail subjects had VO2peak <18 ml/kg/min, and 3) 12/25 frail subjects had impairment in IADLs.
Table 1.
Clinical, Physical Function and Body Composition Characteristics of Subjects (mean ± SD)
| FRAIL (n=25) |
NON-FRAIL (n=15) |
p | |
|---|---|---|---|
| Clinical Characteristics | |||
| Age, y | 59 ± 7 | 57 ± 5 | 0.29 |
| Female, n% | 7 (28) | 4 (27) | 0.93 |
| CD4 count, cells/ml | 598 ± 254 | 552 ± 235 | 0.59 |
| Duration of HIV, y | 18 ± 5 | 15 ± 7 | 0.17 |
| Viral Load (<50), copies/ml, n% | 19 (76) | 11 (73) | 0.85 |
| Current PI Use, n% | 15 (60) | 8 (53) | 0.48 |
| Current N(t)RTI Use, n% | 24 (96) | 14 (93) | 0.71 |
| Stavudine Exposure, n% | 16 (64) | 7 (47) | 0.50 |
| Hypertension, n% | 19 (76) | 8 (53) | 0.11 |
| Diabetes, n% | 9 (36) | 2 (13) | 0.13 |
| Hyperlipidemia, n% | 13 (52) | 6 (40) | 0.15 |
| Physical Function | |||
| Physical Performance Test | 29 ± 2 | 33 ± 1 | 0.0001 |
| Peak aerobic power, ml/kg/min | 18 ± 6 | 23 ± 5 | 0.01 |
| Functional Status Questionnaire | 29 ± 4 | 32 ± 3 | 0.04 |
| Obstacle course, sec | 10 ± 1 | 8 ± 1 | 0.0001 |
| One leg limb stand, sec | 16 ± 12 | 31 ± 15 | 0.001 |
| Gait speed, m/min | 89 ± 10 | 102 ± 14 | 0.001 |
| Knee strength, Nm | 64 ± 18 | 70 ± 22 | 0.35 |
| Muscle quality, Nm/kg | 7.2 ± 1 | 8.2 ± 1 | 0.02 |
| Body Composition | |||
| Body mass index, kg/m2 | 31 ± 8 | 26 ± 3 | 0.04 |
| Waist circumference, in | 42 ± 6 | 37 ± 5 | 0.02 |
| Fat mass, kg | 26 ± 9 | 20 ± 5 | 0.04 |
| Lean mass, kg | 55 ± 8 | 50 ± 16 | 0.19 |
| Lean, % | 67 ± 8 | 66 ± 18 | 0.79 |
| Trunk fat, kg | 15 ± 5 | 10 ± 3 | 0.004 |
| Appendicular/Trunk fat ratio | 0.61 ± 0.2 | 0.73 ± 0.2 | 0.09 |
| Trunk/Total fat ratio | 0.60 ± 0.1 | 0.55 ± 0.1 | 0.06 |
| Appendicular/Total fat ratio | 0.35 ± 0.1 | 0.39 ± 0.1 | 0.14 |
PI, Protease Inhibitors; N(t)RTI, Nucleoside/Nucleotide Reverse Transcriptase Inhibitors.
There were no statistically significant differences among the FR and NF regarding age, gender, CD4 cell count, viral load and stavudine exposure. There was a trend of higher prevalence of the metabolic diseases (hypertension, hyperlipidemia and diabetes) in the FR group compared to the NF group. Compared with the NF group, the FR group had significantly lower scores in PPT (33 vs. 29), VO2peak (23 ml/kg/min vs.18 ml/kg/min), and FSQ (32 vs. 29). Specific tests of physical function (static and dynamic balance, walking speed, muscle quality) were significantly lower in the FR group compared to the NF group. More importantly, compared to the NF group, the FR group had significantly higher BMI (26 vs. 31), waist circumference (37 in vs. 42 in), FM (20 kg vs. 26 kg) and trunk fat (10 kg vs. 15 kg). Although the FR group trended to have a larger absolute amount of LM, LM relative to body weight (i.e., percent LM) was similar in both the groups. The FR group trended to exhibit severe lipodystrophy characteristics as measured by appendicular to trunk fat, trunk to total fat and appendicular to total fat ratios as compared to the NF group. Even after controlling for age, CD4 count and duration of HIV, both trunk fat (r= −0.34; p=0.045) (Figure 1) and IMF to total fat ratio (r=−60; p=0.02) (Figure 2) remained significantly inversely correlated with PPT score.
