Abstract
Objectives
To characterize the ability of high intensity focused ultrasound (HIFU) to achieve thrombolysis in vitro and investigate the feasibility of this approach as a means of restoring blood flow in thrombus occluded arteries in vivo.
Materials and Methods
All experiments were approved by the institutional animal care committee. Thrombolysis was performed with a 1.51MHz focused ultrasound transducer with pulse lengths of 0.1–10 ms and acoustic powers up to 300W. In vitro experiments were performed with blood clots formed from rabbit arterial blood and situated in 2mm diameter tubing. Both single location and flow-bypass recanalization experiments were conducted. In vitro clot erosion was assessed with 30MHz ultrasound, with debris size measured with filters and a Coulter counter. In vivo clots were initiated in the femoral arteries of rabbits (n = 26). Cavitation signals from bubbles formed during exposure were monitored. In vivo flow restoration was assessed with 23MHz Doppler ultrasound.
Results
At a single location, in vitro clot erosion volumes increased with exposure power and pulse length, with debris size reducing with increasing pulse length. Flow bypass experiments achieved 99.2% clot erosion with 1.1% of debris above 0.5mm in size. In vivo, 10ms pulses were associated with bleeding, but at 1ms it was feasible to achieve partial flow restoration in 6/10 clots with only 1/10 showing evidence of bleeding. In all cases, thrombolysis occurred only in the presence of cavitation.
Conclusion
HIFU thrombolysis is feasible as a means of restoring partial blood flow in thrombus occluded arteries in the absence of thrombolytic agents. The potential for bleeding with this approach requires further investigation.
Keywords: High Intensity Focused Ultrasound, Thrombolysis, Ischemic Stroke
Introduction
The dissolution of blood clots is relevant to the treatment of myocardial infarction, deep venous thrombosis, peripheral artery disease and acute ischemic stroke. In the case of stroke, the only clinically approved medical treatment involves the infusion of tissue plasminogen activator (tPA). tPA must however be administered within three hours of stroke onset, which reduces patient eligibility.1,2 It is also limited in its effectiveness and is associated with increased risk of intracerebral hemorrhage.3–5 While catheter-based approaches such as mechanical thrombectomy have demonstrated their utility for clot removal, these are more invasive approaches that carry with them risks of vascular damage.6,7
The use of ultrasound to potentiate thrombolysis has been investigated for several decades, initially in the context of cardiology and, more recently, for stroke applications.8 Ultrasound is a promising technique for promoting clot breakdown as it can improve lysis rates and thereby reduce the amount of thrombolytic agent required. The large majority of sonothrombolysis research has focused upon low intensity ultrasound in conjunction with thrombolytic agents9–14 and has demonstrated significant improvements in clot lysis over thrombolytic agents alone in vitro, in vivo and in clinical trials. However, conjunctive ultrasound treatments that enhance the efficacy of thrombolytic agents are limited by the need to administer treatment within several hours of stroke onset and will likely not eliminate the risks associated with tPA.
Sonothrombolysis has also been achieved with injected microbubble contrast agents15–22 where there is now clear evidence ultrasound stimulated bubble oscillations can promote clot lysis in the presence or absence of tPA. The clinical feasibility of this approach has been shown, though the results of a recent clinical trial22 that was terminated early highlights the need for an improved understanding of the mechanisms involved, treatment monitoring, and a determination of safe operating conditions.
To date little work has been done to investigate the use of high intensity focused ultrasound (HIFU) as a stand-alone technique for thrombolysis. It is hypothesized that this approach may be feasible as a means of restoring flow in thrombus occluded vessels and thereby obviate the need for thrombolytic agents and injected microbubbles. The clinical concept will be to employ this approach as a noninvasive means of restoring or improving flow in thrombus occluded vessels in the context of acute stroke by focusing ultrasound through the skull using transcranial therapeutic array transducers. As a first step towards investigating the feasibility of this approach, several groups have shown its ability to rapidly disrupt unconstrained clots in vitro,23–25 using relatively short 0.5–1 MHz ultrasound pulse durations (0.05–0.2 ms). Initial work with short histotripsy pulses (0.05ms) at 1 MHz has indicated flow improvement can be achieved in partially and fully occluded larger arteries26. This work suggests that clot degradation is being achieved by the formation and subsequent oscillation (cavitation) of small bubbles, as indicated by changes in brightness on ultrasound images during treatment.
In the present study, guided by results of preliminary experiments,27 the feasibility of in vivo 1.5 MHz HIFU thrombolysis to achieve flow restoration in occluded vessels was investigated. Experiments were first performed in vitro, to examine the effects of ultrasound amplitude and pulse length on erosion volumes and debris sizes. In vivo investigations were then performed on a rabbit femoral artery clot model, with blood flow assessed with the use of 23 MHz ultrasound pulsed-wave Doppler (PWD).
Materials and Methods
Ultrasound
Therapeutic pulses were generated with a spherically focused air-backed 1.51 MHz transducer (10 cm focal length, 10 cm diameter). Pulses were initiated with a function generator, (model AFG 3102, Tektronix, Beaverton, Oregon), amplified (model A-500, ENI, Rochester) and passed through an electrical impedance matching circuit. The therapy transducer was attached to a 3-axis position stage and oriented upwards from below the clot at an angle of 15°. All experiments were performed in a degassed water tank at room temperature. The transducer efficiency, calibrated as described elsewhere28, was 55% and power levels are expressed in terms of acoustic watts. The width and length of the focal zones (−6 dB) were 0.9 and 7.1 mm, as measured with a hydrophone (Precision Acoustics, UK).
Acoustic cavitation signals, arising from the formation and subsequent oscillation of bubbles, were monitored with a 0.52 MHz transducer (10 cm focal length, 4 cm diameter) mounted to the side of the transmit transducer. Signals were recorded with a digitizer (Model ATS460, Alazartech, Montreal, Canada) and for each resulting power spectrum, the energy was integrated between 0.45 and 0.59 MHz. The time dependence of cavitation signal power levels during insonation was also estimated.
In vitro experiments
Two types of in vitro experiments were performed. The purpose of the first was to assess clot erosion at a single transducer beam location where clots were constrained within a thin tube to determine appropriate transmit conditions (amplitude, pulse length). In particular, the size of the eroded region was assessed as a function of transmit conditions along with the dependence of erosion on cavitation. In a second set of experiments clot erosion was examined with a flow bypass approach, where a clot was situated within tubing and eroded along its length by translating the transducer through a series of locations. The purpose of this experiment was to assess the erosion efficiency and the size of the resulting clot debris.
