Abstract
Brain edema remains a challenging obstacle in the management of acute liver failure (ALF). Cytotoxic mechanisms associated with brain edema have been well recognized, but evidence for vasogenic mechanisms in the pathogenesis of brain edema in ALF has been lacking. Recent reports have not only shown a role of matrix metalloproteinase-9 in the pathogenesis of brain edema in experimental ALF but have also found significant alterations in the tight junction elements including occludin and claudin-5, suggesting a vasogenic injury in the blood-brain barrier (BBB) integrity. This article reviews and explores the role of the paracellular tight junction proteins in the increased selective BBB permeability that leads to brain edema in ALF.
Acute liver failure (ALF) is the sudden loss of liver function in the absence of a pre-existing liver disease. In the early stages of ALF, the injured liver may recover. However, the onset of coma and advanced stages of encephalopathy mark(s) the beginning of the lethal course of ALF (Vaquero et al., 2003). It is during this early stage that the blood-brain barrier (BBB) becomes permeable to small molecules (Horowitz et al., 1983). If the BBB dysfunction persists, brain edema and herniation will result. Brain herniation is clinically manifested by fixed and dilated pupils (Blei, 1995). Postmortem examinations show evidence of brain edema in 80% of comatose ALF patients (Ede and Williams, 1986; Silk and Williams, 1979). In the United States, the median waiting time for ALF patients who successfully receive a liver transplant is 3 days (Lee, 2003). In contrast, ALF patients who do not receive a liver transplant die within 5 days of hospital admission (Lee, 2003). The window of opportunity for a life-saving liver transplant is quite narrow.
Brain edema in patients with ALF remains the major cause of death and a major impediment toward being eligible for a life-saving liver transplantation (Chan et al., 2009; Lee et al., 2008; Park et al., 2010). Klatzo categorized the mechanism of brain edema in general as being either cytotoxic, “intracellular swelling without increased permeability of BBB,” or vasogenic, “increased permeability of BBB leading to net gain of fluid,” with water and plasma constituents accumulated in the extracellular region as a consequence of structural BBB injury (Kimelberg, 2004; Klatzo, 1967). Ede and Williams in 1986 further applied the above concepts to patients with ALF (Ede and Williams, 1986). In 1992, Kato and colleagues demonstrated swollen astrocytes and their endfoot (markers of cytotoxic effects) but observed only minimal ultrastructural alterations in brain capillaries of patients who died of ALF. In fact, the brain capillary