Abstract
In gray matter, cerebral endothelium is known to provide trophic support for neighboring cells such as neurons. However, signaling from cerebral endothelium to white matter cells remains to be elucidated. Here, we show that vascular endothelial growth factor (VEGF-A) secreted from cerebral endothelial cells promotes the migration but not the proliferation of oligodendrocyte precursor cells (OPCs). Cultured OPCs were obtained from newborn rat cortex, and treatment with conditioned culture media of cerebral endothelial cells increased the OPC proliferation and migration. Importantly, co-treatment with anti-neutralizing antibody for Flk-1 (VEGF-receptor2) inhibited OPC movement but did not affect OPC propagation. Western blot and flow cytometry analyses confirmed that our cultured cerebral endothelial cells produced VEGF-A and our cultured OPCs expressed Flk-1. Taken together, our current data suggest that cerebral endothelium is supportive for oligodendrocyte lineage cells and VEGF-A may participate in the endothelium-OPC cell-cell signaling. This phenomenon may be important for white matter homeostasis.
Keywords: oligodendrocyte precursor cell, vascular endothelial growth factor, migration, proliferation, cerebral endothelial cell, neurovascular unit, oligovascular niche, white matter
Introduction
Traditionally, the cerebrovascular system was thought as inert pipes for delivering blood flow to the brain. However, recent findings reveal another aspect of this system, i.e. cerebral endothelial cells can produce trophic factors to maintain brain homeostasis within the context of the neurovascular unit [7, 14, 17]. This concept is well established for gray matter. Signaling between cerebral endothelium and neuronal precursor cells may work for sustaining neurogenesis and angiogenesis even in the adult brain [3, 6, 9, 25, 26]. Cross-talk between the vascular and neuronal compartments in the so-called “neurovascular niche” is mediated by an exchange of soluble signals including growth factors [7, 14, 23].
This concept of cell-cell signaling has been extended to white matter. We recently proposed that a corresponding “oligovascular niche” may exist in white matter, wherein cerebral endothelial cells promote the proliferation of oligodendrocyte precursor cells (OPCs) [1]. In addition, we have also reported that vascular endothelial growth factor (VEGF-A) may facilitate the OPC migration [8]. But where does VEGF-A come from? Here, we tested the hypothesis that cerebral endothelial cells might modulate OPC function via secreting VEGF-A.
Materials and Methods
Cell Culture
OPCs were prepared as previously described [1, 8]. Briefly, cerebral cortices from 1-2 day old Sprague-Dawley rats were dissected, minced, and digested. Dissociated cells were plated in poly-D-lysine-coated 75-cm2 flasks, and maintained in Dulbecco's Modified Eagle's medium containing 20% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin. After the cells were confluent (~10 days), the flasks were shaken for 1 hour on an orbital shaker (220 rpm) at 37°C to remove microglia. The medium was changed with a new medium and shaken overnight (~ 20 hours). The medium was then collected and plated on non-coated tissue culture dishes for 1 hour at 37°C to eliminate possible contamination by astrocytes and microglia. The non-adherent cells were collected and replated in Neurobasal Media containing glutamine, 1% penicillin/streptomycin, 10 ng/mL PDGF, 10 ng/mL FGF, and 2% B27 supplement onto poly-DL-ornithine-coated plates. Four to 5 days after plating, the OPCs were differentiated into mature oligodendrocytes by switching culture media to Dulbecco's Modified Eagle's medium containing 1% penicillin/streptomycin, 10 ng/mL CNTF, 50 ng/mL T3, and 2% B27 supplement. Human brain microendothelial cells were obtained from Cell System Corporation and maintained in EGM-2MV containing EGM-2MV SingleQuots kit onto collagen-coated culture plates.
Preparation of endothelial conditioned media
To prepare endothelial-conditioned media, we used cells at 90-95% confluence, grown in Neurobasal Media containing glutamine, 1% penicillin/streptomycin and 2% B27 supplement for 24 hours. Conditioned medium was collected and filtered using 0.20 um filter. Control media was prepared with the same culture medium derived from empty wells without endothelial cells.
