Abstract
Objective
Nosocomial infection / sepsis occurs in up to 40% of children requiring long stay intensive care. Zinc, selenium, glutamine, metoclopramide (a prolactin secretalogue), and or whey protein supplementation have been effective in reducing infection and sepsis in other populations. We evaluated whether daily nutriceutical supplementation with zinc, selenium, glutamine, and metoclopramide, compared to whey protein would reduce the occurrence of nosocomial infection / sepsis in this at-risk population.
Design
Randomized double blinded comparative effectiveness trial.
Setting
Eight pediatric intensive care units in the NICHD Collaborative Pediatric Critical Care Research Network.
Patients
Two hundred and ninety three long stay intensive care patients (age 1–17 years) expected to require more than 72 hours of invasive care.
Interventions
Patients were stratified according to immunocompromised status and center and then randomly assigned to receive daily enteral zinc, selenium, glutamine and IV metoclopramide (n = 149 ZSGM), or daily enteral whey protein (n = 144 WHEY) and IV saline, for up to 28 days of intensive care unit stay. The primary endpoint was time to development of nosocomial sepsis / infection. The analysis was intention to treat.
Measurements and Main Results
There were no differences by assigned treatment in the overall population with respect to time until the first episode of nosocomial infection / sepsis (median WHEY 13.2 days vs ZSGM 12.1 days, p=0.29 by log rank test) or the rate of nosocomial infection / sepsis (4.83/100 days WHEY vs. 4.99/100 days ZSGM, p = 0.81). Only 9% of the 293 subjects were immunocompromised and there was a reduction in rate of nosocomial infection / sepsis with ZSGM in this immunocompromised group (6.09/100 days WHEY vs 1.57/100 days ZSGM, p value = 0.011).
Conclusions
Compared with WHEY supplementation, ZSGM conferred no advantage in the immunecompetent population. Further evaluation of ZSGM supplementation is warranted in the immunocompromised long stay PICU patient.
Keywords: Whey protein, zinc, selenium, glutamine, prolactin, nosocomial infection / sepsis
INTRODUCTION
Despite implementation of CDC recommendations and bundled interventions for preventing catheter-associated blood stream infection, ventilator associated pneumonia, and urinary catheter-associated infections, nosocomial infection / sepsis remains a significant cause of morbidity in long stay critically ill children. Critical illness stress induces lymphopenia and lymphocyte dysfunction associated with hypoprolactinemia (1) and deficiencies in zinc and selenium (2, 3) and amino acids (4, 5). Because lymphocyte integrity is important for the immune response to fight infection, standard nutritional practice in critically ill children includes zinc, selenium, and protein. It is unknown whether additional supplementation is needed in this population at risk for stress induced lymphocyte dysfunction and nosocomial infection / sepsis.
Metoclopramide, a prolactin secretalogue, given at the dosage commonly used for gastrointestinal prokinesis maintains prolactin levels in the high normal range in children. In mechanically ventilated adults, metoclopramide delayed time to onset of nosocomial pneumonia by 50%, but had no effect on the rate of nosocomial pneumonia or mortality (6). In malnourished children, zinc supplementation reduced morbidity and mortality with severe pneumonia (7, 8) or diarrhea (9–11), and reduced infectious disease mortality in small for gestational age infants (12). Selenium supplementation (13) or glutamine-enriched enteral nutrition (14) also reduced the risk of nosocomial sepsis in preterm neonates. Enteral glutamine safely maintains TH1 lymphocyte function for bacterial killing (15, 16).
Essential amino acids are also important to overall immune function and lymphocyte function in particular (5). Whey protein provides all the essential amino acids needed to maintain immune function in immune cells. Experimental animal studies show that whey protein supplementation facilitates maturation of the immune system and is protective against rotavirus (17–21). Randomized human clinical studies of whey protein have demonstrated improved lymphocyte function and reduced co-infection in HIV infected children, reduction in infection in severely burned children, and improved immunologic response to immunization in the elderly (22–26).
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) investigators hypothesized that critical illness stress-induced immune suppression (CRISIS) related nosocomial infection / sepsis would be more effectively prevented by prophylactic supplementation of ‘standard’ nutritional practice with added zinc, selenium, glutamine and metoclopramide, (ZSGM) than by prophylactic supplementation with added amino acid (whey protein WHEY). The CPCCRN designed a randomized, double blinded comparative effectiveness trial with the primary hypothesis that daily ZSGM would prolong the time to developing nosocomial infection / sepsis compared to daily WHEY. In this paper we report the results of the CRISIS prevention trial.
MATERIALS AND METHODS
This randomized, double-blinded comparative study was performed on 2 parallel groups of children at eight Pediatric Intensive Care Units (PICU) in the CPCCRN. The Institutional Review Boards of all CPCCRN centers, approved the protocol and informed consent documents. Parental permission was provided for each subject. An independent Data Safety Monitoring Board (DSMB) was appointed by the NICHD, and two interim safety and efficacy analyses were planned. The study was carried out under an Investigational New Drug (IND) application from the Food and Drug Administration (IND 74,500), for which the DCC Principal Investigator (JMD) acted as the sponsor. The study was registered with ClinicalTrials.gov, number NCT00395161.
Patients were eligible for enrollment if they: 1) were greater than 1 year and less than 18 years of age; 2) were within the first 48 hours of PICU admission; 3) had an endotracheal tube, central venous catheter (new or old, tunneled or not tunneled), or urinary catheter; 4) were anticipated to have an indwelling arterial or venous catheter for blood sampling during the first three days of study enrollment, and 5) were anticipated to have venous access and an enteral feeding tube for the administration of study drugs.