In vitro clots were placed in polyester tubing (2 mm diameter, 0.01 mm wall thickness, Advanced Polymers Inc., Salem, NH). Prior to clot initiation the tubing was primed with degassed saline, mounted to a custom acrylic holder (Fig. 1) and a 2 mm arterial clamp was placed in the center of the tube. Clots were formed in a manner similar to previously described approaches9 using blood drawn from the auricular artery of New Zealand white rabbits into citrated Vacutainer tubes (3.2% sodium citrate, Becton Dickinson, USA). 600 μL of citrated blood was mixed with 40 μL of degassed bovine thrombin (25 NIH units/ml in saline; Sigma Aldrich, St. Louis, Mo) and 75 μL of degassed CaCl2 (100 mmol/L; Sigma Aldrich, St. Louis, Mo) and then injected proximal to the vascular clamp. Immediately after injection, a second vascular clamp was placed 3 cm proximal to the first vascular clamp to isolate the clot. Clots were then incubated at 37 °C for 1 hour, and the holder was then attached to a platform located within the water tank withabsorbing material placed above the clot.
Fig. 1.
Schematic of the in vitro apparatus. The therapy transducer focused acoustic energy onto a clot constrained within a tube that was mounted to an acrylic holder (tube not shown). High frequency ultrasound imaging monitors clot erosion before and after treatment. A separate passive cavitation detection transducer was mounted to the side.
Alignment of the ultrasound beam with the thrombus was achieved using pulse echo measurements with the therapeutic transducer. In vitro ultrasound exposures used pulse lengths of 0.1 and 1ms, acoustic powers from 120–185 W and sonication durations of 5 and 20 s at each exposure location. Note that this power range was selected based on pilot experiments that determined that at 100 W no cavitation was evident, and by 200 W, cavitation was produced when degassed saline was present within the tube. Duty cycle was fixed at 0.1% for all treatments.
For the single location study, 3D 30 MHz ultrasound (Vevo 770, Visualsonics, Toronto, Canada) imaging was employed to quantify clot erosion. Volumetric images along 30 mm longitudinal segments were acquired pre- and post-treatment with the eroded clot boundary being delineated based on the lower echogenicity of the eroded region relative to the intact clot.
The thrombus debris suspension (homogenate volume) was collected from the lyzed region of the tube with a 27G needle (0.21 mm inner diameter) and measured with a Coulter Counter (Multisizer 3; Beckman Coulter, Fullerton, CA) to obtain estimates of debris size distributions. Particles were sized between 2–60 μm (100 μm aperture) and binned in three groups: 2–10 μm, 10–30 μm and 30–60 μm. We note that a pilot study with an 18G needle (1.2 mm inner diameter) resulted in no Coulter counter blockages, indicating that no debris larger than 100 μm was present, consistent with microscope observations of the suspension. A 27G needle was therefore employed in these studies. Statistical comparisons were made using a Student’s t-test. In vitro experiments were conducted by C.W. (a physicist with 3 years ultrasound experience and D.G. a physicist with 13 years of biomedical ultrasound experience).
Statistical comparisons between erosion widths and erosion volumes and particle sizes at different exposure levels were made with an unpaired Student’s t-test using the SPSS software package. P-values are indicated and those less than 0.05 were considered to indicate significant differences between the means of groups.
Flow bypass experiments were conducted using an approach similar to that described in previous studies.29,30 Clots (25 mm in length) were situated within tubing as described above, with a parallel 2.4 mm inner diameter tube providing bypass flow. The flow circuit was perfused with gravity fed degassed saline (flow rate 0.5 ml/s in bypass tube). Downstream, three successive nylon membrane filters 500, 200 and 53 μm (Spectrum Labs, CA, USA) retrieved larger debris fragments and the resulting filtered suspensions were collected and underwent Coulter counter analysis. The treatment regimen for a given axial location along the vessel was to expose three times with the focus located at −0.75, 0 and 0.75 mm lateral offsets with respect to the vessel axis. Exposures began at the distal end of the clots and the transducer was then moved 1 mm along the vessel axis and this process was repeated. After each experiment, filters and tubing were retrieved, desiccated for 36 h and then weighed. These results were used to estimate the volume fractions of clot trapped in the filters and remaining within the tube, based on calibration experiment estimates of wet to dry clot weight ratios.
In vivo
All experiments were carried out according to protocols approved by the Institutional Animal Care Committee. In vivo clots were formed in a rabbit femoral artery, based on procedures reported in for example.31 New Zealand white rabbits (Charles-River, Canada) weighing 3.0–4.0 kg were anaesthetized with xylazine (10 mg/kg) and ketamine (40 mg/kg). The femoral artery was exposed through surgical resection of the overlying skin and muscle layers. A 40% stenosis was induced 2–3 mm proximal of the femoral artery bifurcation with a 4.0 silk suture. A 1 cm segment of the artery, beginning 1 mm proximal to the suture, was occluded with vascular clamps and subjected to external compressions with blunt forceps to produce endothelial injury. 0.2 ml of blood was extracted from the rabbit auricular artery primed with 20 μL of 0.2 NIH units/μL degassed thrombin, mixed and injected into the clamped segment. Thirty minutes after injection the clamps were removed and 23 MHz PWD was used to verify a complete blockage of flow. Immediately after the occlusion was confirmed, a bolus injection of heparin (200 units/kg) was administered to prevent the clot from propagating proximally.