Immunocytochemistry
After the cells reached 70-80% confluence, they were washed with ice-cold PBS (pH 7.4), followed by 4% paraformaldehyde for 30 min. After being further washed three times in PBS containing 0.1% Triton X-100, they were incubated with 1% bovine serum albumin in PBS for 1 h. Then cells were incubated with primary antibodies against OPC markers A2B5 (1:200) and NG2 (1: 200), an astrocyte marker GFAP (1:200), endothelial markers CD31 (1:200), vWF (1:200), and VE-cadherin (1:200), a smooth muscle cell marker αSM (1:200), and tight junction markers claudin-5 (1:200) and ZO-1 (1:200) at 4°C overnight. After washing with PBS, they were incubated with secondary antibodies conjugated with fluorescein isothiocyanate for 1 h at room temperature. Finally, nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI), and cells were rinsed and coverslips were placed. Immunostaining was analyzed with a fluorescence microscope (Nikon, Japan) interfaced with a digital charge-coupled device camera and an image analysis system.
Western Blotting
Cell culture lysates were prepared in Pro-PREPTM Protein Extraction Solution (BOCA SCIENTIFIC). Samples were heated with equal volumes of SDS sample buffer (Novex) and 10 mM DTT at 95°C for 5 min, then each sample (20 μg per lane) was loaded onto 4–20% Tris-glycine gels. After electrophoresis and transferring to polyvinylidene difluoride membranes (Novex), the membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 0.2% I-block (Tropix) for 90 min at room temperature. Membranes were then incubated overnight at 4°C with monoclonal anti-MBP antibody (1:1000, an oligodendrocyte marker), anti-VEGF-A antibody (1:1000) or anti-β-actin antibody (1:5,000) followed by incubation with peroxidase-conjugated secondary antibodies and visualization by enhanced chemiluminescence (Amersham).
In vitro tube formation assay
The standard Matrigel assay was used to assess the spontaneous formation of capillary-like structures by the human brain endothelial cells obtained from Cell System Corporation. Standard 24-well plates were coated with 150 μL of cold Matrigel and allowed to solidify at 37°C for 30 mins. Cells (5 × 104 cells/well) were incubated at 37°C for 18 hr with or without 5% FBS.
FACS analysis
OPC suspensions were pre-blocked with 3% BSA and then incubated with the following primary antibodies against NG2 (1:200, Millipore) and VEGFR2/Flk1/KDR (1:100, Abcam). Fluorescent-tagged Fab specific secondary antibodies from Jackson laboratories were incubated for 30 min at room temperature. Labeled cell populations were measured by FACSCalibur (BD biosciences). FACS data were analyzed by Cellquest pro software (BD biosciences). FACS analysis were performed using a variety of controls including unstained samples, isotype antibodies and single stained samples for determining appropriate gates, voltages, and compensations required in multivariate flow cytometry.
OPC migration
When OPCs were reached at 70-80% confluent, a wound was made across the cells with a cell scraper (MIDSCI, MO). Then the cultures were washed once with Neurobasal Medium. After treatment with endothelial-conditioned media, cells were allowed to migrate for 24 hours. Then, the number of OPCs that moved across the injury line was counted in 1.5 mm2 regions of interest.
Determination of cell proliferation
Cell proliferation/survival was assessed by WST reduction assay (Dojindo). This assay is based on the detection of dehydrogenase activity of viable cells. Twenty four hours after treatment of endothelial conditioned media, the cells were incubated with 10% WST solution for 1 hour at 37°C. Then the absorbance of the culture medium was measured with a micro-plate reader at a test wavelength of 450 nm and a reference wavelength of 630 nm. The OPC proliferation (%) was calculated based on the results from control-media-treated OPCs.
Statistical analysis
All experiments were performed in duplicate, repeated at least 3 times independently. Quantitative data were analyzed by One-way ANOVA followed by Tukey's Multiple Comparison Test. Data are expressed as mean ± SD. A value of p < 0.05 was considered significant.
Results and Discussion
The current study was designed to use cultured OPCs and cerebral endothelial cells for dissecting the mechanism of endothelium-to-OPC signaling in vitro. For this purpose, we first characterized our cell culture platforms for OPCs and endothelial function. We cultured OPCs from neonatal rat brains. Immunostaining confirmed that these cells were positive for the OPC markers A2B5 and NG2 (Fig. 1a). The lack of GFAP-positive cells showed that our procedures successfully separated OPCs from astrocytes (Fig. 1a). Importantly, our OPCs were functional because they can be matured into myelin-basic-protein-positive oligodendrocytes over time (Fig. 1b).