Patients were excluded from enrollment if they: 1) had a known allergy to metoclopramide; 2) were expected to have planned removal of endotracheal tube, central venous, and urinary catheters, within 72 hours after study enrollment, 3) had suspected intestinal obstruction, 4) had intestinal surgery or bowel disruption, 5) had other contraindications to the enteral administration of drugs or nutrients, 6) received chronic metoclopramide therapy prior to enrollment, 7) had a known allergy to whey (cow’s milk) or soy based products, 8) had been discharged from the PICU in the previous 28 days, 9) had been previously enrolled in this study, or 10) had a positive pregnancy test. Patients were also excluded if their parents indicated a lack of commitment to aggressive intensive care therapies.
The FDA required an interim analysis review by the DSMB before authorizing enrollment of infants younger than 1 year. After the first interim analysis, the DSMB deferred this authorization until a second interim analysis could be reviewed. At the time of the second review, the trial was terminated for futility. Therefore, no infants younger than 1 year were enrolled in the trial. The study commenced in April 2007 and terminated in November 2009.
After informed consent was obtained from parents, subjects were randomized by telephone according to an a priori design using randomized blocks of variable length, stratified according to center and immunocompromised status. Immunocompromised status was defined by the known presence of AIDS, cancer, transplantation, primary immune deficiency, or chronic immune suppressant therapy. Children were randomized in a 1:1 ratio into the two arms of the trial in these stratified groups. All patients, medical and nursing staffs, clinical site monitors, and most DCC staff remained blinded throughout the study period. The DCC biostatistician (RH) prepared DSMB reports and reviewed results in the two arms, but remained blinded to actual group assignment throughout the study period. Central and clinical site research pharmacists and the pharmacy site monitor were unblinded throughout the study.
Subjects were randomized to receive enteral whey protein powder and IV saline (“WHEY” group) or enteral zinc, selenium, and glutamine and IV metoclopramide (“ZSGM” group). Subjects assigned to the WHEY group received 0.3 g/kg Beneprotein™ each morning, and IV saline every 12 hours. Subjects assigned to the ZSGM group received zinc (20 mg), selenium (40 μg age 1–3, 100 μg age 3–5, 200 μg age 5–12, 400 μg adolescent) and glutamine (0.3 g/kg) each morning, and IV metoclopramide (0.2 mg/kg, maximum 10 mg) every 12 hours. All study drugs were shipped from a central pharmacy (University of Utah) and dispensed by site research pharmacists. Subjects received study drug until discharge from the PICU, or for 28 days from the time of randomization, whichever occurred earlier. Enteral drugs were administered by feeding tube, and discontinued if the feeding tube was removed or if contraindications to enteral feeding developed during the study. Intravenous drugs were discontinued if the IV was removed.
The hypothesis of the CRISIS Prevention Trial was that daily prophylaxis with enteral zinc, selenium, and glutamine, and intravenous metoclopramide would delay the time (hours) between admission to the PICU and occurrence of nosocomial infection / clinical sepsis in PICU patients who have endotracheal tubes, central venous catheters, or urinary catheters. Nosocomial events were defined as occurring at least 48 hours after PICU admission, during the hospital stay until 5 days after discharge from the PICU; for children remaining in the PICU for more than 28 days after randomization, events were counted until day 33. The study protocol required that patients be randomized within 48 hours of PICU admission and that study drug administration begin within 72 hours of PICU admission. According to Center for Disease Control (CDC) definitions Clinical sepsis occurs when patients older than 1 year develop fever (≥38 degrees centigrade), hypotension (≤ 90 mm Hg), or oliguria (≤ 20 cc/hr) and a clinician initiates antibiotic therapy with no positive microbiological evidence and no other recognized cause. Nosocomial infection occurs when microbiologically (culture, antigen, PCR, or antibody) proven infection is observed in a patient with fever, hypothermia, chills or hypotension. The treatment arm blinded CPCCRN investigators adjudicated the presence or absence of a nosocomial clinical sepsis or infection event for every subject. Each case was reviewed independently by two investigators and presented in detail so that consensus for all outcomes was attained. All participants in this adjudication process were blinded to treatment arm through the study period.
Secondary outcome variables of this study included the rate of nosocomial infection / sepsis per 100 PICU days, number of antibiotic-free days, incidence of prolonged lymphopenia (ALC ≤ 1,000 mm3 for ≥7 days), serum prolactin, zinc, and selenium levels before treatment and after 7 days of treatment, and the safety indicators 28 day mortality and adverse events. Serum zinc and selenium levels were classified as deficient if they were below the pediatric reference ranges of the core laboratory. Zinc deficiency was defined as a level <0.60 μg/mL in children aged 10 years or less, and < 0.66 μg/mL in children aged at least 11 years. Selenium deficiency was defined as a level <70 ng/mL in children aged 10 years or less, and <95 ng/mL in children aged at least 11 years. Prolactin deficiency was defined as a level of <3 ng/mL across all ages. Glutamine levels were not measured because they are not considered indicative of total body reserves.
The sample size was calculated to provide 90% power to detect an inverse hazard rate of 1.5, using a two-sided non-parametric test (logrank test) with Type I error (alpha) of 0.05. This required accrual of subjects until 263 had nosocomial events; the estimated total sample size was 600 subjects based on initial event rate estimates. A logrank test with a two-sided alpha = 0.05, stratified by immune compromised status, was used to compare the primary endpoint of freedom from nosocomial infection or sepsis (from time of admission to the PICU until up to five days following discharge from the PICU) between treatment arms. Outcome rates over time are presented using Kaplan-Meier freedom from event curves. In the subgroup of immunocompromised WHEY patients, median time to nosocomial infection / sepsis was derived at the 50.5% event free time point.