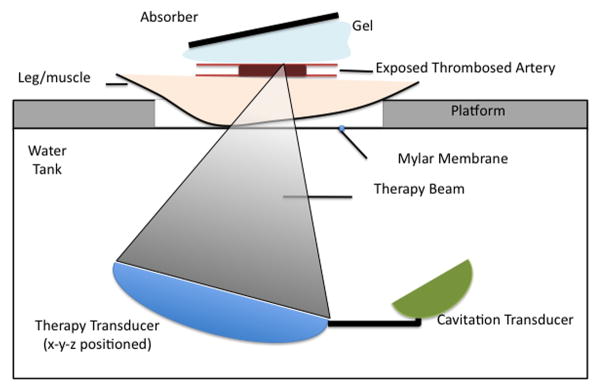
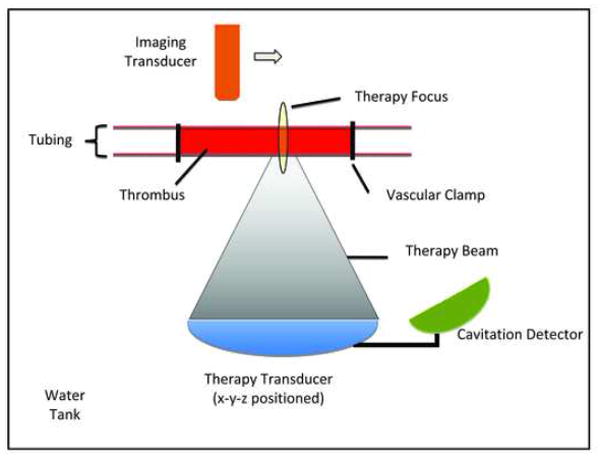
The subjects were arranged in a supine position (hair removed from bottom of thigh) on a platform located atop a water tank and exposures were conducted through a mylar acoustic window (Fig. 2). In this configuration, the therapy beam passed through skin and a layer of muscle before reaching the vessel. Alignment of the ultrasound beam with the clot was achieved by first co-aligning the beams of two spatially separated laser pointers to the therapy transducer focus with liquid crystal temperature sensitive film and then using this calibrated beam intersection point to guide the beam to the vessel.
Fig. 2.
Schematic of the in vivo apparatus. The subject was placed supine on platform located atop a water tank. The therapy and cavitation detection transducers were situated below and the ultrasound passes through a mylar acoustic window to focus upon the clot. An x-y-z position stage was employed to exposure sequential regions along the clot.
The exposure regimen for a given axial location along the vessel was to expose three times with the focus located at −0.75, 0 and 0.75 mm lateral offsets with respect to the vessel axis. The transducer was then moved 1 mm along the vessel axis and this process was repeated. Exposures were conducted from ~1–2 mm proximal to the clot, moving distal to the point of bifurcation.
23 MHz PWD (6 dB beam width 0.24 mm) was used to assess the in vivo flow status within the clot region before and after treatment with the imaging probe at an angle of ~70–80° with respect to the vessel axis. The Doppler sample volume was adjusted to have a length equal to the vessel diameter within the image and the pulse repetition frequency (PRF) was set between 2–10 kHz with the lowest clutter filter setting. Flow was assessed at regular axial locations (1 mm) from the distal bifurcation to the proximal end of the clot region. At each axial location along the vessel, the PWD sample volume was placed at 4–5 lateral positions across the vessel to assess for the presence or absence of flow, as indicated by the PWD spectrum. Partial flow restoration was determined if at least one of the lateral positions demonstrated flow at all of the axial locations. Although PWD is highly sensitive to flow, due to the small beam width (0.24 mm) relative to the vessel size (1–1.5 mm) it could not be employed to quantitatively assess total blood flow changes. It should also be noted that, due to the high levels of echogenicity of blood at high frequencies, B-scan imaging is not well suited to assessing clot erosion in vivo in situations where flow restoration has been achieved and was therefore not employed for this purpose. After exposures, the exposed region was visually assessed for the presence of bleeding. For control experiments, animals underwent the same procedures as described above, without therapeutic ultrasound exposures.
Results
In vitro single location
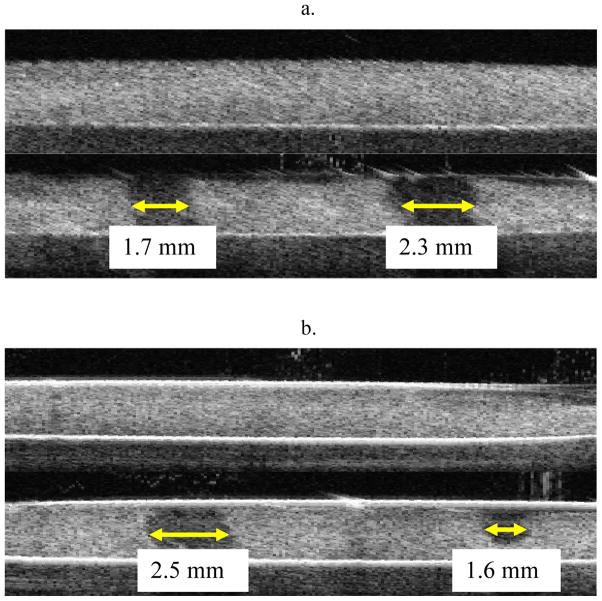
Example pre- and post-treatment images of HIFU initiated clot erosion are presented in Fig. 3, where the eroded regions are evident as hypoechogenic areas. Fig. 3A illustrates the dependence of clot erosion on sonication duration for a 1 ms, 185 W pulse of exposure duration 5 s (left) and 20 s (right). Fig. 3B shows the dependence of clot erosion on pulse duration for a 185 W, 20 s exposure at 1 ms (left) and 0.1 ms (right). A quantification of the in vitro results are summarized in Table 1 for erosion widths and Table 2 for erosion volumes (n = 7 per condition). No clot lysis was evident at 120 W and erosion widths and volumes were observed to increase with pulse length (0.1 versus 1 ms), transmit power (160 versus 185 W) and exposure duration (1 ms pulse). In particular, the volumes for the 1 ms pulse (160 and 185 W) were significantly larger (p<0.0001) than for the 0.1 ms case, despite the same total energy being delivered. Increasing power from 160 to 185 W (20 s duration) produced significantly larger erosion volumes in the 0.1 ms (p<0.001) but not the 1ms case. Increasing exposure duration for the 1ms pulse (160 and 185 W conditions) also produced significant (p<0.02) increases. It was found that for the 1 ms case (185 W) the erosion widths exceeded the 3 dB beam-width by a factor of 2.7.
Fig. 3.
Pre- and post-treatment erosion images from two separate HIFU treatments a) 1 ms pulse, 185 W at: 5 s exposure (left) and 20 s exposure (right) and b) 185 W, 20 s exposure at: 1 ms (left) and 0.1 ms (right). Thrombolysis had occurred in the hypoechogenic regions.
Table 1.
Summary of in vitro erosion widths (mean and standard deviation) as a function of power, pulse length and exposure duration.
| Power | 5 s Exposure, 1 ms | 20 s Exposure, 1 ms | 20 s Exposure, 0.1 ms |
|---|---|---|---|
| 120 W | 0 mm | 0 mm | 0 mm |
| 160 W | 1.64 ± 0.38 | 2.36 ± 0.51 | 1.10 ± 0.10 |
| 185 W | 1.68 ± 0.44 | 2.46 ± 0.49 | 4.20 ± 0.60 |
Table 2.