Figure 1. Characterization of oligodendrocyte precursor cells (OPCs) and brain endothelial cells.
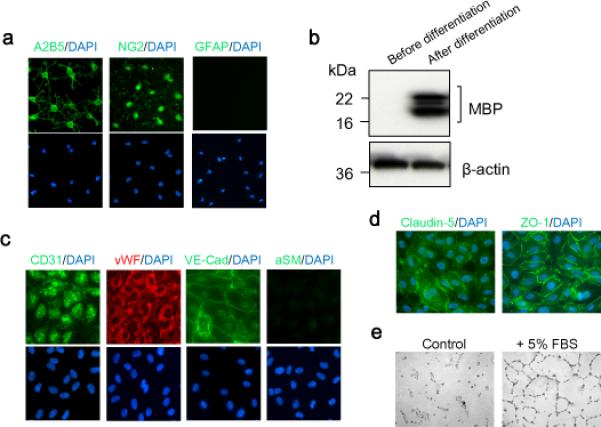
(a) Immunostaining confirmed the expression of OPC markers A2B5 and NG2, but not astrocyte marker GFAP in our OPCs. (b) OPCs were functional because they were matured into myelin-basic-protein-positive oligodendrocytes after differentiation. (c) Primary human brain microendothelial cells expressed the endothelial markers CD31, vWF and VE-cadherin (VE-Cad) but not the smooth muscle cell marker alpha smooth muscle (αSM). (d) Cells expressed tight-junction proteins, claudin-5 and ZO-1. (e) In vitro angiogenesis assay confirmed that cells were capable to form vessel-like tubes.
For our endothelial platform, we used primary human brain microendothelial cells, and confirmed that these cells expressed the endothelial markers CD31, vWF and VE-cadherin (VE-Cad) but not the smooth muscle cell marker alpha smooth muscle (αSM) (Fig. 1c). Consistent with a cerebral origin, these cells expressed tight-junction proteins, claudin-5 and ZO-1 (Fig. 1d). Functionally, our cerebral endothelial system was intact since they were capable of angiogenesis using an in vitro Matrigel tube formation assay (fig. 1e).
As noted before, our basic hypothesis in this study was that cerebral endothelial cells can secrete VEGF-A and endothelial-derived VEGF-A modulates OPC function including migration and proliferation. Cerebral endothelial cells are well known to produce VEGF-A both in vitro and in vivo [4, 5, 10]. Here, we confirmed that our cerebral endothelial cultures can release VEGF-A into culture media (Fig. 2a). Correspondingly, flow cytometry verified that our OPCs were positive for the VEGF receptor Flk-1 expression (Fig. 2b). Flk-1 (also known as KDR or VEGF-receptor 2) is primarily responsible for VEGF-induced angiogenesis, but recently, we have demonstrated that Flk-1 may also participate in OPC homeostasis [8].
Figure 2. Brain endothelial cells secret VEGF-A and OPCs express the receptor, Flk1.
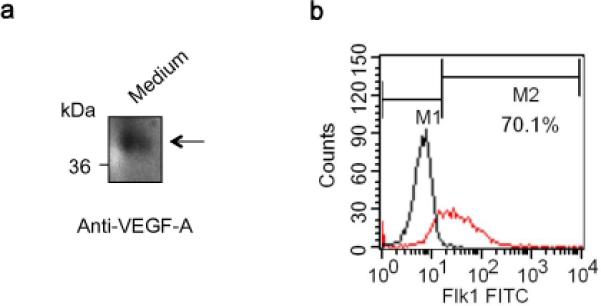
(a) Primary human brain microendothelial cells were maintained as described in Materials and Methods section. Western blot analysis with endothelial-conditioned media (Endo-CM) confirmed that our brain endothelial cells released VEGF-A to the culture media. (b) Flow cytometry analysis verified that OPCs were positive with the VEGF receptor Flk-1. Black and red lines represent OPC population stained with phenotype control IgG-FITC (M1 region) and Flk-1-FITC (M1+M2 region), respectively. Over 70% of our OPCs (M2 region) were strongly positive with Flk-1.
