Abstract
Background
The effects of gastric bypass surgery on the secretion of the anorexogenic gut-derived hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), independent of caloric restriction and due to different dietary macronutrients, is not well-characterized. This study examines the effects of a mixed-nutrient or high-fat liquid meal on the postprandial stimulation of GLP-1 and PYY following gastric bypass or equivalent hypocaloric diet.
Methods
Total PYY and active GLP-1 were measured fasting and at multiple points after standardized mixed-nutrient and high-fat liquid meals in two matched groups of obese subjects. The meal stimulation tests were performed before and 14.6±3.3 days after gastric bypass (GBP, n=10) and before and after a 7-day hypocaloric liquid diet matching the post-GBP diet (Control, n=10).
Results
Mixed-nutrient and high-fat postprandial GLP-1 levels increased following GBP (mixed-nutrient peak: 85.0±28.6 pg/ml to 323±51 pg/ml, p<0.01; high-fat peak: 81.8±9.6 pg/ml to 278±49 pg/ml, p<0.01), but not after diet (mixed-nutrient peak: 104.4±9.4 pg/ml to 114.9±15.8 pg/ml, p=NS; high-fat peak: 118.1±16.4 pg/ml to 104.4±10.8 pg/ml, p=NS). The postprandial PYY response also increased after GBP but not diet, though the increase in peak PYY did not reach statistical significance (GBP mixed-nutrient peak: 134.8±26.0 pg/ml to 220.7±52.9 pg/ml, p=0.09; GBP high-fat peak: 142.1±34.6 pg/ml to 197.9±12.7 pg/ml, p=0.07; diet mixed-nutrient peak: 99.8±8.0 pg/ml to 101.1±13.3 pg/ml, p=NS; diet high-fat peak: 105.0±8.8 pg/ml to 103.1±11.8 pg/ml, p=NS). The postprandial GLP-1 response was not affected by the macronutrient content of the meal. However, following GBP the mixed-nutrient PYY AUC0–120 was significantly greater than the high-fat PYY AUC0–120 (22081±5662 pg/ml•min versus 18711±1811 pg/ml•min, p=0.04).
Conclusions
Following GBP there is an increase in the postprandial stimulation of PYY and GLP-1 that is independent from caloric restriction. The phenomenon of ‘bariatric surgery-induced anorexia’ may be linked to the increased levels after GBP.
Keywords: Bariatric surgery, Gastric bypass, Peptide YY, Glucagon-like peptide-1, Meal stimulation
INTRODUCTION
Obesity currently affects roughly one third of Americans, and the prevalence of obesity is increasing at an alarming rate[1]. While many people are able to achieve initial weight loss through diet and/or exercise, few are able to sustain such weight loss over time. As such, bariatric surgery is now regarded as the only means to sustained weight loss for individuals with class II and III obesity[2].
The Roux en-Y gastric bypass, in which a small gastric pouch is created and roughly 100–150 cm of the small intestine is bypassed, is the most popular weight-loss surgery currently performed in the United States. This procedure is thought to produce weight loss through both food restriction, as a result of the small gastric pouch, and malabsorption, as a result of intestinal bypass. However, it has repeatedly been noted that prior to any significant weight loss, many patients experience complete resolution or improvement of many comorbidities, including diabetes, hypertension, and hyperlipidemia[3], suggesting that gastric bypass surgery is more than just a weight-loss surgery, but is actually a metabolic surgery[4]. As such, over the past few years there has been a surge of research investigating the mechanism of weight-loss and alterations in metabolism that result from bariatric surgery. One such metabolic factor that is thought to contribute to gastric bypass-induced weight loss is appetite regulation and satiety.
The mechanism of appetite regulation and energy expenditure involves a series of complex interactions between nutrients, gut-derived hormones, and the brain[5]. Two of the most well-studied gut-derived peptides are glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Both GLP-1 and PYY are secreted from L cells in the distal small bowel in response to nutrient intake[6,7]. They are thought to decrease appetite through central mechanisms, via involvement in hypothalamic appetite regulation, as well as through peripheral mechanisms, functioning as mediators of the ileal break and delaying the transport of food through the gastrointestinal tract[5,6,7]. Multiple studies have shown that obese individuals have decreased basal and postprandial PYY levels[8,9] as well as a decreased postprandial GLP-1 response[10,11,12] as compared to lean individuals. These diminished gut-hormone levels may result in decreased feelings of satiety and contribute to the development of obesity. Indeed, the exogenous administration of PYY[8,9,13] has been shown to increase satiety and decrease food intake, an effect which is additive with concomitant GLP-1 infusion[13].