endothelial cell and its tight junction were relatively intact (Kato et al., 1992). Similar findings were observed in animal models of ALF (Gove et al., 1997; Traber et al., 1987). Since there is no gross structural damage seen in the brain capillary membrane, the concept of vasogenic brain edema became unpopular, and for many years a cytotoxic event was considered to be the main mechanism in the pathogenesis of brain edema in ALF. The accepted tenet of cytotoxic brain edema in ALF has been recently challenged (Blei, 2007; Nguyen et al., 2006).
Increased BBB permeability occurs without overt BBB breakdown in ALF
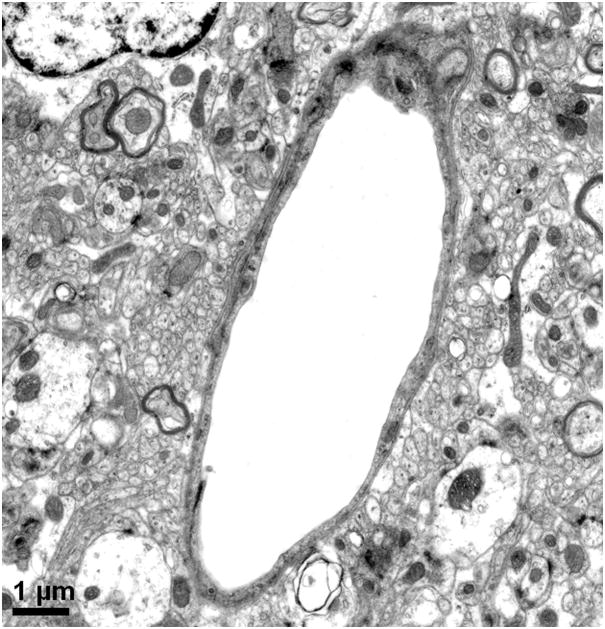
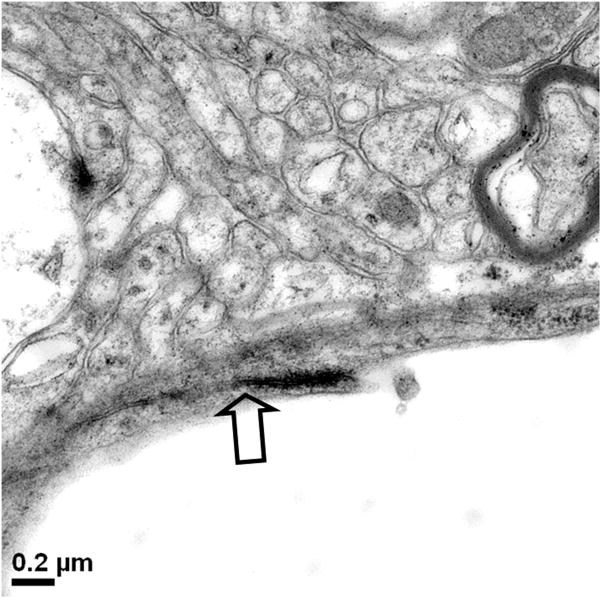
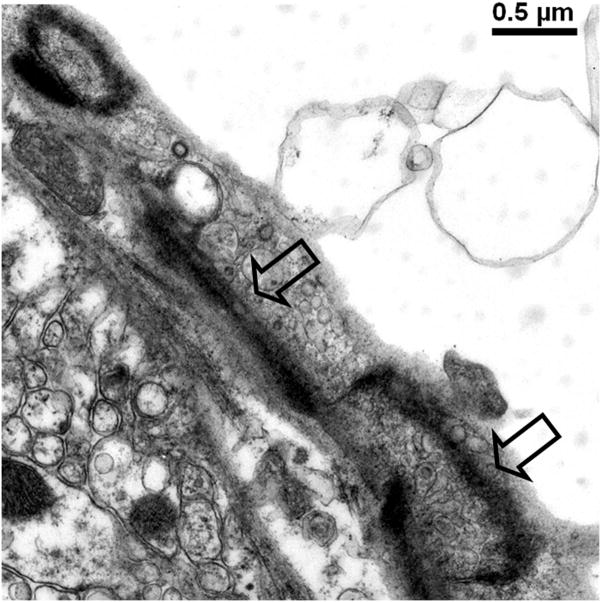
In 1977, Livingston and colleagues showed the extravasation of Trypan blue in brains of rats with D-galactosamine-induced ALF (Livingstone et al., 1977). Dixit and Chang in 1990 similarly showed that Trypan blue extravasates into brain parenchyma in rats with ALF-induced with D-galactosamine (Dixit and Chang, 1990). The increased permeability to the intravascularly injected dye molecules increased with the degree of hepatic encephalopathy. These studies along with others consistently reveal only subtle alterations in the BBB of ALF animals and are consistent with the minimal BBB changes observed in human patients who died of ALF (Kato et al., 1992). Interestingly, Horowitz et al. demonstrated increased uptake of α-aminoisobutyric acid, a non-metabolized amino acid, into brains of ALF rats prior to clinical onset of overt hepatic encephalopathy (Horowitz et al., 1983). In addition to astrocytic swelling, extravascular and interstitial edema are also consistently observed in brains of humans and animals with ALF (Gove et al., 1997; Kato et al., 1992; Traber et al., 1987). Electron microscopic examinations of the BBB in brain capillaries of ALF subjects show minimal ultrastructural perturbations, Figure 1a and b, including an increase in cytoplasmic vesicles and thickened basal lamina (Gove et al., 1997; Kato et al., 1992; Lv et al., 2010). In addition, we have noted that although the brain capillary tight junction remains physically intact in ALF, the tight junction could be lengthened and be tortuous in shape, Figure 1c and d.
Figure 1.
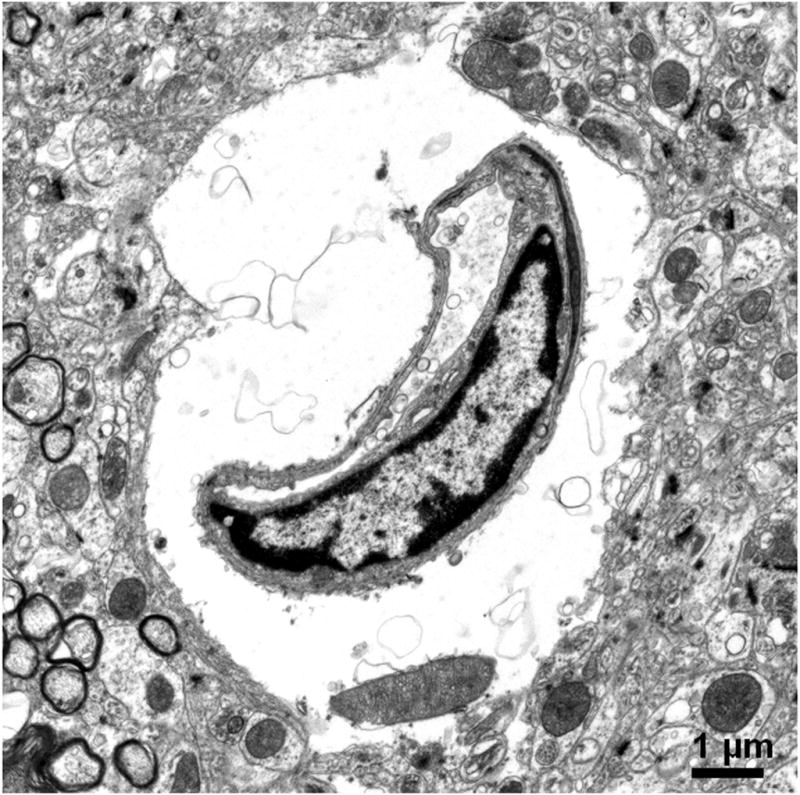
Electron micrographs of (a) brain capillary with astrocytic endfoot process in normal mouse; (b) collapsed brain capillary surrounded by swollen astrocytic endfoot processes in mouse with ALF; (c) typical linear tight junction in brain capillary from normal mouse; (d) distorted and lengthened tight junction in brain capillary of mouse with ALF. Arrowheads mark the path of the tight junction.