In pre-specified analyses complementary to the primary analysis, rates of infection were analyzed using Poisson regression analyses, which count multiple events for a single subject. Additionally, numbers and proportions of antibiotic free days during the PICU stay were compared between study arms using the Wilcoxon rank-sum test. Incidence of prolonged lymphopenia and all-cause mortality and adverse events at 28 days after randomization were analyzed using the χ2 test or exact analogues when numbers of events were small. Four ZSGM-assigned subjects (one of whom received no study treatment) had unknown 28-day survival status.
Five factors (immunocompromised status, postoperative status, gender, race/ethnicity, and center) were prespecified for subgroup analysis, and the DSMB subsequently added another factor (infection or sepsis at study entry). The intention to treat approach was used for all study analyses of efficacy. Analysis by treatment received was performed for the safety outcomes of mortality and occurrence of adverse events.
RESULTS
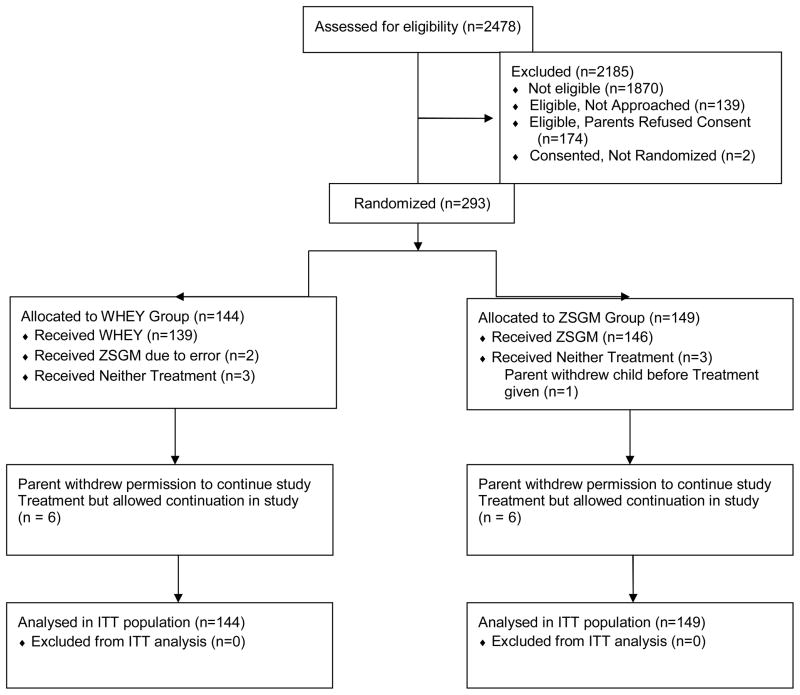
We enrolled 293 subjects (Figure 1). Enrollment was terminated for futility after the second interim analysis indicated that the conditional power to determine a beneficial effect by ZSGM, compared to the WHEY therapy, was < 10%. Figure 1 shows the screening, enrollment, randomization, and study completion results.
Figure 1.
Screening, enrollment, randomization, and study completion.
Table 1 shows the epidemiologic and clinical characteristics of the study population at the time of enrollment in both treatment arms. The median age of children was 7 years, and less than 10% were immunocompromised on entry. Baseline characteristics were equally distributed between the study arms. Among patients assigned to WHEY, 46.5% received parenteral nutrition and 89.6% received enteral nutrition, compared to 43.0% and 90.6% of patients assigned to ZSGM. The proportion of patients who were NPO during one or more PICU study days was 55% in the WHEY arm and 53% in the ZSGM arm with the average proportion of PICU study days being NPO at 14% and 13%, respectively.
Table 1.
Epidemiologic and Clinical Characteristics at Admission
| Factor | WHEY Group N=144 |
ZSGM Group N=149 |
|---|---|---|
|
| ||