Summary of in vitro erosion volumes (mean and standard deviation) as a function of power, pulse length and exposure duration.
| Power | 5 s Exposure, 1 ms | 20 s Exposure, 1 ms | 20 s Exposure, 0.1 ms |
|---|---|---|---|
| 120 W | 0 mm3 | 0 mm3 | 0 mm3 |
| 160 W | 5.2 ± 1.2 mm3 | 7.4 ± 1.6 mm3 | 2.4 ± 0.5 mm3 |
| 185 W | 5.3 ± 1.4 mm3 | 7.7 ± 1.5 mm3 | 4.2 ± 0.6 mm3 |
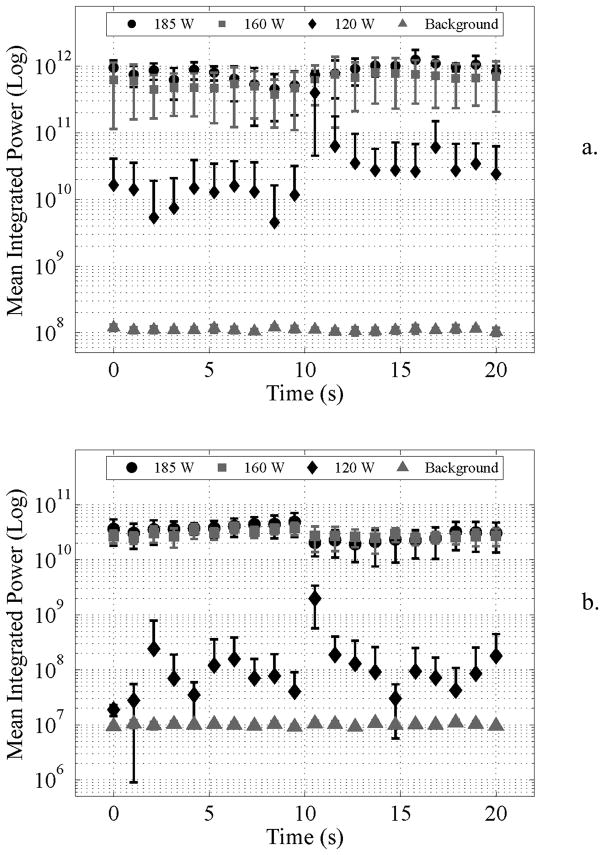
The results for the in vitro cavitation recordings are shown in Fig. 4 (n = 7 per condition). Fig. 4A shows the quantified spectral power for the 1 ms pulse 20 s exposure duration. At 120 W the power fluctuates, but was on average 25.7 dB above the background noise level. At 160 and 185 W cavitation was sustained throughout treatment 37.4 and 38.7 dB above noise levels. Fig. 4B shows the quantified spectral power for 0.1 ms pulses. At 120 W the cavitation power was sporadic, and on average not significantly above background noise. As the transmit power was increased to 160 and 185 W, cavitation was sustained throughout treatment, and was 34.6 and 35.0 dB above the baseline level respectively.
Fig. 4.
Integrated cavitation power (0.45–0.59 MHz) for a) 1 ms pulse and b) 0.1 ms pulse. It was observed that for either pulse length exposures at 160 and 185 W were significantly above background power, whereas at 120 W it was closer to the background level. In a) it was seen that the integrated power fluctuated considerably. Mean and standard deviations are shown and the absence of lower error bars indicates a highly varying quantity whose standard deviation was larger than the mean value.
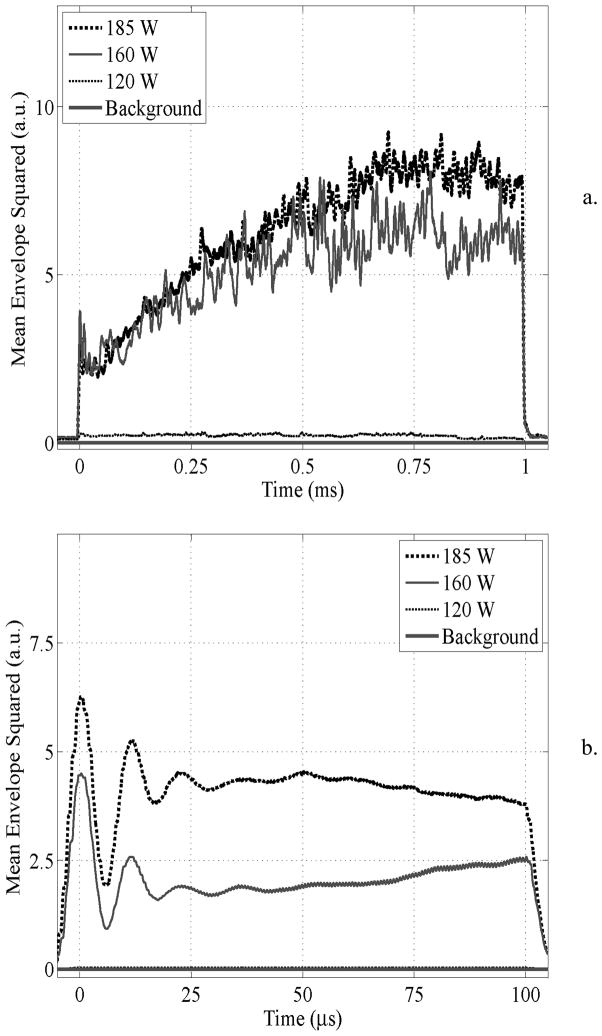
The evolution of the time domain cavitation signal power during an insonation pulse is shown in Fig. 5 (n = 7 clots). For the 1 ms case an increase in power was observed until ~0.7 ms into treatment for the 160 and 185 W pulses. In particular, the cavitation power increased by a factor of 1.7 and 2.7 between the 0.1 and 1.0 ms pulse lengths for the 160 and 185 W cases respectively. For 0.1 ms pulses at 160 and 185 W, the power remains relatively constant throughout its duration after undergoing a transient response at the outset.
Fig. 5.
Time domain cavitation signal power for a) 1 ms and b) 0.1 ms pulses. For the 1 ms pulse duration, power increased over the course of the pulse whereas for 0.1 ms pulses the power reached a plateau.