Gastric bypass surgery results in augmented postprandial GLP-1 and PYY levels [12,14,15,16,17,18]. It has been proposed that due to bypass of a significant portion of the small bowel and increased delivery of unabsorbed nutrients to the GLP-1 and PYY-producing cells of the distal small bowel, gastric bypass surgery allows for an amplified postprandial gut-hormone response and increased satiety [18], thereby contributing to the dramatic and sustained weight-loss that results from gastric bypass surgery. However, most studies investigating GLP-1 and PYY levels following bypass surgery are conducted after individuals have lost a significant amount of weight[12,14,15,16]. It is therefore possible that the observed changes in gut-hormone levels are due to actual changes in weight and adiposity, rather than a result of the altered gastrointestinal anatomy and the surgery itself. Furthermore, it is possible that caloric restriction and alterations in daily diet, as is necessary following gastric bypass, may influence the hormonal response to a test meal.
Our aim was to determine the direct effects of gastric bypass surgery on the secretion of the anorexogenic gut-derived hormones GLP-1 and PYY. In this study we measured hunger perceptions and basal and postprandial GLP-1 and PYY levels in obese individuals prior to surgery and two weeks following gastric bypass surgery, before substantial weight loss. To control for changes in gut-hormone release that may be due caloric restriction, obese control patients consumed a similar calorie-restricted liquid diet as post-gastric bypass patients for one week prior to the test meal. In addition, as studies indicate that macronutrient composition can affect gut-hormone secretion[19,20,21,22,23,24], to further characterize the effect of different macronutrients on postprandial GLP-1 and PYY levels, all study participants completed two meal-stimulation tests, one with a mixed-nutrient content, and one of equal calories but much higher fat content.
METHODS
Subjects
A total of 20 morbidly obese patients with non-insulin-dependent type 2 diabetes participated in this prospective case-control study: 10 patients underwent gastric bypass surgery and 10 patients were age and BMI matched controls. Patients who underwent gastric bypass surgery were recruited from the Duke Metabolic and Weight Loss Surgery Center after they were approved for surgery. Matched controls were recruited by advertisement. All patients were between the ages of 18 and 65 and met NIH criteria for weight-loss surgery (BMI over 40 or a BMI over 35 with significant co-morbidities). Exclusion criteria included previous esophageal, gastric, pancreatic, small bowel, or large bowel operations, hemoglobin A1c over 10.0%, use of insulin, dipeptidyl peptidase-IV inhibitors, or GLP-1 analogues, tobacco use, known alcohol or substance abuse within six months of enrollment, inability to provide informed consent, or exclusion based on the Duke Metabolic and Weight Loss Surgery Program criteria for surgery (exclusion based on psychology evaluation or deemed medically unfit for surgery). The study was approved by the Duke University Institutional Review Board and all participants provided written, informed consent to participate.
Study Protocol
Subjects completed a mixed-nutrient stimulation study at baseline and then 2–3 weeks after GBP (surgery group) or after a 7-day low-calorie liquid diet (diet group). For each study visit, following a 12-hour overnight fast, patients presented to the study center where their height and weight were measured. Subjects were asked to drink an 8-ounce standardized liquid mixed-nutrient meal of Ensure High Protein with protein powder supplement (262 kcal, 20g protein 32%, 31g carbohydrates 47%, 6g fat 21%) or an equal calorie and volume high-fat meal supplement (262 kcal, 14.4 g protein 22%, 23.2 g carbohydrates 33%, 13.1g fat 45%). The liquid supplement was provided to the subjects at 5-minute intervals over a total of 20 minutes to accommodate for the reduced gastric capacity of subjects after GBP. Blood samples were collected from a forearm intravenous catheter immediately prior to drinking the liquid meal, and every 30 minutes thereafter for 2 hours.
Roux-en-Y Gastric Bypass Protocol
A Roux-en-y gastric bypass was performed using a laparoscopic approach via 6 ports. Briefly, a linear stapler was used to create a 30-ml gastric pouch. An ante-colic, retro-gastric Roux-en-y gastrojejunostomy, 100-centimeter long Roux limb, and 30-centimeter biliopancreatic limb were created. There were no complications and all patients were discharged between post-operative days 1 and 3. Diabetic medications were adjusted on an individual basis upon hospital discharge by a member of the surgical team. Following surgery, subjects were advised to follow a standard post-gastric bypass liquid diet including a daily caloric intake of 800–900 kcal and 40–60 g of protein. The diet was monitored by fluid intake records in the 7 days prior to the post-operative meal stimulation tests.
