Abstract
Background
Opioid neurotransmission mediates hedonic value of sweet tastants; their intake may be exaggerated by the consumption of exogenous opioids (e.g., opioid dependence). Sweet Taste Test (STT) is a validated quantitative instrument assessing taste perception and hedonic features of sugar (sucrose) using a randomized and double-blind administration at five different sucrose concentrations ranging from 0.05 to 0.83 M.
Methods
The STT and cue-induced craving procedure were administered to opioid dependent patients (n=15) before and one week after the injection of a long-acting depot-naltrexone (XRNT) preparation.
Results
Analyses of covariance, employing sucrose concentration and its perceived taste as covariates, showed that XRNT therapy significantly reduced the self-reported hedonic and motivational characteristics of sucrose. Greater reductions in both these characteristics were associated with more diminution in the cue-induced opioid craving.
Conclusions
Opioid antagonism in opioid dependent subjects leads to a smaller sweet taste reward, which, in turn, may be proportional to decreased opioid craving. These pilot results support the heuristic value of the STT as a potential marker of the XRNT treatment response and call for further inquiry into potential clinical applications of the test.
Keywords: glucose, opioid, antagonist, reward, motivation, hedonic, Sweet Taste Test, craving, incentive sensitization
Introduction
Sugar is the main source of energy throughout the central nervous system (CNS) that is neither stored nor is produced there, making procurement of sweet tasting and presumably sugar-containing foods essential for survival. This is in part accomplished via opioidergic neurotransmission within the scattered network of the brain reward regions mediating hedonic impacts of sweet and palatable foods (Berridge and Robinson 2003; Berthoud 2003; Kelley et al. 2002; Pecina et al. 2003; Will et al. 2004). Observations in opioid-dependent animals and humans that are maintained on an opioid agonist, methadone, suggest that exogenous opioid intake enhances the overall opioidergic output and may lead to an exaggerated preference for sugar-rich foodstuffs (Nolan and Scagnelli 2007; Pecina and Smith 2010; Sadava et al. 1997; Shin et al. 2010; Stromberg et al. 1997). It is therefore reasonable to expect that opioid antagonism would be conversely associated with a diminished sweet food preference in opioid dependence, but such an effect has not yet been demonstrated.
One way to address this issue is via the Sweet Taste Test (STT), which is a validated procedure involving collection of subjective self-reports on hedonic and motivational value of sucrose (i.e., table sugar comprised of glucose and fructose), administered at various concentrations in a randomized and double blind fashion (Kampov-Polevoy et al. 1997). Several lines of evidence suggest STT’s usefulness for the assessment of opioid antagonism effects on the sweet taste processing in opioid dependence. In mentally healthy subjects, a μ-opioid antagonist, naltrexone, acutely reduced subjective pleasurable experience normally produced by the sweet taste (Arbisi et al. 1999). From a clinical perspective, in patients with alcohol dependence, where the opioid system is critically implicated (Hillemacher et al. 2011) including allelic variations in specific opioid genes, for example OPRM1 (Oslin et al. 2003), STT performance predicted naltrexone effects on the brain responses to alcohol cues (Myrick et al. 2008) along with longitudinal treatment outcomes (Laaksonen et al. 2011).
Here we evaluated the effects of the depot-naltrexone (XRNT) opioid blockade on sugar reward by comparing STT ratings in opioid-dependent patients before and after the XRNT injection. The XR preparation was recently approved by the Food and Drug Administration for the treatment of opioid dependence (Krupitsky and Blokhina 2010). The major therapeutic advantage of the monthly injections over the daily oral intake lies in the improved compliance profile (Hulse et al. 2009; Lobmaier et al. 2008; Lobmaier et al. 2010) and in the alleviation of the subjective expectancy of potential drug effects, that is to say, craving (Childress et al. 1986; Krupitsky and Blokhina 2010; Wilson et al. 2004). We hypothesized that XRNT opioid antagonism would be associated with a reduced reward value of the sweet solutions. Additionally, given the prior alcohol data (Garbutt et al. 2009; Myrick et al. 2008), in conjunction with the conspicuous overlap (Carr 2002; Elman et al. 2006; Grigson 2002) between the homeostatic system involved in the processing of caloric balance and in the motivational circuitry engaged by addictive drugs, we explored a potential relationship between XRNT-induced changes in the STT parameters and subjective craving evoked by visual drug-related cues.