Clot debris size measurements are presented in terms of particle concentration in Table 3 and in terms of fraction of the original clot material in Table 4. In all cases (160 and 185 W) the total volume fraction of measured debris particles was less than 1.1%. The 1 ms, 185 W treatment resulted in the smallest volume fraction of large (10–60 μm) particle debris (0.31%). In all cases, increasing the pulse length (0.1 vs. 1 ms), power (160 vs. 185 W) or exposure duration (5 vs. 20 s) resulted in statistically significant reductions in the number of particle sizes above 10 μm. In particular, the p-values were less than 0.01 when comparing these groups, except for the 160 versus 180 W (1 ms, 20 s) and 0.1 vs. 1 ms (160 W, 20 s) cases where p<0.05.
Table 4.
Mean and standard deviation particle volume measurements as a function of treatment and particle size. Results are expressed in terms of the % of the original clot volume.
| Exposure | 2 – 10 μm | 10 – 30 μm | 30 – 60 μm |
|---|---|---|---|
| 1 ms, 185 W, 20 s | 0.79 ± 0.25 | 0.30 ± 0.14 | 0.03 ± 0.01 |
| 1 ms, 185 W, 20 s | 0.32 ± 0.25 | 0.63 ± 0.29 | 0.11 ± 0.05 |
| 1 ms, 185 W, 20 s | 0.57 ± 0.27 | 0.44 ± 0.20 | 0.07 ± 0.05 |
| 1 ms, 185 W, 20 s | 0.48 ± 0.29 | 0.39 ± 0.21 | 0.05 ± 0.03 |
| 0.1 ms, 185 W, 20 s | 0.16 ± 0.12 | 0.37 ± 0.18 | 0.03 ± 0.02 |
| 0.1 ms, 160 W, 20 s | 0.20 ± 0.12 | 0.48 ± 0.19 | 0.06 ± 0.04 |
In vitro flow bypass
Based on the above results, flow bypass experiments were conducted using 1 ms, 185 W pulses for 20 s at a 1 Hz PRF. A summary of the results is shown in Table 5 (n = 7 clots). The treatment procedures resulted in the erosion of 99.2% of the clot volume. For the Coulter counter measurements (<60 μm) a higher proportion of the particles were below 10 μm in size, but the total volume fraction was higher (5.0%) than for the single location experiments. The filtered particle data indicated that 6.5% of the clot debris was above 53 μm, with 1.1% above 500 μm.
Table 5.
Summary of results for residual thrombus and debris sizes, expressed as a percentage of the original clot volume.
| Thrombolysis Efficiency | 53-μm Filter | 200-μm Filter | 500-μm Filter | 2–5 μm | 5–10 μm | 10–30 μm | 30–60 μm |
|---|---|---|---|---|---|---|---|
| 99.2 ± 2.1 | 3.1 ± 1.2 | 2.3 ± 2.0 | 1.1 ± 0.9 | 4.5 ± 1.4 | 0.5 ± 0.4 | 0.09 ± 0.18 | 0.006 ± 0.004 |
In vivo
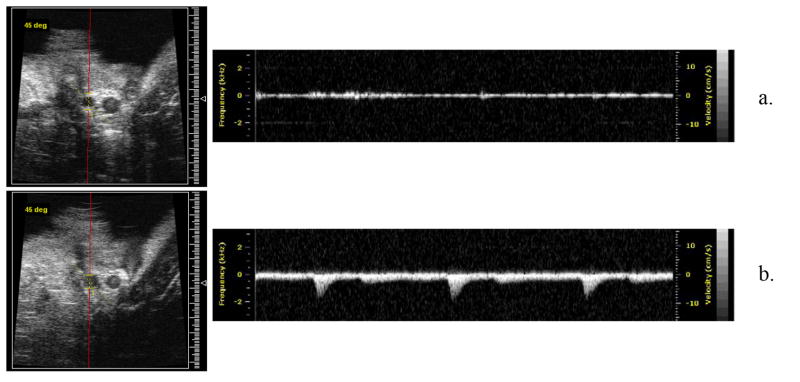
The in vivo results for the 1 ms pulses are summarized in Table 6 (n = 22 rabbits). Guided by the in vitro findings, which revealed that 1 ms pulses, 185 W, 20 s duration created the largest erosion volumes and smallest particle debris of the conditions investigated, these acoustic parameters were initially used when attempting recanalization in vivo. Experiments conducted at 185 W were not successful in restoring flow (0/5). At 215 W, 1 of 2 treatments were successful and at 300 W 5 of 8 were successful. In addition, 7 control clots were employed, none of which recanalized. No HIFU treatment was able to completely resolve the clot to the extent that PWD flow was observed at all locations along the clot. Instead, recanalization was limited to partial flow restoration, meaning that PWD assessment confirmed the presence of flow in least one lateral location at all points along the vessel axis. A representative occluded rabbit femoral artery that has undergone partial flow restoration after HIFU insonation (1 ms, 300 W, 20 s) is shown in Fig. 6. Fig. 6A shows a B-scan image of the occluded artery before treatment initiation, where PWD indicates that no flow was present within the artery. In Fig. 6B, a typical result observed after HIFU treatment is shown where the PWD spectrum shows clear evidence of pulsatile flow within the vessel. In a subset of animals (n = 2) where PWD had indicated that flow restoration had occurred the vessel was cut immediately proximal to the bifurcation point (distal of the original clot region) and bleeding was observed, confirming that the PWD assessment was indicative of flow restoration.
Table 6.
In vivo flow restoration summary
| Treatment | Flow Restoration |
|---|---|
| Control | 0/7 |
| 1 ms, 185 W | 0/5 |
| 1 ms, 215 W | 1/2 |
| 1 ms, 300 W | 5/8 |
Fig. 6.
Example pre- and post-treatment images of HIFU thrombolysis treatment in a rabbit femoral artery. Successful occlusion was initiated with a lack of flow in the artery (top), where the only PWD signal remaining is due to low level tissue clutter. After HIFU treatment (1 ms, 20 s, 215 W) the occlusion had been recanalized, where flow was clearly evident as a pulsatile arterial signal (bottom).