Control Subject Calorie-Restricted Diet Protocol
Following the baseline meal-stimulation studies, control patients completed a 7-day low-calorie liquid diet, which they were provided, designed to match the post-gastric bypass diet. The diet consisted of four 8-ounce cans of vanilla Ensure High Protein per day, providing a total of 920 kcal/day. Subjects received individual instructions on how to adjust diabetic medications while on the diet. The diet was monitored by daily fluid intake records which were reviewed at the post-diet meal stimulation test.
Hunger and Appetite Assessment
Subjects completed a visual analogue scale (VAS) assessment of hunger at the time of the baseline, 30-minute, 60-minute, and 120-minute blood sample collections. The 100-mm VAS was scored by measuring the distance from the left end of the scale to the mark placed by the subject. A larger value indicates greater sensations of hunger. This VAS has been validated in a previous study[25].
Sample Collection and Hormone Analysis
Blood samples were collected in chilled EDTA tubes. Aprotinin (Sigma-Aldrich, St. Louis, MO) at a final concentration of 1µg/ml blood and dipeptidyl peptidase-IV inhibitor (Millipore, Billerica, MA) 10µl/ml blood were added to the sample, which was then centrifuged at 4°C. The plasma was collected and stored at −80°C until analysis. Total plasma PYY levels and active GLP-1 were measured with a multiplex immunoassay (Millipore Luminex® xMAP®, St. Charles, MO). This assay uses fluorescently-labeled microsphere beads to determine the levels of both the 1–36 and 3–36 forms of PYY and active form of GLP-1 (7–37 and 7–36-amide). The lower limit of detection for the PYY assay is 15 pg/ml and for active GLP-1 is 12 pg/ml.
Statistical Analysis
Data are presented as mean ± SD or mean ± SEM. Outcome variables were plasma total PYY, active GLP-1, and hunger ratings as measured with a VAS. The changes in the outcome variables during the meal stimulation tests were assessed by peak levels (GLP-1 and PYY) and total area under the curve (AUC0–120) calculated using the trapezoidal method. Repeated measures ANOVA with post-hoc Dunnett’s test were used to detect hormonal changes over time during the meal-stimulation tests and compare data within groups relative to baseline. Paired t tests were used to compare data before and after gastric bypass or diet within each group. Unpaired t tests were used for comparisons between diet and gastric bypass groups. Pearson correlation coefficients were calculated to determine the relationship between hormone AUC0–120 and hunger AUC0–120. All tests were 2-tail. P values of less than 0.05 were considered to indicate statistical significance. Statistical analyses were performed with GraphPad Prism version 5.04 (GraphPad Software Inc., La Jolla, CA).
RESULTS
Subject Characteristics and Weight Loss
As seen in Table 1, the diet and gastric bypass cohorts each included 7 women and 3 men. The groups did not differ in subject age, pre-intervention weight, or pre-intervention BMI. While the change in BMI following either hypocaloric diet intervention or gastric bypass were not significantly different, the gastric bypass cohort did have a significantly greater percent weight loss and percent excess weight loss. The length of intervention was also significantly greater for the gastric bypass cohort.
Table 1.
Characteristics of diet and gastric bypass groups before and after intervention
| Diet | Gastric Bypass | P value | |
|---|---|---|---|
| n (females) | 10 (7) | 10(7) | |