| Age in years (median, range) | 7.1 (1–17) | 7.0 (1–17) |
|
| ||
| Female (%) | 55 | 46 |
|
| ||
| PELOD (median; range) | 11 (0–40) | 11 (0–50) |
| PRISM (median; range) | 8 (0–34) | 7 (0–31) |
| OFI (median; range) | 2 (0–5) | 2 (0–6) |
|
| ||
| Immunocompromised (%) | 8 | 9 |
|
| ||
| Postoperative PICU Admit (%) | 28 | 26 |
|
| ||
| Primary Diagnosis (%) | ||
| Asthma | 3 | 1 |
| Cancer | 3 | 1 |
| Cardiac Arrest | 3 | 3 |
| Cardiovascular Disease | 7 | 5 |
| Pneumonia/Bronchiolitis | 22 | 15 |
| Seizures | 3 | 5 |
| Sepsis | 7 | 8 |
| Shock | 3 | 5 |
| Trauma | 17 | 23 |
| HIV | 0 | 1 |
| Hypoxic-ischemic encephalopathy | 1 | 1 |
| Intoxication | 1 | 0 |
| Meningitis | 1 | 2 |
| Transplant | 1 | 0 |
| Other | 28 | 29 |
| Chronic Diagnoses (%) | 49 | 48 |
|
| ||
| Malnutrition (reported as Primary or Secondary Diagnosis) (%) | 1 | 0 |
|
| ||
| Infection Status at Entry (%) | ||
| Existing Infection | 32 | 37 |
| Existing Sepsis | 33 | 29 |
| No Infection or Sepsis | 35 | 34 |
|
| ||
| Existing Lymphopenia (ALC ≤ 1,000/mm3) | 40% (N=114) | 37% (N=126) |
|
| ||
| Baseline Core Laboratory Data (%) | ||
| Zinc Deficiency | 79% (N=138) | 89% (N=141) |
| Prolactin Deficiency | 23% (N=133) | 15% (N=134) |
| Selenium Deficiency | 57% (N=138) | 55% (N=140) |
PELOD – Pediatric Logistic Organ Dysfunction; PRISM – Pediatric Risk of Mortality; OFI-Organ Failure Index; HIE – hypoxic ischemic encephalopathy;
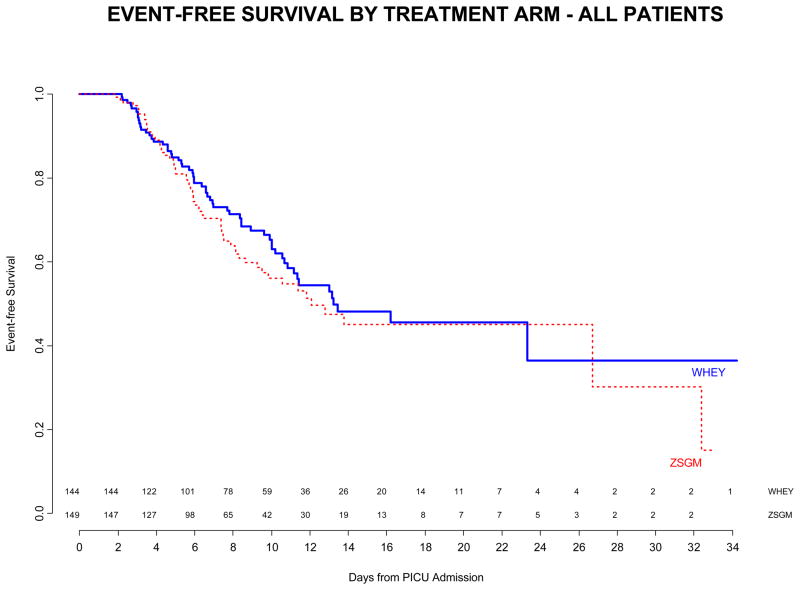
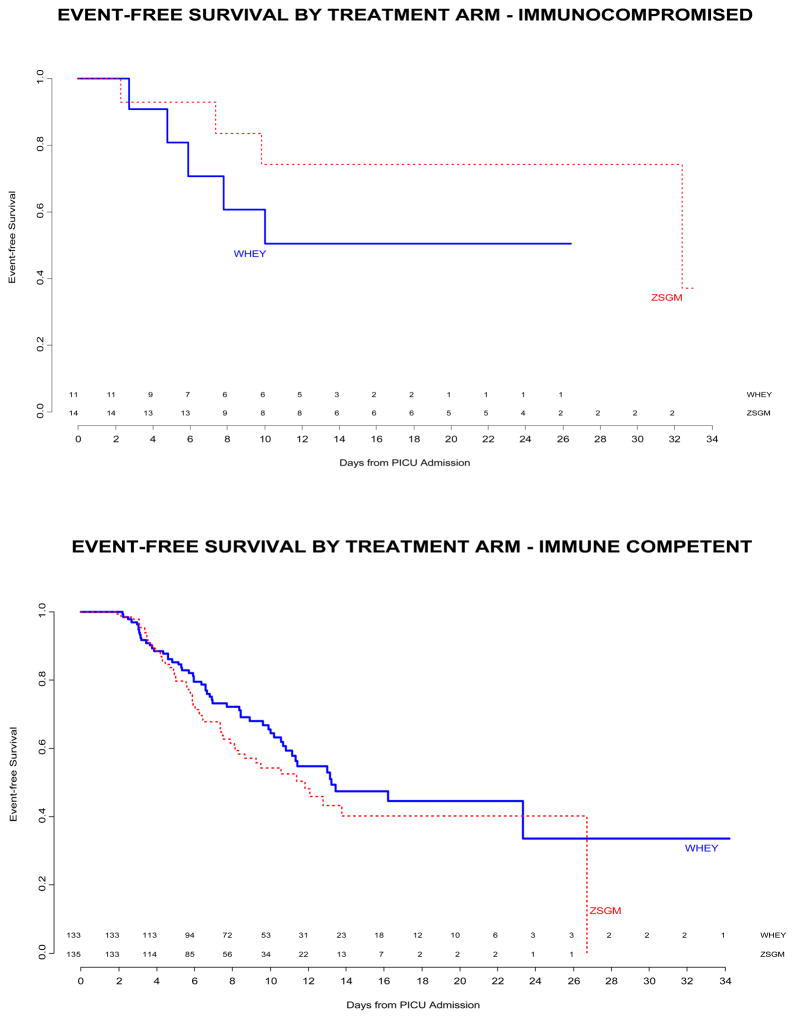
Treatment with ZSGM did not delay the time until nosocomial infection / sepsis compared to treatment with WHEY (median time WHEY 13.2 days vs ZSGM 12.1 days, log rank p=0.29, Figure 2 Top Panel). The median PICU stay was 10 days. Of subjects at risk for an event, approximately 50% in each treatment arm were event-free at 14 days after PICU admission. The effect of immunocompromised status on time to nosocomial infection / sepsis was not significant (median time in immunocompromised patients WHEY 10 days vs ZSGM 32.4 days, and median time in immune competent patients WHEY 13.2 days vs ZSGM 11.8 days; p=0.12 for interaction between treatment group and immunocompromised status in the time-to-event analysis, Figure 2 Middle and Bottom Panels). Other subgroup factors examined were not significantly associated with the primary endpoint.