Figure 1.
Relationship between Physical Performance Test Score and Trunk Fat.
Figure 2.
Relationship between Physical Performance Test Score and Intermuscular Fat to Total Fat ratio.
DISCUSSION
The results of this study suggest that community-dwelling HOA often experience frailty at a much younger age compared to the general older population. Central obesity and fat redistribution are important predictors of frailty among HOA. This is the first study to demonstrate that elevated fat mass, particularly truncal fat, and higher BMI is associated with frailty in the HOA population. Previous studies have shown this relationship in the general older population11;19. Our study is interesting because frailty in HIV-infected patients is usually conceptualized as being a wasting disorder. Previously, frailty in HIV-infected individuals was commonly observed in the setting of wasting and immunocompromised states with lower CD4 count20;21. In contrast, our community-dwelling frail subjects were mostly obese and had immune restoration as indicated by higher CD4 count and suppressed viral load.
With the growing prevalence of obesity, the most common phenotype of frailty in the years to come has been described as the obese, disabled older adult12. Notably, as HIV disease has become a manageable chronic illness, it has been progressively accompanied with an increased prevalence of overweight and obesity more than it is accompanied with wasting22. Additionally, the long-term treatment with ART contributes to the development of lipodystrophy23, which is characterized by fat redistribution with relative increase in abdominal fat and frequently accompanied with metabolic dysfunction such as insulin resistance24. Indeed, HIV-infected individuals have significantly greater amounts of abdominal fat compared to age and BMI matched HIV-uninfected individuals25. Moreover, excess lipid accumulation in muscle and liver also occurs in HIV-infected individuals compared to HIV-uninfected individuals26 – and is compounded by aging27. Therefore, it is plausible that the ART-related deleterious body composition changes that HOA experience have significant functional consequences. However, in this pilot study we did not observe an association between stavudine exposure and frailty, despite stavudine having the greatest effect on mitochondrial toxicity and fat redistribution24. We suspect that it is the cumulative toxicity of antiretroviral agents rather than any one agent alone which explains the relationship between body composition and frailty in this population. Additionally, changes in body composition, particularly the gains in abdominal fat, have cardiovascular disease (CVD) consequences24. Both clinical and subclinical manifestations of CVD have been found to be independently related to frailty 28.
To our knowledge, this is the first study to investigate the relationship between body composition and physical frailty in HOA on ART. The IMF and trunk fat measure we used are surrogate markers of intramyocellular fat and visceral fat respectively29. These markers were inversely related to global physical performance. Our data suggest that excessive visceral fat hypertrophy and lipolysis contribute to fat infiltration in the muscle, and consequently lead to poor muscle quality and a decline in physical function among HOA. We suspect that the relationship between fat redistribution and physical function is a unique finding in the HOA population because to our best knowledge this has not been reported. We speculate that the relationship between fat redistribution and physical function is mediated by adipokines because ART toxicity alters the hemostatic regulation of adipokines30. Future studies should investigate the pathophysiologic mechanism of HIV-related frailty.
In this study, the PPT was the major determinant of frailty, thereby suggesting that compromised dynamic muscle performance is the most important contributor to functional limitations in HOA. Further, the 60% frailty rate that we observed in our study is higher compared to the near-15% showed in another study which used a different criteria to assess frailty.7 Indeed, the prevalence of frailty can vary using different frailty criteria. The higher frailty rate seen in the present study can be explained not only by the different frailty criteria used to assess frailty, but also by the use of a sample of convenience. Additionally, more functional seniors may have been unable to participate in the present study due to being employed, or substance abuse issues and mental health concerns. It is important to note that the predictive value of defining frailty using the various frailty criteria have not been validated in this younger population with specific comorbidities such as HIV. Therefore, the frailty criteria used in this study and previous studies may not predict morbidity or mortality in HOA, particularly when controlling for other comorbidities. Future research is needed to validate frailty measures in the HIV population.