| Intervention time (days) | 9.2±2.6 | 14.6±3.3 | <0.001 |
| Baseline | |||
| Age (yr) | 46.3±6.6 | 49.6±11.2 | 0.6 |
| Weight (kg) | 263.4±47.6 | 280.9±65.1 | 0.5 |
| BMI (kg/m2) | 44.0±8.9 | 45.6±7.6 | 0.7 |
| After intervention | |||
| Change BMI (kg/m2) | −2.4±2.7 | −3.0±1.4 | 0.6 |
| Weight loss (%) | 3.5±1.3 | 6.7±1.2 | <0.001 |
| EWL (%) | 7.4±3.2 | 14.2±2.0 | <0.001 |
Values are presented as mean ± SD. EWL, excess weight loss
Effect of Weight Loss Intervention on Hormonal Response to Test Meal
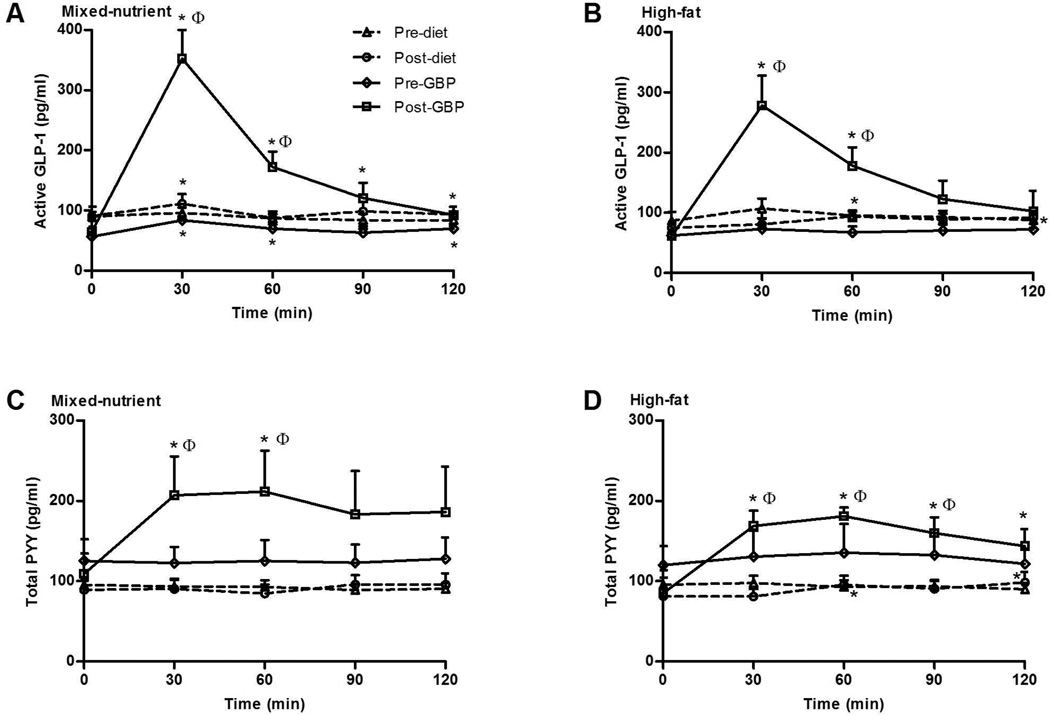
Gastric bypass, but not diet-intervention, resulted in an augmented postprandial GLP-1 response in the mixed-nutrient and high-fat meal stimulation studies (Figure 1). Following gastric bypass, peak GLP-1 levels and AUC0–120 were significantly greater than prior to surgery with both meal stimulation tests (Table 2). The post-bypass stimulated GLP-1 levels at 30 and 60 minutes were also significantly greater than the post-diet GLP-1 levels at these same time points (Figure 1). In contrast to surgical-intervention, diet-intervention alone had minimal effect on GLP-1 levels. Following diet there was a small, but significant, increase from fasting GLP-1 levels at 30 minutes with the mixed-nutrient meal and at 60 and 120 minutes with the high-fat meal (Figure 1). However, diet-intervention did not result in a change in peak GLP-1 levels or GLP-1 AUC0–120 (Table 2). Neither weight loss by diet nor gastric bypass affected fasting GLP-1 levels.
Figure 1.
Active GLP-1 (A and B) and total PYY (C and D) in response to a 262 kcal mixed-nutrient or high-fat liquid meal in morbidly obese subjects before and after GBP (n=10 mixed-nutrient, n=7 high-fat) and before and after diet-intervention (n=10 mixed-nutrient, n=9 high-fat). Data are expressed as mean ± SEM, *P < 0.05 for comparison relative to baseline. ΦP < 0.05 between groups after weight loss by GBP or diet.
Table 2.
Hormonal Response to Meal Stimulation
| Pre-diet | Post-diet | Pre-GBP | Post-GBP | P value | |
|---|---|---|---|---|---|
| Mixed-nutrient | |||||
| Fasting active GLP-1 (pg/ml) | 90.1 ± 7.6 | 90.4 ± 15.8 | 56.4 ± 7.4 | 64.7 ± 8.2 | 0.5240 |
| Peak active GLP-1 (pg/ml) | 104.4 ± 9.4 | 114.9 ± 15.8 | 85.5 ± 7.3 | 323.5 ± 51.4a | 0.0004 |
| AUC active GLP-1 (pg/ml•min) | 10616 ± 926 | 11658 ± 1663 | 8378 ± 751 | 20820 ± 3167a | 0.0018 |
| Fasting total PYY (pg/ml) | 95.2 ± 7.6 | 89.1 ± 11.4 | 125.0 ± 27.3 | 109.0 ± 25.3 | 0.5597 |
| Peak total PYY (pg/ml) | 99.8 ± 8.0 | 101.1 ± 13.3 | 134.8 ± 26.0 | 220.7 ± 52.9 | 0.0904 |
| AUC total PYY (pg/ml•min) | 11023 ± 912 | 10871 ± 1446 | 14901 ± 2855 | 22081 ± 5662 | 0.1460 |
| High-fat | |||||
| Fasting active GLP-1 (pg/ml) | 86.6 ± 15.0 | 74.7 ± 9.6 | 61.8 ± 7.5 | 61.1 ± 7.4 | 0.6070 |
| Peak active GLP-1 (pg/ml) | 118.1 ± 16.4 | 104.4 ± 10.8 | 81.8 9.6 | 278.2 ± 49.4a | 0.0005 |
| AUC active GLP-1 (pg/ml•min) | 11449 ± 1342 | 10379 ± 1078 | 8320 ± 1041 | 19815 ± 3550a | 0.0029 |
| Fasting total PYY (pg/ml) | 95.3 ± 9.2 | 81.0 ± 10.3 | 119.8 ± 23.6 | 85.0 ± 28.6a | 0.1933 |
| Peak total PYY (pg/ml) | 105.0 ± 8.8 | 103.1 ± 11.8 | 142.1 ± 34.6 | 197.9 ± 12.7 | 0.0274 |
| AUC total PYY (pg/ml•min) | 11285 ± 880 | 10682 ± 1263 | 15566 ± 3615 | 18711 ± 1811 | 0.0049 |
Values are presented at mean ± SEM. Fasting, peak, and AUC0–120 (total area under the curve) values during the mixed-nutrient or high-fat meal stimulation test. The reported P value represents the difference between the changes occurring with diet and GBP.