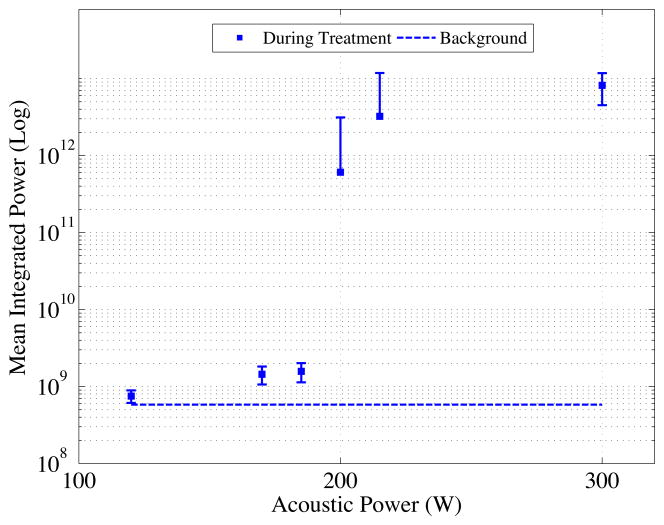
The cavitation power spectra for a 1 ms HIFU insonation as a function of acoustic power in vivo are shown in Fig. 7. In vivo cavitation monitoring was assessed for 18 recordings over 3 animals. From 120 to 170 W small increases in power were observed followed by a steep increase until 215 W and by 300 W the signals were 43 dB above background levels.
Fig. 7.
Integrated cavitation power (0.45–0.59 MHz) from in vivo cavitation recordings. Below 170 W cavitation activity was near baseline. At 200 W a transition was observed and at powers ≥215 W cavitation activity was ~35 dB above baseline is observed. Mean and standard deviations are shown.
Inspections of the exposed femoral arteries following ultrasound exposures indicated that no bleeding occurred for 1 ms pulses for power levels up to 215 W and one minor bleed (of 8 cases) occurred at 300 W. Specifically, a small volume of blood (on the order of 1–2 drops) was observed adjacent to the vessel upon the completion of treatment. When the acoustic gel was removed for further inspection, bleeding ceased within 1–2 minutes. Additional experiments (n = 4, not included in Table 6) were conducted with 10 ms pulses, where at the 215 W power level 3 of 4 vessels exposed exhibited substantial bleeding that persisted after experiments ceased (> 5 minutes) and recanalization experiments were therefore not pursued with this pulse length.
Discussion
The results of this study have shown that stand-alone HIFU thrombolysis is feasible as a means of restoring flow in thrombus occluded arteries in vivo and its effectiveness is highly dependant upon exposure parameters, which are linked directly with levels of induced cavitation.
These and previous results23–26 at 0.5–1 MHz indicate that high intensities are required to perform stand-alone ultrasound thrombolysis. Such exposure conditions can be readily achieved in the context of peripheral vasculature disease. In the case of acute stroke, this will be more challenging due to the distortion of the ultrasound beam by the skull through refraction and phase aberration effects and high levels of attenuation.32 Developments in hemispherical transcranial transducer array approaches have significantly mitigated the defocusing effects of the skull by applying phase and amplitude corrections to array elements and thereby have achieved improved focusing and increased intensity levels.33–37 As the attenuation of ultrasound within the skull increases with frequency, intensity levels will decrease with increasing frequency. While this effect suggests the use of lower frequencies, it must also be considered that bleeding risk increases with decreasing frequency.38 The choice of a 1.5 MHz transmit frequency in the present study was determined in part by the results of a preliminary HIFU thrombolysis study conducted at 0.68 MHz using the same animal model, where the results indicated that flow restoration was possible, but was associated with high rates of bleeding.27 Based on results obtained with a recently reported hemispherical array system,36 which was operated well below its potential maximum power level, it is hypothesized that the required intensity levels for stand-alone transcranial thrombolysis will be viable to achieve at a frequency of 1.5 MHz. A second separate consideration related to frequency is that the size of the focal region will decrease with increasing frequency. In recent work conducted at 0.84 MHz with a novel hemispherical array, we found that ex vivo transcranial ultrasound transmission could produce focal spots with dimensions of 2.6 mm axially and 0.95 mm laterally (−6dB intensity values)36. It is also notable that since cavitation is a pressure threshold dependant phenomenon, it will be possible to make the therapeutically active portion of the focal region smaller than this. Further, as the focal size scales inversely with frequency, it is anticipated that millimeter to sub-millimeter focal regions will therefore be possible to achieve at 1.5 MHz with this technology. With such a level of localization it may be possible to reduce or eliminate the potential for damage to the vascular wall and surrounding tissue by situating the focus within the clot under MRI guidance. This was not the case in the present study, as the axial length of the focus encompassed both clot and vessel wall. This situation is known to lead to vascular damage and has been previously reported for longer pulses that are of sufficiently high amplitude to produce cavitation39,40.
Direct evidence of the link between clot erosion and cavitation was observed, whereby if cavitation was not sustained above a threshold level, clot erosion and flow restoration was not achieved. These results are generally consistent with the evidence from histotripsy studies on other tissue types and with in vitro clots where bubble clouds, as indicated by changes in ultrasound images, have been shown to be present at the transducer focus during tissue erosion.24,25,41,42. They are also consistent with the results reported in43,44 where HIFU was employed below the cavitation threshold, and no clot degradation was observed unless tPA was present. The mechanism for degradation is associated with the violent oscillation of bubbles that are locally formed and then subsequently stimulated to oscillate by the incident high amplitude ultrasound field. These results therefore highlight the need for cavitation monitoring in preclinical studies as well as in a future clinical context.
Although clot erosion was associated with the presence of high levels of cavitation, erosion volumes varied considerably for conditions that met this criterion. The superior performance of the 1 ms relative to the 0.1 ms pulse may be associated with the higher cumulative cavitation levels present over the longer 1 ms pulse (Fig. 3). It should be noted though that previous studies have shown that the capacity of short ‘histotripsy’ pulses (<0.01 ms) to erode tissue is also dependant in a complex manner on the absolute power level45,46, and that such pulses have been shown to be capable of eroding clots25,26. The interval between pulses and a more detailed comparison of these approaches is therefore warranted.
It was observed that a higher level of acoustic power was required to initiate cavitation in vivo than in vitro, and it is hypothesized that this is the reason why flow restoration was not observed in vivo until 215 W. This is likely due to differences in attenuation along the beam path length that will act to reduce the intensity level at the focus.47–49 In vitro experiments were conducted in degassed water which is weakly attenuating (3.7 × 10−5 dB/cm at 1.5 MHz) whereas in vivo experiments were conducted through ~3 cm of muscle tissue (0.54 dB/cm at 1.5 MHz)50.