Methods
The study protocol was approved by the Institutional Review Boards of the University of Pennsylvania and the City of Philadelphia. Participants were 15 (2F) daily intravenous opioid users, 34.3 ± Standard Deviation (SD) = 8.2 years of age, who met the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision (DSM-IV-TR) criteria for opioid dependence and selected XRNT among several options for court-mandated drug treatment in the Philadelphia court system (Cornish et al. 1997). Written informed consent was obtained after complete description of the study to the subjects. They were explicitly allowed to ask any questions they had about the study and were thoroughly explained that giving or withholding their consent would not be associated with any repercussion with regard to their legal status or future treatment at any medical facilities affiliated with the University of Pennsylvania. The subjects underwent the study procedures in return for a participation fee.
All participants were diagnosed using a best estimate format, based on all available sources of information, including clinical history, interview, the Structured Clinical Interview for DSM-IV (First 2002) and the Addiction Severity Index 5th Edition (McLellan et al. 1992). Subjects were briefed about the expected loss of subjective efficacy of opioid self-administration that would result from the XRNT treatment, as well as the dangers of attempting to overcome the opiate receptor blockade with higher than usual opioid doses (Paronis and Bergman 2011; Ruan et al. 2010).
Inclusion criteria were: 1) intravenous heroin self-administration within eighteen months prior to enrollment; 2) 21-55 years of age; 3) parole or probation for non-violent drug-related offences (e.g., illegal drug possession, sale and/or retail theft in order to procure the drug). Exclusion criteria included a history of head injury, loss of consciousness, brain tumor, seizures or cerebrovascular accident, as well as medical illnesses, use of any prescription medications (including prescribed opioid analgesics) that could affect alertness or circulatory system, current DSM-IV-TR Axis I psychiatric disorders other than opioid and nicotine dependence. Prior to the XRNT administration, naloxone challenge test (0.6 mg IV) was performed to ascertain opioid abstinence, which was assessed thereafter via bi-weekly urine toxicology screens. The XRNT treatment involved a monthly open-label intramuscular injection of Depotrex® (Biotek, Inc, Wellesley, MA) that contained 228 mg of slow-release naltrexone.
The STT was administered within a week after the XRNT injection and was conducted as described by Kampov-Polevoy and colleagues (Kampov-Polevoy et al. 2004) with a slightly modified rating scale and an additional rating question (#3, below). Timing of the test was before noon and at least 90 min after breakfast along with smoking and teeth cleaning (about 8:30 am). Five concentrations of sucrose solution (0.05, 0.10, 0.21, 0.42 and 0.83 M) were each administered three times (trials) in a random order, for a total of 15 samples. Subjects were instructed to sip the solution, swish it around in their mouths, and spit it out. They were then asked to rate the solution, rinse their mouth with distilled water, and proceed to the next solution. Each subject was asked to rate each solution on the following likert-type scales: 1) Please rate the taste of this drink (−5: very bitter; +5 very sweet); 2) Please rate how much you like the taste of this drink (−5: not at all; +5: very much) and 3) Please rate to what extent you want to have more of this drink (−5: not at all; +5: very much). The STT change scores were determined by subtracting PRE-from ON-XRNT ratings (taste, liking and wanting) averaged across the three tasting trials and the five sucrose concentrations.