Next, we conducted a media transfer approach that was previously used to dissect soluble factor coupling in the neurovascular and oligovascular niches [1, 7]. OPCs and cerebral endothelial cells were grown separately and endothelial-conditioned media (Endo-CM) was then obtained and added to OPCs for the OPC migration (Fig. 3a). Control OPCs were treated with the same culture medium derived from empty wells without endothelial cells. Treatment with Endo-CM significantly amplified the OPC migration (Fig. 3b). Importantly, Flk-1 neutralizing antibody significantly inhibited the Endo-CM-induced OPC migration (Fig. 3b). Taken together, these findings indicate that endothelial-VEGF-A may play a central role in the endothelial-induced OPC migration.
Figure 3. Endothelial conditioned media promoted OPCs migration via Flk1.

(a) OPCs and cerebral endothelial cells were grown separately, and endothelial-conditioned media (Endo-CM) was then obtained and added to OPCs. Control media was prepared with the same culture medium derived from empty wells without endothelial cells. The OPC migration was assessed at 24-hour later as described in the Materials and Methods section. (b) Treatment with Endo-CM significantly amplified the OPC migration. The Flk-1 neutralizing antibody (1 μg/mL) inhibited the Endo-CM-induced OPC migration. *P<0.05
Finally, we tested if endothelial-VEGF-A could enhance the OPC proliferation as well. The media transfer approach was used again, and we assessed the cell propagation with a WST assay (fig. 4a). Endo-CM increased the rate of OPC proliferation (Fig. 3b). But the Flk-1 antibody did not affect the OPC numbers (Fig. 4b), suggesting that VEGF-A may not contribute to endothelial-OPC proliferation signaling.
Figure 4. Endothelial conditioned media induced OPCs proliferation was not inhibited by the blockade of Flk1.
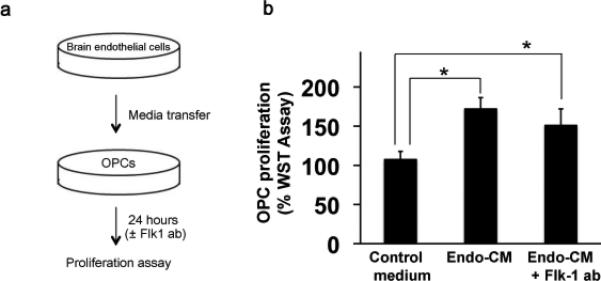
(a) OPCs and cerebral endothelial cells were grown separately, and endothelial-conditioned media (Endo-CM) was then obtained and added to OPCs. Control media was prepared with the same culture medium derived from empty wells without endothelial cells. The OPC proliferation was assessed by the WST assay at 24-hour later as described in the Materials and Methods section. (b) Endo-CM was confirmed to increase the rate of the OPC propagation compared to the control media. But, the Flk-1 antibody (1 μg/mL) did not affect the OPC proliferation. *P<0.05
In recent years, it has been well accepted that the cerebrovascular system comprises much more than just inert plumbing for the brain. Cerebral endothelial cells produce molecular signals that nourish neuronal precursors for ongoing neurogenesis in adult brain [19]. In the present study, we showed that (i) conditioned media from cerebral endothelial cells promoted both OPC migration and proliferation, (ii) endothelial-derived VEGF-A played a central role in the OPC migration, and (iii) endothelial-VEGF-A did not participate in the OPC proliferation. These findings support the idea that cerebral endothelial cells can also nourish neighboring cells in white matter. Because OPC proliferation and migration are both key steps for myelination, cerebral endothelial cells would work for ongoing oligodendrogenesis as well as neurogenesis.
OPCs are generally accepted as a key cell type for maintaining myelin homeostasis in white matter. OPCs multiply and migrate from the ventricular zone to their destination to form myelin sheaths during development [2, 13]. While the population is relatively small, OPCs are still widely distributed in the adult brain to maintain and renew myelin sheaths. After brain injury, OPCs are proliferated rapidly and guided to the damaged area for myelin repair through differentiation (i.e. oligodendrogenesis) [18]. Moreover, OPCs also have the potential of maturing into some subsets of cortical projection neurons [22]. Hence, the capability of cerebral endothelial cells to facilitate OPC proliferation and migration may have broad relevance in understanding not only gray matter development, but also white matter recovery in pathology as well.