A significant factor that must be considered with ultrasound thrombolysis is the size and concentration of the resulting clot debris, as this has the potential to block microvessels in distal vascular beds. From this perspective, the resulting debris should ideally undergo hemolysis or be comprised of particles that are sufficiently small to pass through a capillary bed. For the parameters investigated in this paper, longer pulses and higher powers produced a significantly reduced number of larger (10–60 μm) particles, and these comprised less than 0.75% of the volume fraction of the resulting suspension. While particles larger than 60 μm were not explicitly measured, the absence of blockages of the Coulter counter indicated that for the single location experiments, particles were less than 100 μm in size. The flow model, more relevant to in vivo conditions, indicated that larger particles (>53 μm) were present in the resulting debris, though they were still a small fraction of the original clot volume (6.5%). This is significant as this debris will then propagate distally and potentially lodge in smaller vessels thereby blocking a portion of the distal vascular bed. Given that insonation at single locations within the clot appears to produce smaller particle sizes, we hypothesize that the origin of larger particles in the flow model may be associated with when the proximal end of the clot is insonated and fluid breakthrough occurs. This was likely exacerbated by the time duration associated with manual repositioning between locations. In this regard it is useful to note that the eventual application of these approaches will be with array systems, which will have the capability to rapidly scan over the clots may mitigate these effects in future implementations.
This study has a number of limitations. First, the clot model involved an endothelial injury process to promote adherence to the vascular wall and as such may not be representative of embolic clots in stroke that are mechanically lodged within vessels. It is hypothesized that in these latter situations flow restoration may in fact be more readily achieved. It is therefore of interest to conduct experiments with such clot models in the brain51. In the context of these experiments it will be important to perform histologic evaluations for damage of the vessel and surrounding tissue, which were not reported in the present study due to the relative isolation of the vessel from the surrounding tissue and that the vessel itself underwent a degree of damage from the injury process. While the present results indicated that bleeding rates were low for the 1 ms case (1/10), a histologic evaluation of this is required in relevant tissues, such as brain in the context of an acute stroke model. In the context of future animal experiments it will be of particular interest to conduct work in large animal (e.g. pig) acute stroke models using transcranial hemispherical array technology under MRI guidance, where the reduced focal volume size relative to that of the target vessels may reduce or eliminate the potential for bleeding. Further, while Doppler ultrasound is an established approach for assessing blood flow in larger vessels it will be important to employ other imaging methods such as angiography and MRI in future stroke work. Angiography was not employed in the present study as it would require the local release of contrast with a catheter situated within the femoral artery, which in turn would necessitate the use of heparin, which was not compatible with the type of in situ clots employed here. MRI approaches will be particularly useful in future stroke experiments to assess flow restoration and reperfusion. In such experiments, reperfusion measurements will reflect not only flow restoration due to recanalization of the original occluded artery, but may also give insight into the potential effects of emboli propagating to the distal vascular bed, which were not assessed here. Finally, this study has focused upon the feasibility of using HIFU to dissolve clots and has made no comparisons with established tPa approaches or with developing and promising low intensity ultrasound approaches to potentiate tPa with or without microbubbles. It is in this context that the success rate and degree of flow restoration needs to be evaluated. Ultimately, the determination of the clinical viability of this approach relative to others will require the detailed comparative assessment of the lytic effectiveness (speed and degree of recanalization), emboli effects, the safety to both the target vessel and surrounding tissue, and issues relating to ease of clinical use.
Table 3.
Mean and standard deviation particle concentration measurements as a function of treatment and particle size for the single focal location experiments.
| Exposure | 2 – 10 μm [107] | 10 – 30 μm [106] | 30 – 60 μm [104 ] |
|---|---|---|---|
| 1 ms, 185 W, 20 s | 15.1 ± 5.3 | 1.7 ± 0.8 | 1.0 ± 0.5 |
| 1 ms, 185 W, 20 s | 6.4 ± 4.7 | 2.9 ± 1.3 | 3.0 ± 2.1 |
| 1 ms, 185 W, 20 s | 10.8 ± 6.1 | 3.4 ± 1.6 | 3.0 ± 2.4 |
| 1 ms, 185 W, 20 s | 3.5 ± 0.8 | 2.8 ± 1.6 | 2.0 ± 1.0 |
| 0.1 ms, 185 W, 20 s | 3.4 ± 2.4 | 2.3 ± 1.3 | 2.0 ± 1.0 |
| 0.1 ms, 160 W, 20 s | 4.1 ± 0.3 | 2.6 ± 1.2 | 3.0 ± 2.1 |
Acknowledgments
The funding for this grant was provided by NIH grant number: R01EB009032
References
- 1.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Kirmani JF, Sayed MA, et al. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology. 2005;64:2115–2120. doi: 10.1212/01.WNL.0000165951.03373.25. [DOI] [PubMed] [Google Scholar]
- 3.NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke tPA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Tsuji K, Lee SR, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 5.Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49:93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke - Results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 8.Pfaffenberger S, Devcic-Kuhar B, Kastl SP, et al. Ultrasound thrombolysis. Thromb Haemost. 2005;94:26–36. doi: 10.1160/TH04-12-0818. [DOI] [PubMed] [Google Scholar]
- 9.Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636–2643. [PubMed] [Google Scholar]
- 10.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi F, Blinc A, Braaten J, Francis CW. Ultrasound increases flow through fibrin gels. Thromb Haemost. 1995;73:495–498. [PubMed] [Google Scholar]
- 12.Pfaffenberger S, Devcic-Kuhar B, El-Rabadi K, et al. 2MHz ultrasound enhances t-PA-mediated thrombolysis: comparison of continuous versus pulsed ultrasound and standing versus travelling acoustic waves. Thromb Haemost. 2003;89:583–589. [PubMed] [Google Scholar]
- 13.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 14.Holland CK, Vaidya SS, Datta S, Coussios CC, Shaw GJ. Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thromb Res. 2008;121:663–673. doi: 10.1016/j.thromres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birnbaum Y, Luo H, Nagai T, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug - Recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–134. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- 16.Culp WC, Porter TR, Xie F, et al. Microbubble potentiated ultrasound as a method of declotting thrombosed dialysis grafts: experimental study in dogs. Cardiovasc Intervent Radiol. 2001;24:407–412. doi: 10.1007/s00270-001-0052-4. [DOI] [PubMed] [Google Scholar]