Contrary to prior belief, the above observations suggested an important role of vasogenic mechanisms in the pathogenesis of brain edema in ALF (Blei, 2007). In 2006, using a reproducible model of ALF in mice with 100% lethality induced with azoxymethane, we showed that brain extravasation of Evans blue dye across the BBB and brain water in ALF are significantly increased and that BBB permeability and brain water are attenuated with monoclonal antibodies that are specific for active matrix metalloproteinase-9 (MMP-9) (Nguyen et al., 2006). Similar findings were demonstrated in rats with D-galactosamine induced ALF (Yamamoto and Nguyen, 2006). The results suggest the importance of a vasogenic mechanism in the development of brain edema in ALF. These findings have been recently corroborated. Filipo and colleagues showed that in rats with D-galactosamine-induced ALF, vasogenic edema caused by BBB permeability is an early step in the development and progression of brain edema in ALF (Cauli et al., 2011).
Other recent studies have also provided evidence supporting the role of vasogenic edema in ALF (Cauli et al., 2011; Chavarria et al., 2010; Kristiansen et al., 2010; Lv et al., 2010). MRI studies of human patients have also recently confirmed the vasogenic element in brain edema in liver failure (Kale et al., 2006; Rai et al., 2008). However, the molecular basis for failure in BBB integrity in ALF remains unknown. Even less understood is whether BBB dysfunction leads to increased permeation of small molecules, such as amino acids and ammonia, with subsequent cytotoxic manifestations or if BBB dysfunction is a consequence of the cytotoxic insult.
BBB organization: Endothelial cell and its tight junction
The BBB is made up of brain capillary endothelial cells and their junctional complexes (Abbott et al., 2010; Vorbrodt and Dobrogowska, 2003). The endothelial cell spreads itself on the basal lamina, covering the entire luminal surface of the capillary with the two surface edges being sealed with junctional molecules forming the tight junction of the BBB. The endothelial cell on the luminal side of the basal lamina, together with the pericyte and astrocytic endfoot enveloping the inner face of the basal lamina, and surrounding neurons collectively represent a neurovascular unit that tightly regulates any exchange between the circulating blood and the central nervous system.
In a normal state, brain capillary endothelial cells have various enzymes to break down and prevent entry of various compounds, a low level of vesicular transport, and a variety of nutrient transporters. The tight junction, i.e. the paracellular cleft between the two cellular surfaces of the endothelial cell, is highly regulated by elements of tight junction proteins including occludin, claudin-5, junctional adhesion molecules (JAM), and cadherins. Tight junction molecules are transmembrane proteins and are associated with the intracellular cytoskeleton via the peripheral junctional molecules zona occludin 1 (ZO-1) and its associates ZO-2 and ZO-3 and catenin isoforms. The tight junction segregates the apical or luminal plasmalemma from the basal or abluminal one, maintaining cellular polarity. The tight junction limits the paracellular diffusion of small molecules, regulating entry of circulating molecules into the brain parenchyma. The endothelial cell and its tight junction is thus the first barrier of the neurovascular unit.
Occludin was the first discovered element of the tight junction (Furuse et al., 1993; Vorbrodt and Dobrogowska, 2003). It is about 65 kD with C- and N-terminals within the cytoplasm and four membrane-spanning segments. The two extracellular loops participate in the paracellular path. Occludin is expressed in various tissues and organs including epithelial and endothelial layers. Occludin is not essential for tight junction strands, since deletion of the occludin gene did not stop the formation of the tight junction (Sandoval and Witt, 2008). On the other hand, occludin is a reliable marker for tight junction in immunohistochemical evaluation (Vorbrodt and Dobrogowska, 2003). Occludin integrity is essential for normal barrier function (Balda et al., 2000; Bamforth et al., 1999; Sandoval and Witt, 2008).