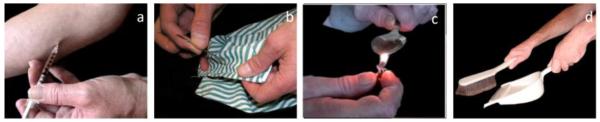
The drug cues symptom provocation procedure (Figure 1), described elsewhere (Langleben, Ruparel et al. 2008), was performed before and after the XRNT injection 1-2 days apart from the STT. Briefly, participants viewed images of heroin preparation or injection (“Drug”) and images of housework and office work (“Neutral”), presented in a fixed pseudorandom order. The two stimuli classes were matched for luminosity and semantic content and had uniform black background. None of the stimuli contained human faces. A total of 96 unique Drug and Neutral stimuli were shown once, for 1.5 seconds. Stimuli were separated by a variable inter-trial interval (ITI), during which the “baseline” image (white crosshair on black background) was displayed. Opioid craving was assessed before and after each session with a 10-point (0-9) Subjective Craving Scale ((SCS, (Childress et al. 1999; Langleben et al. 2008)) and a change score was calculated for each session (dSC) and between the PRE and POST dSC’s (ΔdSC). The latter scores were used in the correlation analysis with the STT measures, utilizing the Pearson product-moment coefficient.
Figure 1.
Examples of images used in the cue-induced craving procedure. The subjects visually processed images of heroin preparation or injection (a and c) and images of housework and office work (b and d), presented in a fixed and pseudorandom order. The two stimuli classes were matched for luminosity and semantic content. All images had uniform black background and none contained human faces (see the Text).
Separate analyses of covariance (ANCOVA) were performed for each dependent variable viz., subjective rating for taste, for liking and for wanting. Each ANCOVA entailed two within-subjects factors namely, Drug and Trial. The former had two levels (pre-XRNT and on-XRNT) while the latter had three levels (first, second and third). To control for the possibility that a difference in a subjective rating merely reflected a difference in concentration or taste perception, these variables were employed as covariates. Specifically, taste ratings analyses were covaried for sucrose concentrations, while liking and wanting analyses were covaried for both taste ratings and for sucrose concentrations. Data were summarized as Mean ± SD. All analyses were two-tailed with α < 0.05 set as the threshold for statistical significance.
Results
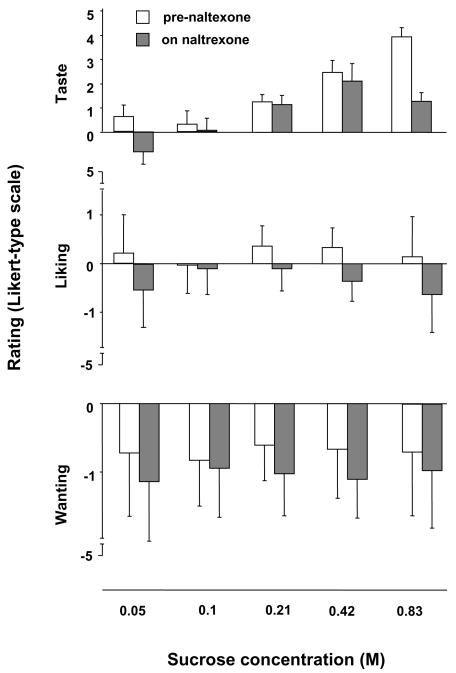
Subjects’ means ± SD for each subjective rating are displayed in Figure 2. Five subjects were “sweet likers” as they rated the highest available sucrose concentration solution (0.83M) as the most likable one, while the rest of the sample (n=10) preferred solutions with lower sucrose concentrations and therefore were “sweet-dislikers” (Garbutt et al, 2009). XRNT led to a significant reduction in the subjective sweet taste perception (F=4.32; df=1,2, 428; p=0.04) as well as in liking (F=15.39; df=1,2; 427, p=0.0001) and in wanting (F=9.86; df=1,2; 427, p=0.002) ratings (i.e., drug effect) but no trial effects (p>0.2) or trial by drug interaction (p>0.18). Notably, sucrose concentration covariate emerged as a significant predictor for the liking (F=14.24; p<0.001) but not for the wanting (F=0.02; p=0.89) effect. Conversely, perceived taste was a significant predictor for both liking and wanting (p<0.001).
Figure 2.
Subjective self-rating for the perceived taste of the drink (−5: very bitter; +5 very sweet) along with liking the taste of the drink (−5: not at all; +5: very much) and wanting to have more of the same drink (−5: not at all; +5: very much). The data were obtained pre- and on XRNT pharmacotherapy. Results across the three sampling points were averaged and expressed as mean ± standard deviation.