- 17.Nishioka T, Luo H, Fishbein MC, et al. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: An in vitro and in vivo study. J Am Coll Cardiol. 1997;30:561–568. doi: 10.1016/s0735-1097(97)00182-4. [DOI] [PubMed] [Google Scholar]
- 18.Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. Journal of Ultrasound in Medicine. 2001;20:1313–1325. doi: 10.7863/jum.2001.20.12.1313. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Coussios CC, McAdory LE, et al. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32:1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores R, Hennings LJ, Lowery JD, Brown AT, Culp WC. Microbubble-augmented ultrasound sonothrombolysis decreases intracranial hemorrhage in a rabbit model of acute ischemic stroke. Invest Radiol. 2011;46:419–24. doi: 10.1097/RLI.0b013e31820e143a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2011;46:202–7. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina CA, Barreto AD, Tsivgoulis G, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Annals Neurology. 2009;66:28–38. doi: 10.1002/ana.21723. [DOI] [PubMed] [Google Scholar]
- 23.Westermark S, Wiksell H, Elmqvist H, Hultenby K, Berglund H. Effect of externally applied focused acoustic energy on clot disruption in vitro. Clin Sci (Lond) 1999;97:67–71. [PubMed] [Google Scholar]
- 24.Rosenschein U, Furman V, Kerner E, Fabian I, Bernheim J, Eshel Y. Ultrasound imaging-guided noninvasive ultrasound thrombolysis: preclinical results. Circulation. 2000;102:238–245. doi: 10.1161/01.cir.102.2.238. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy - histotripsy. Ultrasound Med Biol. 2009;35:1982–1994. doi: 10.1016/j.ultrasmedbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Xu Z. Noninvasive Treatment of Deep Venous Thrombosis Using Pulsed Ultrasound Cavitation Therapy (Histotripsy) in a Porcine Model. J Vascular Intervention. 2011;22:369–377. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hynynen K, McDannold N. MRI-guided focused ultrasound for local tissue ablation and other image-guided interventions. In: Wu J, Nyborg W, editors. Emerging therapeutic ultrasound. Toh Tuck Link, Singapore: World Scientific Publishing Co. Pte. Ltd; 2006. pp. 192–194. [Google Scholar]
- 28.Hynynen K, Vykhodtseva NI, Chung AH, Sorrentino V, Colucci V, Jolesz FA. Thermal effects of focused ultrasound on the brain: determination with MR imaging. Radiology. 1997;204(1):247–253. doi: 10.1148/radiology.204.1.9205255. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Hulsbeck S, Bangard C, Schwarzenberg H, Gluer CC, Heller M. In vitro effectiveness study of three hydrodynamic thrombectomy devices. Radiology. 1999;211:433–439. doi: 10.1148/radiology.211.2.r99ma06433. [DOI] [PubMed] [Google Scholar]
- 30.Salazar GM, Faintuch S, Gladstone SR, Lang EV. In Vitro Analysis of Downstream Particulates with Mechanical Thrombectomy Devices: Comparison of 20-kHz Sonothrombolytic and Rotating Dispersion Wire Systems. J Vasc Intervent Radiol. 2009;20:634–639. doi: 10.1016/j.jvir.2008.12.416. [DOI] [PubMed] [Google Scholar]
- 31.Gold HK, Yasuda T, Jang IK, Guerrero JL, Fallon JT, Leinbach RC, Collen D. Animal models for arterial thrombolysis and prevention of reocclusion. Erythrocyte-rich versus platelet-rich thrombus. Circulation. 1991;83:IV26–40. [PubMed] [Google Scholar]
- 32.Fry FJ, Barger JE. Acoustical properties of human skull. J Acoust Soc Am. 1978;63:1576–1590. doi: 10.1121/1.381852. [DOI] [PubMed] [Google Scholar]
- 33.Clement GT, Sun J, Giesecke T, Hynynen K. A hemisphere array for non-invasive ultrasound brain therapy and surgery. Phys Med Biol. 2000;45:3707–3719. doi: 10.1088/0031-9155/45/12/314. [DOI] [PubMed] [Google Scholar]
- 34.Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47:1219–1236. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 35.Pernot M, Aubry JF, Tanter M, Thomas JL, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol. 2003;48:2577–2589. doi: 10.1109/TMI.2010.2076829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song J, Hynynen K. Feasibility of using lateral mode coupling method for a large scale ultrasound phased array for noninvasive transcranial therapy. IEEE Trans Biomed Eng. 2010;57:124–33. doi: 10.1109/TBME.2009.2028739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery. 2010;66:323–332. doi: 10.1227/01.NEU.0000360379.95800.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 39.Hynynen K, Chung AH, Colucci V, Jolesz FA. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol. 1996;22:193–201. doi: 10.1016/0301-5629(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 40.Hynynen K, Colucci V, Chung A, Jolesz F. Noninvasive arterial occlusion using MRI-guided focused ultrasound. 1996;22:1071–1077. doi: 10.1016/s0301-5629(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 41.Xu Z, Hall TL, Fowlkes JB, Cain CA. Optical and acoustic monitoring of bubble cloud dynamics at a tissue-fluid interface in ultrasound tissue erosion. J Acoust Soc Am. 2007;121:2421–2430. doi: 10.1121/1.2710079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Raghavan M, Hall TL, et al. High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy--histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:2091–2101. doi: 10.1109/TUFFC.2007.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frenkel V, Oberoi J, Stone MJ, et al. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model. Radiology. 2006;239:86–93. doi: 10.1148/radiol.2391042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone MJ, Frenkel V, Dromi S, et al. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res. 2007;121:193–202. doi: 10.1016/j.thromres.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006;32:115–129. doi: 10.1016/j.ultrasmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: The role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am. 2005;117:424–435. doi: 10.1121/1.1828551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muir TG, Carstensen EL. Prediction of non-linear acoustic effects at biomedical frequencies and intensities. Ultrasound Med Biol. 1980;6:345–357. doi: 10.1016/0301-5629(80)90004-6. [DOI] [PubMed] [Google Scholar]
- 48.Hynynen K. The role of nonlinear ultrasound propagation during hyperthermia treatments. Med Phys. 1991;18:1156–1163. doi: 10.1118/1.596626. [DOI] [PubMed] [Google Scholar]
- 49.Starritt HC, Duck FA, Humphrey VF. Forces acting in the direction of propagation in pulsed ultrasound fields. Phys Med Biol. 1991;36:1465–1474. doi: 10.1088/0031-9155/36/11/006. [DOI] [PubMed] [Google Scholar]
- 50.Duck FA. Physical properties of tissue: a comprehensive reference book. London: Academic Press Ltd; 1990. [Google Scholar]
- 51.Chapman DF, Lyden P, Lapchak PA, et al. Comparison of TNK with wild-type tissue plasminogen activator in a rabbit embolic stroke model. Stroke. 2001;32:748–752. doi: 10.1161/01.str.32.3.748. [DOI] [PubMed] [Google Scholar]