Claudin-5 was discovered in 1998. It has a similar distribution and pattern to occludin but is smaller in size, about 25 kD. There are 15 claudins, ranging from claudin-1 to -15. Claudin-1 and -5 are known to associate with brain endothelial cells. Claudin-5 is essential for the formation of tight junction strands (Vorbrodt and Dobrogowska, 2003). Similar to occludin, claudin-5 is critical for barrier function (Sandoval and Witt, 2008). Both occludin and claudin-5 are positioned in the apical portion toward the capillary lumen. Recently, additional JAM were found to be important integral components of the tight junction (Sandoval and Witt, 2008). However, the role of JAM remains to be further elucidated.
Toward the basal portion of the tight junction, cadherins hold the cellular surfaces together. A loss of cadherins results in cell disengagement (Kiptoo et al., 2011). There are more than 40 cadherins. The vascular endothelial cadherin (VE-cadherin) or cadherin-5 is an important determinant of microvascular integrity, both in vitro and in vivo (Vorbrodt and Dobrogowska, 2003). The exact role of VE-cadherins in BBB in ALF remains to be explored.
Subtle perturbations in tight junction are associated with BBB selective permeability
In general, pathological increase in selective permeability, particularly for polar small molecules, is a direct consequence of failure of integrity of the paracellular tight junction (Abbott et al., 2010; Sandoval and Witt, 2008). The role of the tight junction in the fine regulation of small polar molecules was assessed (Dickson et al.) with electron microscopic investigations. In 1970, Brightman, Klatzo, Olsson, and Reese administered peroxidase into mouse intracranial ventricle and found that the peroxidase permeated the basal lamina; it penetrated the paracellular endothelial cleft but was stopped by the tight junction complexes (Brightman et al., 1970). In contrast, Bouldin and Krigman perfused via the ascending aorta and demonstrated that the tight junction restricts lanthanum ion diffusion in the paracellular cleft of the cerebral capillaries (Bouldin and Krigman, 1975). In mice treated with intravenous bradykinin agonist RMP-7, which increases BBB permeability, the lanthanum ion penetrated the paracellular cleft by 310 fold (Sanovich et al., 1995). These studies underscore an important role of the tight junction in regulating small polar molecules across the BBB.
Paracellular permeability results from a deregulation of the protein composition and function of tight junction complexes. Wong and Gumbiner showed that synthetic peptides corresponding to the extracellular loops of occludin reversibly disrupted the tight junction barrier without changes in the cellular morphology or changes in the tight junction (Wong and Gumbiner, 1997). Short interfering RNA of occludin in MDCK cells leads to an increase of permeability to large organic cations (Yu et al., 2005). Similarly, mutant occludin peptide conjugate induces a transient and reversible disruption of the blood-testis barrier in rats (Wong et al., 2007). Modulating the occludin results in size-selective permeability, which allows small molecules about 500 Da but not larger than 1.9 kDa (Jin et al., 2008).
Claudin-5 also plays an important role in BBB function. In mice with deletion of claudin-5, ultrastructural examinations show intact tight junctions; however, the BBB is selectively permeable to molecules that are <800 Da (Nitta et al., 2003). These mice die shortly after birth and they do not have brain edema (Nitta et al., 2003). In wild-type mice, similar increase in BBB permeability to small molecules is observed when claudin-5 interfering RNA is administered (Campbell et al., 2008). These results illustrate three facts: (i) a subtle alteration in the tight junction component leads to selective permeability without structural breakdown; (ii) compromised BBB can be lethal due to a loss of precise brain ionic regulation that is essential for normal brain function; (iii) in the presence of a BBB defect, there may not be brain edema if there is no oncotic gradient such as ammonia and glutamate across the BBB, which would be taken up by the astrocytes, wherein glutamate and ammonia convert to glutamine by glutamine synthetase. Glutamine serves as an osmolyte, attracting water influx into the astrocyte resulting in astrocytic swelling.
These results collectively suggest that the increased selective permeability in ALF may result from a deregulation of tight junction proteins. From microvessels isolated from brains of mice with azoxymethane-induced ALF, we observed a loss of ZO-2 prior to the onset of advanced encephalopathy (Shimojima et al., 2008). In 2009, we demonstrated that tight junction proteins including occludin, claudin-5, and their accessory associates ZO-1 and ZO-2 are significantly altered in brains of mice with azoxymethane-induced ALF (Chen et al., 2009). It should be noted that the change in the tight junction protein occludin was not reproduced by another laboratory using the azoxymethane-induced model (Bémeur et al., 2010). In contrast, in a surgical model of ALF induced by portacaval shunt and hepatic artery ligation, occludin was observed to decrease in brains of ALF rats (Sawara et al., 2009). Lv and colleagues recently report significant alterations in tight junction components in mice induced with D-galactosamine and liposaccharide (Lv et al., 2010). Further investigations are needed to determine whether a perturbation in tight junction integrity would result in an increase of small molecules like ammonia.