Figure 2.
Top Panel - Freedom from nosocomial sepsis according to assigned treatment for all randomized patients. Numbers above the horizontal time axis denote number of patients remaining at risk at each time point. p=0.29 for logrank test comparing curves between study arms, stratified by immune competent status. Middle Panel - Freedom from nosocomial infection / sepsis according to assigned treatment for patients immunocompromised at study entry. Numbers above the horizontal time axis denote number of patients remaining at risk at each time point. p=0.24 for logrank test comparing curves between study arms. Lower Panel - Freedom from nosocomial infection / sepsis according to assigned treatment for patients who were immune competent at study entry. Numbers above the horizontal time axis denote number of patients remaining at risk at each time point. p=0.16 for logrank test comparing curves between study arms.
There was no difference in the rate of nosocomial infection or clinical sepsis per 100 PICU days between the ZSGM and WHEY groups (Table 2, p=0.81). Examining study days in the PICU, median number of antibiotic-free days (2 vs 1, p = 0.09) and proportion of days (17% vs 10%, p = 0.19) did not differ between subjects assigned to WHEY versus ZSGM. There was no significant difference in the incidence of prolonged lymphopenia (ALC ≤ 1,000 mm3 for ≥7 days) between subjects assigned to WHEY 12/144 (8.3%) versus ZSGM 5/149 (3.4%), p = 0.07. In the study population, 41% receiving WHEY and 42% receiving ZSGM developed nosocomial infection or sepsis. Approximately one third of patients developed nosocomial infection and one fifth developed sepsis. Days of invasive lines, urinary catheterization, and mechanical ventilation, and rates of site-specific infections based on denominators of ventilator days, urinary catheter days, and central venous catheter days were not significantly different between the treatment arms (Table 2). Distribution of events, sites of infection, and infecting organisms were also generally similar between the treatment groups (Table 3).
Table 2.
Rates of nosocomial infection/sepsis, days of invasive lines, urinary catheterization and mechanical ventilation by treatment group
| Variable | WHEY Group N=144 |
ZSGM Group N=149 |
p-value |
|---|---|---|---|
|
| |||
| Total Number of Events (Infection or Sepsis) | 116 | 110 | |
| Total Number of PICU Days | 1993 | 1793 | |
| Total Number of Study Daysa | 2402 | 2205 | |
| Mean No. of Events/Patient/100 Study Days (95% Confidence Interval) | 4.83 (4.01,5.77) | 4.99 (4.12,5.99) | 0.81 |
|
| |||
| Therapeutic Risk Factors | |||
| Days in PICU (mean/median) | 13.8/11 | 12/9 | 0.16 |
| Ventilator Days (mean/median) | 9.4/6 | 7.9/5 | 0.13 |
| Central Venous Catheter Days (mean/median) | 10.2/7 | 9.1/7 | 0.49 |
| Endotracheal Tube Days (mean/median) | 8.8/6 | 7.3/5 | 0.14 |
| Urinary Catheter Days (mean/median) | 8.0/6 | 6.9/5 | 0.54 |
|
| |||
| Total Number of Ventilator Days | 1352 | 1171 | |
| Total Number of Respiratory Infections | 43 | 53 | |
| Mean No. of Respiratory Infections/Patient/100 Ventilator Days (95% Confidence Interval) | 3.18 (2.33,4.24) | 4.53 (3.43,5.87) | 0.08 |
|
| |||
| Total Number of Urinary Catheter Days | 1155 | 1023 | |
| Total Number of Urinary Tract Infections | 12 | 8 | |
| Mean No. of Urinary Tract Infections/Patient/100 Urinary Catheter Days (95% Confidence Interval) | 1.04 (0.57,1.76) | 0.78 (0.37,1.48) | 0.54 |
|
| |||
| Total Number of Central Venous Catheter Days | 1465 | 1353 | |
| Total Number of Bacteremia Infections | 11 | 11 | |
| Mean No. of Bacteremia Infections/Patient/100 Central Venous Catheter Days (95% Confidence Interval) | 0.75 (0.40,1.30) | 0.81 (0.43,1.41) | 0.85 |
Study days=days in PICU plus additional days after PICU discharge that patient was followed for events(5 days unless patient was discharged from hospital earlier).
Table 3.
Nosocomial infection/sepsis, and sites of nosocomial infection by treatment group
| Variable | WHEY Group N=144 |
ZSGM Group N=149 |
|---|---|---|
|
| ||
| Patients with Events | ||
| One or More Events (%) | 41 | 42 |
| Nosocomial Infection (%) | 31 | 35 |
| Nosocomial Sepsis (%) | 22 | 17 |
|
| ||
| Total Number of Infections | 73 | 83 |
|
| ||
| Site of Infection | ||
| Lower Respiratory | 41 | 52 |
| Upper Respiratory | 2 | 1 |
| Urinary Tract | 12 | 8 |
| Skin or Soft Tissue | 6 | 6 |
| Bacteremia | 11 | 11 |
| Other | 1 | 5 |
|
| ||
| Total Number of Infecting Organisms | N=100 | N=107 |
| Fungi | 16 | 21 |
| Candida albicans | 6 | 4 |
| Candida tropicalis | 4 | 3 |
| Yeast | 0 | 7 |
| Candida glabrata | 1 | 3 |
| Candida lusitanae | 2 | 2 |
| Other | 3 | 2 |
| Gram-Negative Bacilli | 43 | 40 |
| Pseudomonas aeruginosa | 14 | 14 |
| Haemophilus influenzae | 5 | 8 |
| Stenotrophomonas maltophilia | 4 | 3 |
| Enterobacter cloacae | 4 | 2 |
| Klebsiella pneumoniae | 3 | 3 |
| Other | 13 | 10 |
| Gram-Positive Bacilli | 1 | 1 |
| Clostridium dificile | 1 | 1 |
| Gram-Negative Cocci | 2 | 2 |
| Moraxella catarrhalis | 2 | 2 |
| Gram-Positive Cocci | 35 | 37 |
| Staphylococcus aureus | 13 | 18 |
| Staphylococcus coagulase negative | 1 | 8 |
| Enterococcus faecalis | 2 | 2 |
| Staphylococcus Epidermidis | 3 | 1 |
| Other | 16 | 8 |
| Virus | 2 | 3 |
| Undetermined | 1 | 3 |
In the immunocompromised population the rate of nosocomial infection / sepsis was reduced in the ZSGM group compared with the WHEY group (unadjusted p = 0.006 for interaction between treatment group and immunocompromised status; Table 4). The causes for immune compromise in WHEY compared to the ZSGM groups were Bone Marrow Transplant 2 vs 3, other organ Transplant 5 vs 1, Cancer 1 vs 3, Human Immunodeficiency Virus 0 vs 1, severe neutropenia 1 vs 1, chronic high dose steroids/immune suppressants 1 vs 3, congenital immunodeficiency 1 vs 1, and therapeutic hypothermia 0 vs 1.