The conclusions of our study were limited due to small sample size. It should be noted, however, that we have characterized our subjects comprehensively in regards to their physical function and body composition. Although our study lacked HIV-uninfected controls, we observed that these subjects have significant functional impairments that are comparable in a physiologic sense to that which is sometimes seen in HIV-uninfected subjects 15–20 years older. We realize that the cross-sectional design can only identify associations. Nonetheless, our findings offer insight regarding the functional implications related to the altered body composition in HOA. These findings are particularly important because aging with HIV is an emerging issue in the developed world. HOA represents a new subset of the older population who are at high risk of frailty-related negative consequences. These negative consequences not only increase the burden on clinical care by geriatricians and other health professionals, but also create an additional demand for access to what is an already overburdened health care delivery service. In light of the rise of aging and obesity trends within the HIV-infected population, the benefits and feasibility of interventions such as exercise and diet therapy to ameliorate frailty should be a focus of future clinical research.
ACKNOWLEDGMENTS
We thank the participants; and Dr. Stephen Dewhurst for his encouragement and support of this work.
Funding source. NIH grants P30 AI78498, AG020493 and John A Harford Foundation Center for Excellence in Geriatric Medicine and Training.
Sponsor's Role: None.
Footnotes
Conflict of Interest:
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Study concept and design: AEL, WJH, KS; Acquisition of participants and data: LM, TNH, JFP. Analysis and interpretation of data: KS, TNH Preparation of manuscript. KS, AEL, WJH.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mack KA, Ory MG. AIDS and older Americans at the end of the Twentieth Century. J Acquir Immune Defic Syndr. 2003;(33 Suppl 2):S68–S75. doi: 10.1097/00126334-200306012-00003. [DOI] [PubMed] [Google Scholar]
- 3.Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention. [16:20, Table 10]. 2009; 2005. Centers for Disease Control and Prevention: HIV/AIDS Surveillance Report 2004. Ref Type: Generic. [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N, Mandel R, Fain MJ. Frailty: An emerging geriatric syndrome. Am J Med. 2007;120:748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Oursler KK, Sorkin JD, Smith BA, et al. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22:1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 7.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 8.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: Workshop on HIV infection and aging: What is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc. 2009;57:2129–2138. doi: 10.1111/j.1532-5415.2009.02494.x. [DOI] [PubMed] [Google Scholar]
- 10.Beaufrere B, Morio B. Fat and protein redistribution with aging: Metabolic considerations. Eur J Clin Nutr. 2000;(54 Suppl 3):S48–S53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- 11.Villareal DT, Banks M, Siener C, et al. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 12.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villareal DT, Smith GI, Sinacore DR, et al. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 2011;19:312–318. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villareal DT, Shah K, Banks MR, et al. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: A one-year randomized controlled trial 2. J Clin Endocrinol Metab. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 17.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol. 1992;47:M106–M110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- 18.Brown M, Sinacore DR, Binder EF, et al. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 19.Zamboni M, Turcato E, Santana H, et al. The relationship between body composition and physical performance in older women. J Am Geriatr Soc. 1999;47:1403–1408. doi: 10.1111/j.1532-5415.1999.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 20.Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50:299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009;18:1965–1974. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calmy A, Hirschel B, Cooper DA, et al. A new era of antiretroviral drug toxicity. Antivir Ther. 2009;14:165–179. doi: 10.1177/135965350901400203. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro G. Visceral fat as target of highly active antiretroviral therapy-associated metabolic syndrome. Curr Pharm Des. 2007;13:2208–2213. doi: 10.2174/138161207781039661. [DOI] [PubMed] [Google Scholar]
- 25.Kosmiski L, Kuritzkes D, Hamilton J, et al. Fat distribution is altered in HIV-infected men without clinical evidence of the HIV lipodystrophy syndrome. HIV Med. 2003;4:235–240. doi: 10.1046/j.1468-1293.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 26.Torriani M, Thomas BJ, Barlow RB, et al. Increased intramyocellular lipid accumulation in HIV-infected women with fat redistribution. J Appl Physiol. 2006;100:609–614. doi: 10.1152/japplphysiol.00797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 29.Snijder MB, Visser M, Dekker JM, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: A comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord. 2002;26:984–993. doi: 10.1038/sj.ijo.0801968. [DOI] [PubMed] [Google Scholar]
- 30.Hammond E, Nolan D. Adipose tissue inflammation and altered adipokine and cytokine production in antiretroviral therapy-associated lipodystrophy. Curr Opin HIV AIDS. 2007;2:274–281. doi: 10.1097/COH.0b013e3281c10df7. [DOI] [PubMed] [Google Scholar]