Transcellular BBB permeability in ALF
Compared with the tight junction, endothelial cells are spread over a much larger surface area of the brain capillary. The transport across the endothelial cell includes passive diffusion for lipid soluble and non-polar molecules; solute carriers including glucose, amino acids, and nucleosides; and receptor and adsorptive mediated transcytosis (Abbott et al., 2010). Glutamate and glucose transporters are significantly altered in brains of ALF animals (Belanger et al., 2006; Desjardins et al., 2001; Knecht et al., 1997). Increased vesicles have been observed in brain endothelial cells of animals and patients with ALF (Gove et al., 1997; Kato et al., 1992; Traber et al., 1987). However, direct correlation of transcellular endocytosis and vesicles to brain edema is poor.
Aquaporin-4 has been implicated in the development of brain edema in ALF (Eefsen et al., 2010; Rama Rao et al., 2010). However, the role of aquaporin channels in ALF is uncertain (Wright et al., 2010).
Extracranial and systemic factors inciting BBB permeability in ALF
It is believed that breakdown products of injured and necrotic hepatocytes are released into the systemic circulation affecting the cardiovascular integrity and stability (Bihari et al., 1986; Oldhafer et al., 1999; Ringe et al., 1993). Among the neurotoxic factors are the inflammatory cytokines including tumor necrosis factor, interleukins-1 and -6 interferon and ammonia (Bemeur et al., 2010b; Jalan, 2003; Jiang et al., 2009b). The systemic inflammation is exacerbated by superimposed infection (Wright et al., 2007a) which is commonly found in ALF. Tumor necrosis factor alpha has been shown to play an important role in the regulation of BBB permeability in human patients and animals with ALF (Lv et al., 2010). Selective deletion of cellular receptors of interleukin-1 and tumor necrosis factor alpha attenuate the development of encephalopathy and brain edema in experimental ALF (Bemeur et al., 2010a). In addition to peripheral inflammation, neuroinflammation of the components of the neurovascular unit in the brain may be an independent pathway leading to the development of encephalopathy and edema (Butterworth, 2011; Jiang et al., 2009a; Wright et al., 2007b).
Ammonia has long been associated with hepatic encephalopathy and brain edema. Arterial ammonia level directly correlates to the risk of impending brain herniation due to brain edema (Bernal et al., 2007). Since 99% of ammonia exists in ionic form at physiologic pH, the cytotoxic mechanism by itself does not fully account for the influx of small molecules such as water and ammonia into the brain parenchyma (Cooper, 2011; Ott and Larsen, 2004). How a small polar molecule like NH4+ traverses BBB in ALF remains incompletely understood.
BBB permeability in ALF is potentially reversible
BBB permeability that is responsible for the development of brain edema in ALF potentially resolves once liver function returns following a timely liver transplantation. However, in the immediate posttransplant period, during the time that the new liver graft is gaining its full potential function, intensive support for the brain is required to avoid brain herniation. Brain edema persists during the early posttransplant period (Azoulay et al., 2001). If the brain injury is beyond repair at the time of transplantation or the brain injury progresses during the transplantation, death or a vegetative state may occur during the posttransplant period (Azoulay et al., 2001; Castellote et al., 2006; Nafidi et al., 2007; Rentsch et al., 2010; Tantawi et al., 1996). Therefore, therapeutic measures that can effectively prevent and/or reverse the development of brain edema in ALF are of paramount importance.
The nature of brain injury reversibility that is associated with ALF is also seen with regeneration of the native liver. Although it is uncommon, it has been reported that brain edema resulting from severe traumatic liver injury resolved as the liver recovered from the injury (Mussack et al., 2005).
Matrix metalloproteinase-9 (MMP-9) induces BBB dysfunction resulting in increased permeability and brain edema
MMP-9, also known as gelatinase B, is one of 24 endopeptidases that belong to the matrix metalloproteinase family. MMP-9 plays an important role in numerous physiologic and pathologic processes. Specifically, MMP-9 can digest capillary endothelial BBB constituents and TJ proteins. For example, MMP-9 degraded occludin and claudin-5 in focal cerebral ischemia (Yang et al., 2007) and altered occludin, claudin-5, and ZO-1 and -2 levels in early diabetic retinopathy (Giebel et al., 2005; Hawkins and Davis, 2005; Hawkins et al., 2007). Clinically, MMP-9 has been shown to be involved in the development of brain edema in various CNS conditions, including multiple sclerosis, infectious encephalitis, brain ischemia/stroke, and traumatic or cold brain injuries. MMP-9 knockout mice subjected to cerebral ischemia or trauma had attenuated BBB permeability accompanied by protection of neurological function (Asahi et al., 2001; Wang et al., 2000). Similar protective effects were demonstrated in rats with focal cerebral ischemia when MMP-9 was inhibited with either a prototype MMP inhibitor BB-94 (Asahi et al., 2000) or an MMP-9 monoclonal antibody (Romanic et al., 1998). Recent data increasingly corroborate the independent role of MMP-9 in CNS injury in stroke (Ramos-Fernandez et al., 2011). MMP-9 thus plays a key role regulating the BBB integrity in CNS diseases and injuries.