Table 4.
Rates of nosocomial infection/ sepsis /100 days by treatment group and immunocompromised status
| IMMUNOCOMPROMISED PATIENTS
| |||
|---|---|---|---|
| Variable | WHEY Group N=11 |
ZSGM Group N=14 |
p-value |
|
| |||
| Total Number of Events (Infection or Sepsis) | 12 | 4 | |
| Total Number of PICU Days | 165 | 217 | |
| Total Number of Study Days | 197 | 255 | |
| Mean No. of Events/Patient/100 Study Days (95% Confidence Interval) | 6.09 (3.33, 10.32) | 1.57 (0.53,3.73) | 0.011 |
| IMMUNE COMPETENT PATIENTS
| |||
|---|---|---|---|
| Variable | WHEY Group N=133 |
ZSGM Group N=135 |
p-value |
|
| |||
| Total Number of Events (Infection or Sepsis) | 104 | 106 | |
| Total Number of PICU Days | 1828 | 1576 | |
| Total Number of Study Days | 2205 | 1950 | |
| Mean No. of Events/Patient/100 Study Days (95% Confidence Interval) | 4.72 (3.87,5.69) | 5.44 (4.47,6.55) | 0.30 |
Among subjects with available baseline values, zinc deficiency was present at baseline in 235/280 (84%), selenium deficiency in 156/278 (56%), and prolactin deficiency in 68/284 (24%) (Table 1). Among WHEY and ZSGM subjects, standard of care included the common use of zinc (44% vs 39%), selenium (40% vs 38%), glutamine (8% vs 7%), primarily as part of routine TPN, and metoclopramide (3% vs 5%) for facilitation of nasoduodenal tube placement or gastroesophageal reflux, respectively. Seven day levels of all three measures were significantly higher in ZSGM patients than WHEY patients, and change from baseline was also higher (p < 0.001 for all six comparisons). At seven days, zinc deficiency was present in 19/83 (23%) ZSGM vs 36/80 (45%) WHEY, selenium deficiency in 10/84 (12%) ZSGM vs 23/80 (29%) WHEY, and prolactin deficiency in 3/84 (4%) ZSGM vs 14/81 (17%) WHEY. Controlling for presence of baseline deficiencies, ZSGM subjects with seven-day measures showed significantly lower seven-day deficiency rates compared to WHEY subjects for zinc (p=0.001 by Cochran-Mantel-Haenszel test), selenium (p=0.009), and prolactin (p=0.014).
Overall 28 day mortality was 8.1% among the 284 children who received WHEY or ZSGM and had known 28-day status. There was no significant difference in 28 day mortality by treatment received between WHEY and ZSGM (8/139 [5.8%] vs 15/145 [10.3%], p = 0.16). Among the 287 children receiving treatment, there were 2624 adverse events, including 113 serious adverse events with no significant differences by treatment received for WHEY and ZSGM. Among 139 subjects receiving only WHEY treatment, adverse events were reported in 126 (90.6%) and serious adverse events in 37 (26.6%) while among 148 subjects receiving ZSGM regimen, adverse events were reported in 135 (91.2%) and serious adverse events in 39 (26.4%). There were also no differences in specific adverse events including diarrhea (WHEY 12.2% vs ZSGM 12.2%), dysrhythmias (arrhythmia, extrasystole, nodal rhythm; WHEY 4.3% vs ZSGM 4.1%), and abnormal movement (akathisia, choreoathetosis, dyskinesia, dystonia; WHEY 2.9% vs ZSGM 2.0%).
DISCUSSION
Similar to previous literature we observed that nosocomial infection / sepsis occurred in over 40% of long stay PICU patients with less than 50% of these children being free of nosocomial infection or sepsis at 14 days, and median time to nosocomial infection or sepsis being just short of 14 days (27). Similar to previous reports we also found a high incidence of critical illness stress related zinc and selenium deficiency, as well as prolactin deficiency in 24% and lymphopenia in nearly 40% of the patients (1, 28). The observation that nearly 95% of subjects had deficiencies at enrollment supports the study design which used the multimodal ZSGM supplement strategy, and analysed the effects on both the pre-hoc stratified immune competent and immunocompromised populations. We observed more frequent resolution of zinc, selenium, and prolactin deficiencies at seven days with ZSGM but this pharmacokinetic effect was not matched with the hypothesized pharmacodynamic effect. The ZSGM supplement did not prevent persistent lymphopenia or nosocomial infection / sepsis compared with essential amino acid supplementation from whey protein in the overall study population.