P < 0.05, effect of weight loss within each group (diet or GBP). GBP indicates gastric bypass; GLP-1, glucagon-like peptide-1; PYY, peptide YY, AUC, area under the curve
Prior to intervention, neither the surgery nor control cohorts had a postprandial PYY response at any time point (Figure 1). Following diet-intervention, the control cohort had a small PYY response at 60 and 120 minutes with the high-fat meal, but failed to have any response with mixed-nutrient meal. In contrast, stimulated PYY levels after gastric bypass were greater than to prior to surgery, with augmented levels compared to baseline at 30 and 60 minutes with the mixed-nutrient meal and at 30, 60, 90, and 120 minutes with the high-fat meal (Figure 1). Furthermore, the post-bypass stimulated PYY levels at 30 and 60 minutes with the mixed-nutrient meal and at 30, 60, and 90 minutes with high-fat meal were significantly greater than the post-diet PYY levels at these same time points (Figure 1). However, with both test meals the change in peak PYY (mixed nutrient p=0.09, high-fat p=0.07) and AUC0–120 (mixed-nutrient p=0.16, high fat p=0.16) as a result of surgery approached, but did not reach, statistical significance (Table 2). Diet-intervention did not have an effect on fasting PYY levels. In contrast, gastric bypass resulted in a significantly lower fasting PYY, though this was only evident in fasting levels measured prior to the high-fat test meal, but not prior to the mixed-nutrient test meal (Table 2).
Effect of Macronutrient Content on Hormonal Response to Test Meal
The macronutrient content of the test meal did not have an effect on peak GLP-1 or AUC120 for either group either before or after intervention. However, following gastric bypass the mixed-nutrient PYY AUC0–120 was significantly greater than the high-fat AUC120 (22081±5662 versus 18711±1811 pg/ml•min, p=0.04).
Hunger Ratings
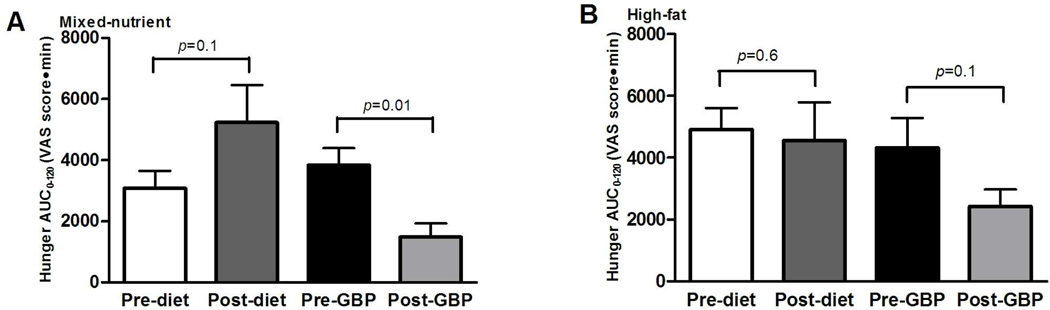
Fasting hunger ratings were not significantly different between the diet and gastric bypass groups prior to any of the meal stimulation studies. Furthermore, following gastric bypass, hunger ratings in the fasted-state did not differ compared with the initial assessment. However, following diet-intervention, fasting hunger ratings were significantly greater than the initial assessment prior to the mixed-nutrient test meal (53.3±11.6 mm versus 25.4±7.8 mm, p=0.03), but not prior to the high-fat test meal (49.2±12.1mm versus 39.3±10.1 mm, p=0.5). Gastric bypass resulted in decreased hunger AUC0–120, though this did not reach significance with the high-fat test meal (Figure 2). In contrast, diet-intervention did not affect hunger AUC0–120. There was a weak negative correlation between hunger AUC0–120 and GLP-1 AUC0–120 (r=−0.25, r2=0.063, p=0.03), though there was no correlation between hunger and PYY response.