Baseline values averaged across the three tasting trials and the five sucrose concentrations for the sweet taste (r=−0.22; df=13; p=0.43), for liking (r=−0.48; df=13; p=0.07) and for wanting (r=−0.48; df=13; p=0.07) were not significant predictors of the XRNT-induced changes in craving evoked by drug-related but not by neutral images. The change scores for craving correlated with those for liking (r=0.58; df=13; P=0.02) and for wanting (r=0.67; df=13; P=0.006) but not with the taste (r=−0.07; df=13; P=0.80) change scores. Lastly, there was a significant relationship between the change scores for liking and wanting of the sweet solutions (r = 0.67; df = 13; p = 0.006).
Discussion
The major finding of this study is that XRNT-treated opioid dependent subjects displayed reductions in both hedonic and motivational responses to sweet solutions. Although the ANCOVAs showed significant effects of sucrose concentration and perceived taste covariates, these did not conceal the observed XRNT-induced liking and wanting declines suggesting a partial specificity of the observed changes. Such specificity is further evident in the fact that changes in the cue-induced craving correlated with those for liking and wanting, but not with the perceived taste change scores. Notably, in addition to the correlation between liking and wanting changes with the naltrexone-induced reductions in craving, there was also a trend toward correlation between the craving reductions and the baseline liking and wanting values (which would have been statistically significant had this result been predicted a priori). This pattern is consistent with the literature on alcohol dependence showing more favorable naltrexone treatment outcomes in patients with heightened baseline sweet liking reports, i.e. the “sweet likers” (Garbutt et al, 2009), and suggests that baseline STT results may also predict treatment outcome in opioid dependent subjects. More research is needed to pursue such a possibility.
The present data replicate early work with STT in which decrements in self-reported sugar liking were reported 60 min after a single oral intake of 50 mg naltrexone (Arbisi et al. 1999). Although there were methodological similarities between the Arbisi et al (1999) and the current study (e.g., STT and hedonic ratings), there were also important differences, including the psychopathological population of opioid dependent subjects, a predominately male sample (c.f., mentally healthy females in the Arbisi et al (1999) report), a motivational rating component, and a longitudinal effect of the depot naltrexone preparation. Together, the current and the previous results (Arbisi et al. 1999) suggest that opioid blockade is associated with a hyposensitive response to the sweet taste reward.
Our results replicate prior reports indicating that opioid antagonism is linked to a diminished appeal of palatable food in general and of sweet taste in particular (Elman et al. 2006; Pecina and Smith 2010) and extend these findings to opioid dependent patients and to a long-acting opioid blocker. Additional issue to be considered in the future work include effects of opioid blockade on fatty and savory palatability components as well as differentiation of the rewarding effects as they are attributed to the caloric value vis-à-vis the taste per se (Smeets et al. 2005).
Another noteworthy finding in our study is that sucrose concentration significantly predicted the liking but not the wanting responses, suggesting a priming and potentially dose-insensitive (c.f. (Mihindou et al. 2011)) effect of sugar with respect to the latter. This observation is in agreement with the incentive motivation theory that postulates that the brain’s appetitive function can be dissociated into hedonic and motivational core processes that are respectively referred to as ‘liking’ and ‘wanting’ (Berridge and Robinson 2003). Drug-induced changes in the mesolimbic dopaminergic circuitry underlying the wanting but not the liking processes are thought to be responsible for heightened incentive salience assigned to drug-related cues and potentially to palatable food-related stimuli. This ’incentive sensitization’ phenomenon is construed to be an animal homolog of human craving. Thus, similarly to the respective reports in the cocaine literature for drug euphoria and craving (Ward, Collins et al. 1999; Elman, Karlsgodt et al. 2002), sweet liking may scale up with the dose while sweet wanting may not be dose-dependent. These impressions, together with the observed correlation between hedonic and motivational sugar ratings and opioid craving raise the possibility of overlapping neuropsychobiological systems engaged by food reward and by drug-related stimuli (Carr 2002; Elman et al. 2006; Spetter et al. 2010). This hypothesis could be tested in drug dependent subjects by juxtaposing responses to neuroimaging probes known to activate the brain circuits subserving food and drug reward.