We stratified the randomization of patients to nutriceutical treatment arms according to immunocompromised status because we thought it was biologically plausible that the TH2 phenotype dominant immunocompromised group of patients would benefit differentially from ZSGM supplementation. In this regard, we did observe a reduction in the nosocomial infection / sepsis rate with use of ZSGM in this at risk population. Because less than 10% of our general PICU population was immunocompromised, the small sample size leads us to view these findings rather cautiously. Repeated study is needed perhaps in a specialized PICU network with a larger immunocompromised population, or in a PICU network with a larger number of centers to properly assess the significance of this signal.
With regard to limitations, several study design and performance variables require the reader’s consideration. First, this trial was performed in the ‘standard practice’ setting. Protein, zinc, and selenium are an accepted part of ‘standard’ pediatric enteral and parenteral nutrition in the intensive care setting (29, 30) and no effort was made to control this nutritional practice. The study results can not be applied to patients who are without any nutrition in the PICU. Second, our trial compared the effectiveness of two nutriceutical strategies to one another, rather than to placebo. The research planning committee wanted to follow prior study designs of glutamine supplementation in newborns which used single amino acids as ‘placebos’ to address potential criticism that an apparent effect in a glutamine arm could be an effect of protein nutrition rather than of glutamine per se. This rationale is problematic because all amino acids have specific immune cell effects and therefore are not true ‘placebos’.5 Whey protein was the only FDA approved amino acid supplement available to us. Because Whey protein is marketed as immune nutrition, we designed a comparative effectiveness trial rather than a true ‘placebo’ controlled trial. A true placebo arm without any zinc, selenium, or protein was considered outside of human subjects standards. Two ongoing adult trials comparing the use of a dopamine 2 antagonist to placebo in mechanical ventilation; and zinc, selenium, and glutamine supplements to placebo in severe sepsis (NCT0013978, NCT00300391) will give information on the effect of these supplements in the absence of concomitant protein supplementation. Third, approximately ten patients in each treatment arm either did not receive the assigned treatment, or they had their treatments stopped prematurely upon parental request. Post-hoc analysis excluding these patients did not change the overall findings of the study. Fourth, there was a low number of antibiotic free days in the subjects enrolled in either arm of this study. This calls into question whether high antibiotic use diminished any effects of the nutriceuticals. However, post hoc analysis found no association between extent of antibiotic use and evidence of treatment effect.
CONCLUSION
Nosocomial infection and sepsis remains a prevalent public health problem in long stay critically ill children. Implementation of CDC recommended practices is the first step in prevention. Our study was performed upon the premise that evaluation of the comparative effectiveness of prophylactic nutritional support strategies could inform further improvement. The novel multimodal strategy designed and employed to reduce critical illness stress induced zinc, selenium, glutamine, and prolactin deficiencies was successful in part in reversing these deficiencies. It conferred no advantage in nosocomial infection and sepsis prevention in immune competent children compared to whey based amino acid supplementation. Further study of the ability of ZSGM supplementation to prevent nosocomial infection and sepsis in the immunocompromised PICU population is warranted.
Acknowledgments
Members of the CPCCRN participating in this study: Children’s Hospital of Pittsburgh, Pittsburgh, PA: Joseph Carcillo, MD, Michael Bell, MD, Alan Abraham, BA, Annette Seelhorst RN, Jennifer Jones RN; University of Utah (Data Coordinating Center), Salt Lake City, UT: J. Michael Dean, MD, MBA, Jeri Burr, MS, RN-BC, CCRC, Amy Donaldson, MS, Richard Holubkov, PhD, Angie Webster, MStat, Stephanie Bisping, RN, Teresa Liu, MPH, Brandon Jorgenson, BS, Rene Enriquez, BS, Jeff Yearley, BS; Children’s National Medical Center, Washington DC: John Berger, MD, Angela Wratney, MD, Jean Reardon, BSN, RN; Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, MD, Sabrina Heidemann, MD, Maureen Frey, PhD, RN; Arkansas Children’s Hospital, Little Rock, AR: KJS Anand, MBBS, DPhil, Parthak Prodhan, MD, Glenda Hefley, MNSc, RN; Seattle Children’s Hospital, Seattle, WA: Jerry Zimmerman, MD, PhD, David Jardine, MD, Ruth Barker, RRT; Children’s Hospital Los Angeles, Los Angeles, CA: Christopher J.L. Newth, MB, ChB, J. Francisco Fajardo, CLS (ASCP), RN, MD; Mattel Children’s Hospital at University of California Los Angeles, Los Angeles, CA: Rick Harrison, MD; University of Virginia Children’s Hospital, Charlottesville VA: Douglas F. Willson, MD; National Institute of Child Health and Human Development, Bethesda, MD: Carol Nicholson, MD, Tammara Jenkins, MSN RN.
Members of the Data Safety Monitoring Board: Jeffrey R. Fineman, MD, Jeffrey Blumer, PhD, MD, Thomas P. Green, MD, and David Glidden, PhD.
Funding
This work was supported by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS):U10HD050096, U10HD049981, U10HD500009, U10HD049945, U10HD049983, U10HD050012 and U01HD049934.