Figure 2.
AUC0–120 (total area under the curve) of hunger VAS scores (millimeters) in response to mixed-nutrient (A) or high-fat (B) liquid meal in morbidly obese subjects before and after GBP (n=10 mixed-nutrient, n=7 high-fat) and before an after a diet-induced weight loss (n=10 mixed-nutrient, n=9 high-fat). A larger value indicates increased hunger sensations. Data are expressed as mean ± SEM. The P values reflect comparison between pre- and post- weight loss intervention.
DISCUSSION
Prior studies have shown that over the long term, and after substantial weight loss, gastric bypass restores the postprandial GLP-1 and PYY response [12,14,16,26]. However, the majority of these studies have been after subjects have lost a significant amount of weight, making it unclear whether the alteration in hormone release is due to surgery itself, or weight loss. A study by le Roux[17] reported an increase in GLP-1 and PYY as early as 2 days after surgery and before significant weight loss. Our study evaluated the postprandial PYY and GLP-1 response at an average of 14.6 days after surgery. Though subjects, as expected, lost weight during this period, this interval was chosen to avoid any effects that the immediate physiologic stress and inflammatory response of surgery may have on gut-hormone levels, as well as to avoid potential side effects of consuming an 8 ounce liquid meal over a short time interval in the early postoperative period.
In this prospective study, we show that gastric bypass results in an augmented postprandial PYY and GLP-1 response, before substantial weight loss and independent of caloric restriction. The gastric bypass subjects had a significantly greater duration of intervention and weight loss than the diet-induced weight lost group, which potentially may have contributed to the increased postprandial PYY and GLP-1 response observed after surgery but not diet alone. However, in an outpatient setting we felt that it would be difficult to achieve subject compliance with a longer duration hypocaloric diet in the control subjects, and thus the study was designed to match daily diet for a seven day period, not necessarily total weight loss during the intervention period.
The mechanism of increased gut-hormone release after gastric bypass is not clear, but is thought to result from an increased and more rapid delivery of nutrients to the GLP-1 and PYY secreting L-cells of the small intestine as a result of bypass of a portion of the proximal small bowel and the decreased gastric emptying and intestinal transit times observed after gastric bypass[18]. To further investigate whether the anatomic rearrangement of gastric bypass itself, and not weight loss, is responsible for the augmented postprandial gut-hormone secretion, recent studies have evaluated the effect of equivalent weight loss by either caloric restriction alone or gastric bypass on gut-hormone secretion. These studies found that gastric bypass, but not diet intervention, resulted in an increased postprandial PYY response[27,28], increased postprandial GLP-1 response[29,30] and decreased hunger AUC[27]. Similarly, our study shows that after gastric bypass there is an increased postprandial GLP-1 and PYY response, as evidenced by increased hormone levels compared to fasting, compared to prior to surgery, and compared to hypocaloric diet intervention. However, in our study the postprandial peak and AUC0–120 PYY response approached, but was not significantly greater than the pre-surgical response. It is likely that with larger patient cohorts, or simply extending the meal-stimulation study from 120 minutes out to 180 minutes, as it was in each of the these other studies[27,28,29,30], the differences would reach significance.
Interestingly, in his study of an equivalent 17-kg weight loss with gastric bypass or diet, Valderas found that diet intervention resulted in the opposite effect of gastric bypass, and produced decreased basal and postprandial PYY levels[27]. A decrease in fasting PYY[31] and similar decrease in fasting and postprandial GLP-1 after diet-induced weight loss[32] have been observed in other studies. Our study did not show a change in either basal or postprandial PYY or GLP-1 levels following diet intervention. In contrast to these other studies, our study investigated the GLP-1 and PYY levels after a 1-week hypocaloric diet, compared with a diet duration of 6 to 12 weeks in the other studies. This discrepancy in results may be due to the varied length of the hypocaloric diet periods, with a longer diet phase allowing for a physiologic adaptation.