In this study, we have experimentally shown that VEGF-A is one of the important mediators for cell-to-cell interactions between cerebral endothelial cells and oligodendrocyte lineage cells. Nevertheless, there are a few caveats that need to be carefully discussed for future studies. First of all, our current report was a proof-of-concept study and all our experiments were conducted in vitro cell culture systems. Future studies are required to define the mechanisms of endothelium-OPC interactions in vivo. Second, other VEGFs may also participate in the OPC function. VEGF-C was reported to promote OPC proliferation via VEGFR-3 but not VEGFR-2 (Flk-1) [12]. Although VEGF-C and VEGF-D are known as ligands for VEGFR-3, these VEGFs can also bind to Flk-1 under some conditions [24]. Future studies should carefully examine roles of the VEGF family network in the endothelium-OPC interactions. Third, we focused on endothelial-derived VEGF-A under normal conditions. Many other growth factors may influence OPC function [15, 16, 20, 21], and expression levels of some growth factors may be downregulated after injury or disease [1, 7]. Therefore, it is important for future studies to carefully dissect the network of endothelium-OPC crosstalk under both normal and disease conditions. Finally, we only examined signal transduction from cerebral endothelial cells to OPCs. But it is possible that endothelium-OPC trophic coupling is a “two-way” phenomenon. In this “oligovascular niche”, OPC-derived factors might help sustain the renewal and differentiation of endothelial precursors for ongoing angiogenesis. Vascular remodeling is an important facet in the chronic phase of brain pathophysiology [3, 11, 19]. Hence, elucidating how trophic signals from OPCs mediate angiogenesis may offer new therapeutic directions for white matte related diseases such as stroke or vascular dementia.
In conclusion, our findings demonstrate that cerebral endothelial cells produce VEGF-A to promote OPC migration. Dissecting the mechanisms of how endothelium-OPC signaling works in white matter will help us understand the white matter remodeling and recovery after injury and disease.
Highlights.
conditioned media from cerebral endothelial cells promoted both OPC (oligodendrocyte precursor cell) migration and proliferation
endothelial-derived VEGF-A played a central role in the OPC migration
endothelial-VEGF-A did not participate in the OPC proliferation
Acknowledgements
Supported in part by the Deane Foundation, MGH ECOR Fund for Medical Discovery, American Heart Association, National Institutes of Health, Research Abroad from the Japan Society for the Promotion of Science, National Research Foundation of Korea, the World Class University Program, and the Global Research Laboratory Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat MA. Molecular organization of axo-glial junctions. Current opinion in neurobiology. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 4.Fischer S, Renz D, Wiesnet M, Schaper W, Karliczek GF. Hypothermia abolishes hypoxia-induced hyperpermeability in brain microvessel endothelial cells. Brain Res Mol Brain Res. 1999;74:135–144. doi: 10.1016/s0169-328x(99)00272-7. [DOI] [PubMed] [Google Scholar]
- 5.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 7.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, Arai K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–1872. doi: 10.1161/01.str.27.10.1865. discussion 1872-1863. [DOI] [PubMed] [Google Scholar]
- 11.Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 12.Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 13.Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 15.Magy L, Mertens C, Avellana-Adalid V, Keita M, Lachapelle F, Nait-Oumesmar B, Fontaine B, Baron-Van Evercooren A. Inducible expression of FGF2 by a rat oligodendrocyte precursor cell line promotes CNS myelination in vitro. Exp Neurol. 2003;184:912–922. doi: 10.1016/j.expneurol.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto Y, Yamauchi J, Tanoue A. Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. J Neurosci. 2008;28:8326–8337. doi: 10.1523/JNEUROSCI.1482-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 19.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohya W, Funakoshi H, Kurosawa T, Nakamura T. Hepatocyte growth factor (HGF) promotes oligodendrocyte progenitor cell proliferation and inhibits its differentiation during postnatal development in the rat. Brain Res. 2007;1147:51–65. doi: 10.1016/j.brainres.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 21.Paez PM, Fulton DJ, Spreur V, Handley V, Campagnoni AT. Multiple kinase pathways regulate voltage-dependent Ca2+ influx and migration in oligodendrocyte precursor cells. J Neurosci. 30:6422–6433. doi: 10.1523/JNEUROSCI.5086-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 26.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]