References
- 1.Felmet KA, Hall MW, Clark RS, et al. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174(6):3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 2.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 3.Stone CA, Kawai K, Kuphra R, Fawzi WW. Role of selenium in HIV infection. Nutr Rev. 2010;68(1):671–681. doi: 10.1111/j.1753-4887.2010.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth E. Immune and cell modulation by amino acids. Clin Nutr. 2007;26(5):535–544. doi: 10.1016/j.clnu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Yin YL, Li D, et al. Amino acids and immune function. Br J Nutr. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 6.Yavagal DR, Karnad DR, Oak JL. Metoclopramide for preventing pneumonia in critically ill patients receiving enteral tube feeding: a randomized controlled trial. Crit Care Med. 2000;28(5):1408–1411. doi: 10.1097/00003246-200005000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet. 2004;363(9422):1683–1688. doi: 10.1016/S0140-6736(04)16252-1. [DOI] [PubMed] [Google Scholar]
- 8.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 9.Baqui AH, Black RE, El Arifeen S, et al. Zinc therapy for diarrhoea increased the use of oral rehydration therapy and reduced the use of antibiotics in Bangladeshi children. J Health Popul Nutr. 2004;22(4):440–442. [PubMed] [Google Scholar]
- 10.Bhatnagar S, Bahl R, Sharma PK, et al. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2004;38(1):34–40. doi: 10.1097/00005176-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Raqib R, Roy SK, Rahman MJ, et al. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am J Clin Nutr. 2004;79(3):444–450. doi: 10.1093/ajcn/79.3.444. [DOI] [PubMed] [Google Scholar]
- 12.Sazawal S, Black RE, Menon VP, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics. 2001;108(6):1280–1286. doi: 10.1542/peds.108.6.1280. [DOI] [PubMed] [Google Scholar]
- 13.Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003;4:CD003312. doi: 10.1002/14651858.CD003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Berg A, van Elburg RM, Westerbeek EA, et al. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: a randomized controlled trial. Am J Clin Nutr. 2005;81(6):1397–1404. doi: 10.1093/ajcn/81.6.1397. [DOI] [PubMed] [Google Scholar]
- 15.Boelens PG, Houdijk AP, Fonk JC, et al. Glutamine-enriched enteral nutrition increases in vitro interferon-gamma production but does not influence the in vivo specific antibody response to KLH after severe trauma. A prospective, double blind, randomized clinical study. Clin Nutr. 2004;23(3):391–400. doi: 10.1016/j.clnu.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Yalcin SS, Yurdakok K, Tezcan I, et al. Effect of glutamine supplementation on diarrhea, interleukin-8 and secretory immunoglobulin A in children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2004;38(5):494–501. doi: 10.1097/00005176-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review) J Nutr Biochem. 2003;14(5):251–258. doi: 10.1016/s0955-2863(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 18.Low PP, Rutherford KJ, Gill HS, et al. Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice. Int Immunopharmacol. 2003;3(3):393–401. doi: 10.1016/S1567-5769(02)00297-7. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Cano FJ, Marin-Gallen S, Castell M, et al. Supplementing suckling rats with whey protein concentrate modulates the immune response and ameliorates rat rotavirus-induced diarrhea. J Nutr. 2008;138(12):2392–2398. doi: 10.3945/jn.108.093856. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Cano FJ, Marin-Gallen S, Castell M, et al. Bovine whey protein concentrate supplementation modulates maturation of immune system in suckling rats. Br J Nutr. 2007;98 (Suppl 1):S80–84. doi: 10.1017/S0007114507838074. [DOI] [PubMed] [Google Scholar]
- 21.Wong CW, Watson DL. Immunomodulatory effects of dietary whey proteins in mice. J Dairy Res. 1995;62(2):359–368. doi: 10.1017/s0022029900031058. [DOI] [PubMed] [Google Scholar]
- 22.Micke P, Beeh KM, Buhl R. Effects of long-term supplementation with whey proteins on plasma glutathione levels of HIV-infected patients. Eur J Nutr. 2002;41(1):12–18. doi: 10.1007/s003940200001. [DOI] [PubMed] [Google Scholar]
- 23.Micke P, Beeh KM, Schlaak JF, et al. Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest. 2001;31(2):171–178. doi: 10.1046/j.1365-2362.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- 24.Moreno YF, Sgarbieri VC, da Silva MN, et al. Features of whey protein concentrate supplementation in children with rapidly progressive HIV infection. J Trop Pediatr. 2006;52(1):34–38. doi: 10.1093/tropej/fmi074. [DOI] [PubMed] [Google Scholar]
- 25.Alexander JW, MacMillan BG, Stinnett JD, et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg. 1980;192(4):505–517. doi: 10.1097/00000658-198010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman SL, Fisher L, German JB, et al. Dairy proteins and the response to pneumovax in senior citizens: a randomized, double-blind, placebo-controlled pilot study. Ann N Y Acad Sci. 2010;1190(1):97–103. doi: 10.1111/j.1749-6632.2009.05264.x. [DOI] [PubMed] [Google Scholar]
- 27.Leclerc F, Leteutre S, Duhamel A, Grandbastien B, Proulx F, Martinot A Gauvin F, Hubert P, Lacroix J. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Resp Crit Care Med. 2005;171:348–353. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 28.Skelton JA, Havens PL, Werlin JL. Nutrient deficiencies in tube fed children. Clin Pediatr. 2006;45(1):37–41. doi: 10.1177/000992280604500106. [DOI] [PubMed] [Google Scholar]
- 29.Jeejeebhoy K. Zinc essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 Suppl):S7–12. doi: 10.1053/j.gastro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Shenkin A. Selenium in intravenous nutrition. Gastroenterology. 2009;137(5 Suppl):S61–69. doi: 10.1053/j.gastro.2009.07.071. [DOI] [PubMed] [Google Scholar]