In contrast to most other study measuring PYY levels after gastric bypass, in this study we found that fasting PYY levels were decreased after surgery. However, this was only evident in the fasting samples collected prior to the high-fat test meal, but not prior to the mixed-nutrient meal. Certainly, we would expect equivalent results from fasting samples collected prior to either test meal as the mixed-nutrient and high-fat studies were conducted on consecutive days. As a potential explanation, ten patients completed the mixed-nutrient meal stimulation studies, whereas due to difficulty in placing the intravenous catheter, only seven of these patients were able to complete the high-fat meal stimulation studies. With an equivalent sized cohort for the high-fat meal stimulation studies, it’s likely that the difference in pre and post-surgery fasting PYY levels would no longer exist. In fact, when incorporating the mixed-meal fasting data for the three missing patients into the high-fat fasting data, there is no longer a difference in fasting PYY as a result of surgery.
Understanding the contribution of different macronutrients on the secretion of anorexogenic gut-hormones may potentially help determine an optimal diet composition for decreased hunger and increased weight loss. In fact, multiple studies have evaluated the differing affects of protein, carbohydrates, and fat on the release of GLP-1 and PYY, though the results of these studies are inconsistent [19,20,23]. A study by Essah found that in obese individuals a 1-week low carbohydrate, high-fat diet, with a test meal of identical composition, results in a greater postprandial PYY response than a low-fat, high carbohydrate diet[22]. A separate study by Helou in which obese subjects completed iso-caloric meal stimulation studies with differing macronutrient compositions, found that the high-carbohydrate meal produced a sustained increase in PYY, the high-fat meal produced a greater early increase in PYY, whereas the high-protein meal produced a delayed increase in PYY[24]. In our study, the fat content of the meal did not affect GLP-1 or PYY levels in obese subjects prior to surgery or obese controls, either before or after weight loss. The total caloric and fat content of the high-fat meal in our study (262 kcal, 45% fat) is lower than that of the other studies (321 to 565 kcal, 50% fat in Helou study and 540 kcal with 75% fat in Essah study), and it’s possible that a greater fat load is needed to elicit a detectable difference in gut-hormone response in obese patients.
To our knowledge, only one prior study has investigated the gut-hormone response to different macronutrients after gastric bypass surgery[21]. In this study by Beckman, 16 women were maintained on either a high-fat or high-protein supplementation diet for 6 weeks after surgery. During this period they consumed two 90 kcal high-protein or high-fat supplements in addition to a normal post-bypass diet. They completed meal-stimulation studies with the same high-fat or high-protein supplement at 2 weeks, 6 weeks, 6 months, and 1 year after surgery. This study did not observe any changes from baseline in PYY and GLP-1 AUC two weeks after surgery, but did see an increase in GLP-1 response with the high-fat supplement at 6 weeks and 1 year, but not with the high-protein supplement. PYY response increased with both the high-fat and high-protein supplement at 6 months, but only remained elevated at one year with the high-fat supplement. Unlike this study, our study found that postprandial PYY and GLP-1 levels were increased two weeks after surgery with both a mixed-nutrient and high-fat meal. In addition, following gastric bypass the high-fat meal resulted in a reduced PYY AUC0–120 as compared with the mixed-nutrient meal, though with the high-fat meal the PYY response was sustained through 120 minutes, whereas with the mixed-nutrient meal by 90 minutes the PYY levels were not significantly different than the fasting level. There are many differences between our study and the study by Beckman which could account for our discrepant results. All subjects in our study were diabetic whereas the Beckman study did not discuss the diabetes status of its subjects. Our study measured GLP-1 and PYY at 30-minute intervals after meal consumption out to 120 minutes whereas the Beckman study measured GLP-1 and PYY at only two time points after meal consumption. These time points varied depending on the hormone measured and the time after surgery. In addition, the test meals in our study were 262 kcal whereas in the Beckman study the test meals were only 90 kcal. It’s possible that at two weeks, the Beckman study did not capture an early increase in GLP-1 or PYY as it did not measure GLP-1 until 60 minutes after meal consumption and did not measure PYY until 90 minutes after meal consumption. The very low total caloric content of the meals in the Beckman study may also have been insufficient to elicit a detectable increase in GLP-1 and PYY levels during many of their study time points.
We had hypothesized based on prior studies[19,22,24] that fat would be a more potent stimulant for GLP-1 and PYY secretion than a mixed-nutrient meal and were somewhat surprised to find no difference in gut-hormone response prior to surgery or in the diet control subjects, and a decreased PYY response with the high-fat diet after gastric bypass. As already discussed, is possible that the macronutrient composition of our high-fat diet was not “extreme enough,” as it was 45% fat whereas in the study by Essah it was 75% fat[22] and in the study by Beckman it was 90% fat[21]. Furthermore, our study measured gut-hormone levels out to 120 minutes after meal intake, whereas the study by Essah continued measurements out to 150 minutes, and Helou continued measurements out to 180 minutes[24]. It is possible that with the prolonged PYY response we observed with the high-fat meal, over a longer observation period the high-fat diet may actually result in an overall increased PYY AUC.