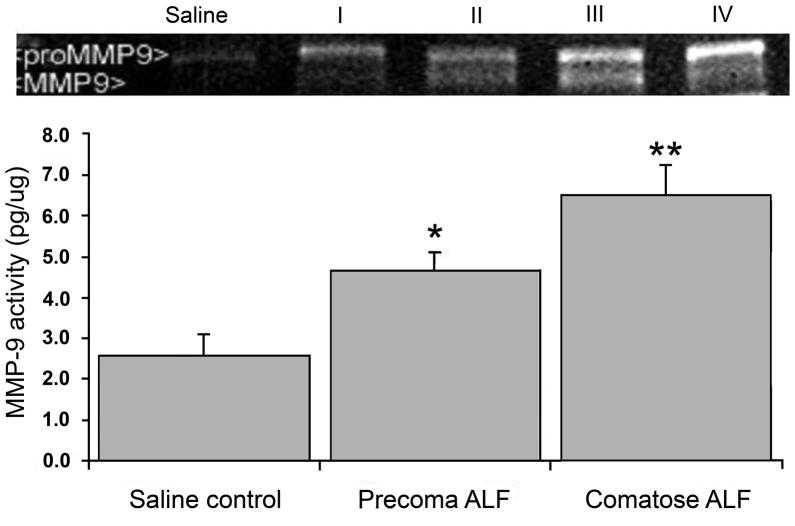
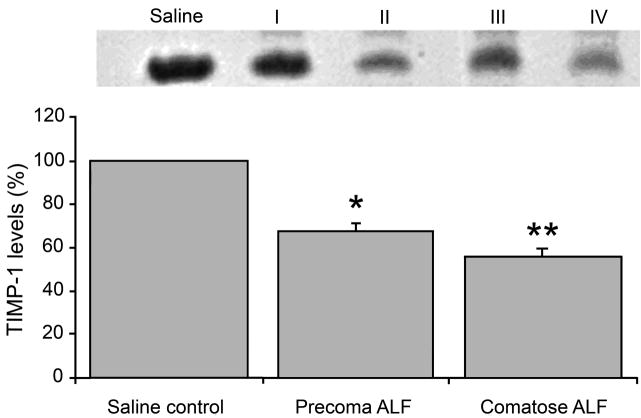
In 2006, we showed that active MMP-9 is upregulated in the circulating sera of the mice and rats with experimentally induced ALF (Nguyen et al., 2006; Yamamoto and Nguyen, 2006) (Figure 2A). The physiologic inhibitor of MMP (TIMP)-1, on the other hand, was significantly decreased in the sera of the ALF animals (Yamamoto and Nguyen, 2006) (Figure 2B). We demonstrated that specific monoclonal antibodies against MMP-9 successfully attenuated brain extravasation and edema in mice with experimentally induced ALF (Nguyen et al., 2006). Felipo and colleagues recently showed that vasogenic edema correlates with MMP-9 upregulation in rats with ALF induced with D-galactosamine (Cauli et al., 2011). In 2009, we demonstrated that MMP-9 induces significant alterations in TJ proteins, particularly occludin, in brain endothelial cells in vitro and in brains of mice with experimentally induced ALF (Chen et al., 2009). The loss of TJ integrity correlates with increased BBB permeability, and inhibiting against MMP-9 attenuates the permeability (Chen et al., 2009). Recently, we show that MMP-9 activates epidermal growth factor receptor and p38 mitogen activated protein kinases on brain endothelial cells leading to a derangement of occludin at the paracellular tight junction (Chen et al., 2011).
Figure 2.
Following induction of acute liver failure (ALF), animals progressed through precoma stages I and II, and comatose stages III and IV of hepatic encephalopathy. Increased MMP-9 activities are observed in systemic circulation accompanied by decreased levels of TIMP-1 in animals with ALF. (A) Using gelatin zymography, both proMMP-9 and MMP-9 are increased significantly in sera of ALF subjects. The MMP-9 activities are highest in comatose stages of ALF. * p<0.05; ** p<0.001. (B) TIMP-1 levels are decreased with increasing stages of ALF. TIMP-1 and –2 are measured with reverse zymography. No TIMP-2 is observed. On the other hand, TIMP-1 steadily disappears in ALF subjects. At comatose stages, TIMP-1 losses are 30% and 50%, respectively. * p<0.001. (J Hepatol 44:1104, 2006; J Surg Res 134:307, 2006)
In addition, we have shown that the brain is not the source of MMP-9 in ALF subjects. Rather, liver-derived MMP-9 present in the lumen of systemic circulation directly influences brain capillary endothelial cells and their neurovascular unit (Nguyen et al., 2006). MMP-9 is released into the systemic circulation from the injured liver within 2 hours of the chemical induction of ALF (Nguyen et al., 2006; Wielockx et al., 2001). Using RT-PCR and zymographic assays, there was no upregulation of MMP-9 within brains of mice with ALF (Nguyen et al., 2006). Therefore, the brain in ALF appears to be an innocent bystander that suffers a secondary injury caused by the circulating MMP-9 and the BBB appears structurally intact. This is in contrast to traumatic and ischemic brain injuries, wherein MMP-9 is produced by the CNS constituents and the neurovascular unit shows significant structural damage (Rosenberg et al., 2001; Rosenberg and Yang, 2007).