A number of caveats need to be factored into the interpretation of our data. First the findings should be considered preliminary, pending replication with a larger sample. Second, mostly men participated in this study. While the sample composition reflects the demographics of intravenous heroin abusers (Puigdollers et al. 2004), gender distribution for non-parenteral routes of administration may be more balanced (Grella and Lovinger 2011). Since gender plays a substantial role in the course of opioid dependence (Cicero et al. 2003; Cicero et al. 2000; de Vos et al. 1995; Fattore et al. 2008; Kelly et al. 2009; Yu et al. 2007), a more gender-balanced group may be an important consideration for the future research.
Third, given the predictable preference and pursuit of pleasant motivational targets, it is plausible that hedonic and motivational aspects of sugar are closely related entities. The findings that liking and wanting changes only accounted for 45% of each others’ variance in conjunction with the opposite directionality of the liking and wanting ratings suggest that these ratings might have captured different aspects of the reward-related sweet taste properties. Nonetheless, introduction of additional paradigms, targeting more objective characteristic of motivational processing such as a computer key press task previously employed by our group (Elman et al. 2005) may complement the presented data.
Moreover, even though self-reports collected here may be distinct from partially subliminal constructs implicated in the animal work and idiomatically termed ‘liking’ and ‘wanting’ (Berridge 2007; Berridge et al. 2009), our results may have clinical significance as subjective liking and wanting ratings for alcohol predicted binge drinking behavior in alcoholics (King et al. 2009). Because the protocol employed does not allow us to firmly disentangle subliminal components, answering this question would require exclusively behavioral or neuromaging procedures that do not involve subjective output. Finally, only a homogeneous sample comprised of intravenous heroin probationers was enrolled, so the results may not necessarily be extrapolated to other types of opioid dependence (e.g., opioid analgesics) or to subjects without legal contingencies.
In conclusion, the presented pilot data suggest that opioid antagonism in opioid dependent patients is associated with a decreased sugar preference, correlated with opioid craving. These data call for further research aimed at understanding a potential role of the STT in formulating the optimal pharmacotherapy for opioid dependence as well as for prevention, early detection and treatment of abnormal eating patterns emerging in the context of excessive opioid use.
References
- Arbisi PA, Billington CJ, Levine AS. The effect of naltrexone on taste detection and recognition threshold. Appetite. 1999;32:241–9. doi: 10.1006/appe.1998.0217. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Neural systems controlling food intake and energy balance in the modern world. Current Opinion in Clinical Nutrition and Metabolic Care. 2003;6:615–20. doi: 10.1097/00075197-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiology and Behavior. 2002;76:353–64. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81:655–60. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–9. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav. 2000;65:91–6. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, O’Brien CP. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14:529–34. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- de Vos JW, Geerlings PJ, van den Brink W, Ufkes JG, van Wilgenburg H. Pharmacokinetics of methadone and its primary metabolite in 20 opiate addicts. Eur J Clin Pharmacol. 1995;48:361–6. doi: 10.1007/BF00194951. [DOI] [PubMed] [Google Scholar]
- Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, Orr SP, Lukas SE, Pitman RK. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Res. 2005;135:179–83. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- First M. Diagnostic and statistical manual of mental disorders. 4th Edition, Text Revision American Psychiatric Association; Washington, DC: 2002. [Google Scholar]
- Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M, Flannery B, Kampov-Polevoy AB. Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol and Alcoholism. 2009;44:293–300. doi: 10.1093/alcalc/agn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Lovinger K. 30-Year trajectories of heroin and other drug use among men and women sampled from methadone treatment in California. Drug and Alcohol Dependence. 2011 doi: 10.1016/j.drugalcdep.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS. Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiology and Behavior. 2002;76:389–95. doi: 10.1016/s0031-9384(02)00758-8. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Heberlein A, Muschler MA, Bleich S, Frieling H. Opioid modulators for alcohol dependence. Expert Opin Investig Drugs. 2011 doi: 10.1517/13543784.2011.592139. [DOI] [PubMed] [Google Scholar]
- Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Archives of General Psychiatry. 2009;66:1108–15. doi: 10.1001/archgenpsychiatry.2009.130. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry. 1997;154:269–70. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 2004;28:1291–8. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Schwartz RP, O’Grady KE, Mitchell SG, Reisinger HS, Peterson JA, Agar MH, Brown BS. Gender Differences Among In- and Out-of-Treatment Opioid-Addicted Individuals. Am J Drug Alcohol Abuse. 2009;35:38–42. doi: 10.1080/00952990802342915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009;33:1044–50. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Blokhina EA. Long-acting depot formulations of naltrexone for heroin dependence: a review. Curr Opin Psychiatry. 2010 doi: 10.1097/YCO.0b013e3283386578. [DOI] [PubMed] [Google Scholar]
- Laaksonen E, Lahti J, Sinclair JD, Heinala P, Alho H. Predictors for the efficacy of naltrexone treatment in alcohol dependence: sweet preference. Alcohol and Alcoholism. 2011;46:308–11. doi: 10.1093/alcalc/agq101. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Lobmaier P, Kornor H, Kunoe N, Bjorndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev: CD006140. 2008 doi: 10.1002/14651858.CD006140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier PP, Kunoe N, Gossop M, Katevoll T, Waal H. Naltrexone implants compared to methadone: outcomes six months after prison release. European Addiction Research. 2010;16:139–45. doi: 10.1159/000313336. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mihindou C, Vouillac C, Koob GF, Ahmed SH. Preclinical Validation of a Novel Cocaine Exposure Therapy for Relapse Prevention. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst Use Misuse. 2007;42:1555–66. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2011;336:488–95. doi: 10.1124/jpet.110.173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. Journal of Neuroscience. 2003;23:9395–402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Smith KS. Hedonic and motivational roles of opioids in food reward: implications for overeating disorders. Pharmacology, Biochemistry and Behavior. 2010;97:34–46. doi: 10.1016/j.pbb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Puigdollers E, Domingo-Salvany A, Brugal MT, Torrens M, Alvaros J, Castillo C, Magri N, Martin S, Vazquez JM. Characteristics of heroin addicts entering methadone maintenance treatment: quality of life and gender. Subst Use Misuse. 2004;39:1353–68. doi: 10.1081/ja-120039392. [DOI] [PubMed] [Google Scholar]
- Ruan X, Chen T, Gudin J, Couch JP, Chiravuri S. Acute opioid withdrawal precipitated by ingestion of crushed embeda (morphine extended release with sequestered naltrexone): case report and the focused review of the literature. J Opioid Manag. 2010;6:300–3. doi: 10.5055/jom.2010.0028. [DOI] [PubMed] [Google Scholar]
- Sadava D, Alonso D, Hong H, Pettit-Barrett DP. Effect of methadone addiction on glucose metabolism in rats. General Pharmacology. 1997;28:27–9. doi: 10.1016/s0306-3623(96)00165-6. [DOI] [PubMed] [Google Scholar]
- Shin AC, Pistell PJ, Phifer CB, Berthoud HR. Reversible suppression of food reward behavior by chronic mu-opioid receptor antagonism in the nucleus accumbens. Neuroscience. 2010;170:580–8. doi: 10.1016/j.neuroscience.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005;82:1011–6. doi: 10.1093/ajcn/82.5.1011. [DOI] [PubMed] [Google Scholar]
- Spetter MS, Smeets PA, de Graaf C, Viergever MA. Representation of sweet and salty taste intensity in the brain. Chemical Senses. 2010;35:831–40. doi: 10.1093/chemse/bjq093. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Meister S, Volpicelli JR, Ulm RR. Morphine enhances selection of both sucrose and ethanol in a two-bottle test. Alcohol. 1997;14:55–62. doi: 10.1016/s0741-8329(96)00107-3. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–60. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]