After gastric bypass, postprandial hunger ratings were decreased compared with before weight loss, whereas diet-induced weight loss did not result in a significant change in postprandial hunger ratings. While restriction due to the small gastric remnant likely contributes to decreased feelings of hunger after surgery, there was also a weak negative correlation between GLP-1 AUC0–120 and hunger AUC0–120. This supports the theory that the increased secretion of anorexogenic hormones following gastric bypass surgery contributes to decreased sensations of hunger, and may ultimately be a mechanism involved in the long-term success of the surgery.
In summary, we show that gastric bypass, and not changes in daily diet or overall caloric restriction, produces augmented postprandial GLP-1 and PYY levels. The changes in the secretion of these anorexigenic hormones as a result of gastric bypass could help explain the long-term weight loss achieved with the surgery, as opposed to a calorie-restricted diet. Further research on the contribution of different macronutrients on gut-hormone secretion after gastric bypass may help define an optimal diet for maximal and sustained weight loss after surgery.
ACKNOWLEDGEMENTS
The study was supported by a SAGES Research Grant Award to Dr. Sarah Evans. Dr. Alfonso Torquati is supported by a National Institute of Health Grant K23DK075907. The authors would like to thank all of the volunteer participants and Dr. Eric DeMaria for referring patients for the study.
Footnotes
DISCLOSURES
Drs. Evans, Pamuklar, Jiang, Park and Mr. Rosko and Mr. Mahaney have no conflicts of interest or financial ties to disclose. Dr. Torquati receives an honorarium from Cinemed and Allergen and research funding from Covidien.
REFERENCES
- 1.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brolin RE. Update: NIH consensus conference. Gastrointestinal surgery for severe obesity; Nutrition; 1996. pp. 403–404. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Doucet E. Gastrointestinal peptides after bariatric surgery and appetite control: are they in tuning? Curr Opin Clin Nutr Metab Care. 2008;11:645–650. doi: 10.1097/MCO.0b013e32830ab9c9. [DOI] [PubMed] [Google Scholar]
- 5.Jayasena CN, Bloom SR. Role of gut hormones in obesity. Endocrinol Metab Clin North Am. 2008;37:769–787. doi: 10.1016/j.ecl.2008.07.001. xi. [DOI] [PubMed] [Google Scholar]
- 6.Frezza EE, Wachtel MS, Chiriva-Internati M. The multiple faces of glucagon-like peptide-1--obesity, appetite, and stress: what is next? A review. Dig Dis Sci. 2007;52:643–649. doi: 10.1007/s10620-006-9096-2. [DOI] [PubMed] [Google Scholar]
- 7.Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008;149:70–78. doi: 10.1016/j.regpep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 9.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 10.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahren B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–878. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 11.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 12.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Ghatei MA, Bloom SR. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146:5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 14.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008;32:1640–1646. doi: 10.1038/ijo.2008.157. [DOI] [PubMed] [Google Scholar]
- 15.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 16.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 18.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 19.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 20.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110:571–584. doi: 10.1016/j.jada.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–4055. doi: 10.1210/jc.2006-2273. [DOI] [PubMed] [Google Scholar]
- 23.Feinle C, Chapman IM, Wishart J, Horowitz M. Plasma glucagon-like peptide-1 (GLP-1) responses to duodenal fat and glucose infusions in lean and obese men. Peptides. 2002;23:1491–1495. doi: 10.1016/s0196-9781(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 24.Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3–36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008;52:188–195. doi: 10.1159/000138122. [DOI] [PubMed] [Google Scholar]
- 25.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 26.Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, Morinigo R, Lacy AM. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94:884–891. doi: 10.1210/jc.2008-1620. [DOI] [PubMed] [Google Scholar]
- 27.Valderas JP, Irribarra V, Boza C, de la Cruz R, Liberona Y, Acosta AM, Yolito M, Maiz A. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab. 2010;95:1069–1075. doi: 10.1210/jc.2009-0983. [DOI] [PubMed] [Google Scholar]
- 28.Olivan B, Teixeira J, Bose M, Bawa B, Chang T, Summe H, Lee H, Laferrere B. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg. 2009;249:948–953. doi: 10.1097/SLA.0b013e3181a6cdb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AF, Koebnick C, Weickert MO, Otto B, Spranger J, Tschop MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab. 2007;92:583–588. doi: 10.1210/jc.2006-1425. [DOI] [PubMed] [Google Scholar]
- 32.Adam TC, Jocken J, Westerterp-Plantenga MS. Decreased glucagon-like peptide 1 release after weight loss in overweight/obese subjects. Obes Res. 2005;13:710–716. doi: 10.1038/oby.2005.80. [DOI] [PubMed] [Google Scholar]