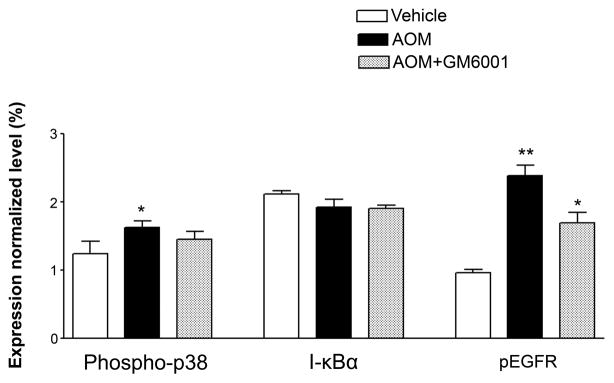
We speculated that endothelial cellular interactions with MMP-9 via surface receptors might play a major role in regulating the BBB integrity in ALF. We assessed the role of EGFR activation and its associated p38 MAPK/NFκB signaling in brains of ALF mice. We showed by western blotting that occludin was significantly altered in the brains of ALF mice and the alteration was restored by a MMP-9 inhibition with GM6001. These results are consistent with our previous report (Chen et al., 2009). Importantly, we observed EGFR activation along with p38 MAPK activation and IκBα degradation in the brains of ALF mice (Figure 3A and B). With confocal microscopy, we substantiated that significant EGFR activation occurred in brains of ALF mice and that EGFR activation was attenuated with GM6001 treatment (Figure 3C). In contrast, brains of normal control mice showed no EGFR activation. These findings will require further evaluation and corroboration in human patients with ALF.
Figure 3.

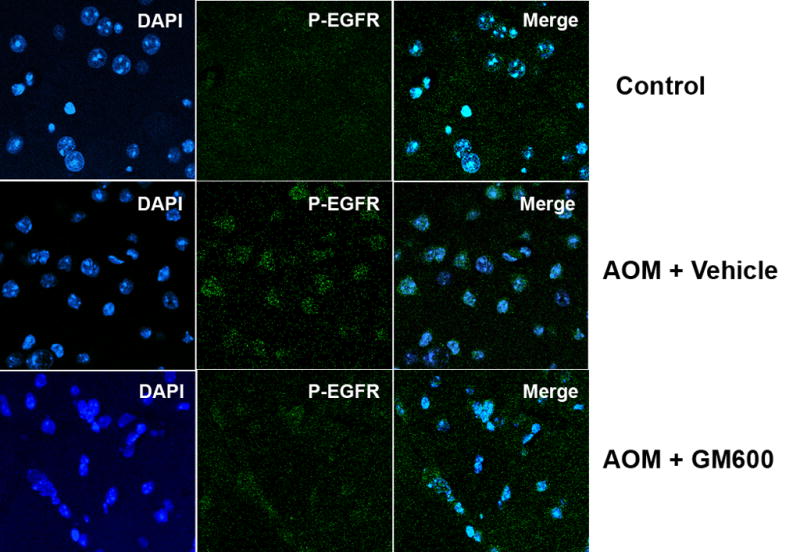
EGFR activation and p38 MAPK/NFκB activation in brains of ALF mice. (A) Western blot of brain samples from control mice, and AOM-induced ALF mice treated with vehicle or with GM6001 (MMP-9 inhibitor). (B) Densitometric quantification of p38 MAPK, IκBα, and p-TyrEGFR expression in brains of control mice and ALF mice treated with and without GM6001. Values are expressed as mean ± SEM with t-test. (N=3,*P<0.05). (C) Confocal microscopic evaluation of brains of control versus ALF mice treated with vehicle or GM6001. Activated EGFR is seen as green and nucleus as blue. (Hepatology 53:1294, 2011).
Summary
Brain edema in ALF is unique. It is associated with BBB dysfunction without overt structural breakdown when examined with conventional light and electron microscopy, and it is potentially reversible once the failing liver is replaced. Emerging evidence suggests that a fine perturbation in tight junction integrity results in selective permeability across the BBB to small polar molecules. A proposed model of subtle modifications in tight junction elements may lead to selective permeability to small polar molecules is presented in Figure 4. Moreover, recent works implicate that the vasogenic pathway is the leading event in the pathogenesis of brain edema in ALF (Cauli et al., 2011). These important advances in the understanding of the molecular pathogenesis of this disease process may potentially lead to effective therapeutic interventions for the prevention and/or reversal of brain edema in ALF.
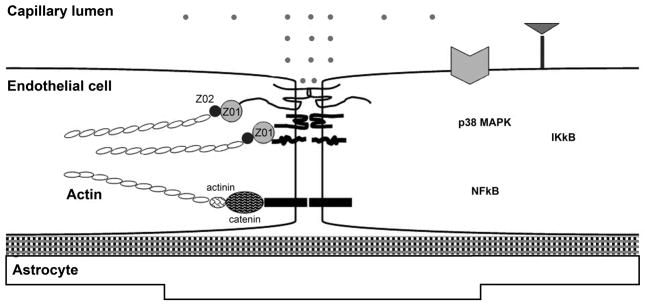
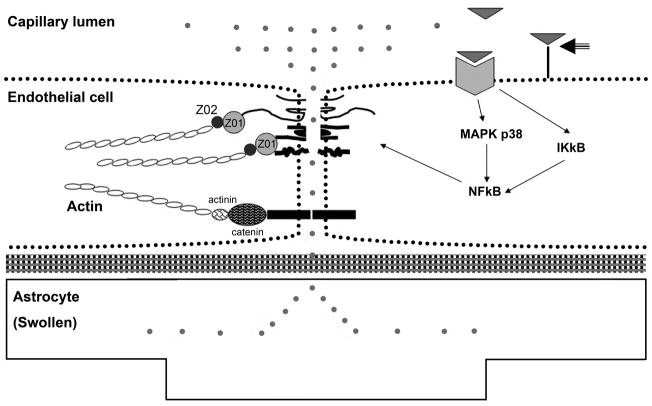
Figure 4.
Schematic diagram of blood-brain barrier (BBB) created by the brain capillary endothelial cell and its paracellular tight junction. Junctional proteins include occludin, claudin-5, junctional adhesion molecule (JAM), and VE-cadherin. Cytosolic junctional accessory molecules include zona occludin (ZO)-1, -2, and -3, and catenins linking the junctional proteins to actin of the cytoskeleton. (A): in normal state, small polar molecules are restricted from entering brain parenchyma with normal endothelial cell resting on basal lamina and normal astrocytic endfoot. (B): in ALF, activation of EGFR and other signaling pathways results in a loss of BBB tight junction integrity. Tight junctional proteins are altered, resulting in increased permeability to small polar molecules, leading to astrocytic endfoot swelling. Figure key shows abbreviations and symbols of Figures 4A and B. (Hepatology 53:1294, 2011).
Highlights.
Brain edema in ALF is associated with BBB dysfunction without overt structural breakdown.
Activation of endothelial cell EGFR receptor by MMP-9 results in subtle perturbations in tight junction integrity leading to increased selective permeability across the BBB in ALF.
A proposed model of the regulation of tight junction selective permeability to small is presented in Figure 4.
Acknowledgments
The electron microscopic figures were provided by Dr. Wenlang Lin in the laboratory of Neuropathology and Microscopy of Dr. Dennis W. Dickson in the Department of Neuroscience at Mayo Clinic in Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Samuel D, Ichai P, Castaing D, Saliba F, Adam R, Savier E, Danaoui M, Smail A, Delvart V, Karam V, Bismuth H. Auxiliary partial orthotopic versus standard orthotopic whole liver transplantation for acute liver failure: a reappraisal from a single center by a case-control study. Ann Surg. 2001;234:723–731. doi: 10.1097/00000658-200112000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem. 2000;78:85–96. [PubMed] [Google Scholar]
- Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci. 1999;112 (Pt 12):1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- Belanger M, Desjardins P, Chatauret N, Butterworth RF. Selectively increased expression of the astrocytic/endothelial glucose transporter protein GLUT1 in acute liver failure. Glia. 2006;53:557–562. doi: 10.1002/glia.20310. [DOI] [PubMed] [Google Scholar]
- Bémeur C, Chastre A, Desjardins P, Butterworth RF. No changes in expression of tight junction proteins or blood-brain barrier permeability in azoxymethane-induced experimental acute liver failure [Letter to the editor] Neurochemistry International. 2010;56:205–207. [Google Scholar]
- Bemeur C, Qu H, Desjardins P, Butterworth RF. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int. 2010a;56:213–215. doi: 10.1016/j.neuint.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Bemeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010b;25:241–249. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–1852. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- Bihari DJ, Gimson AE, Williams R. Cardiovascular, pulmonary and renal complications of fulminant hepatic failure. Semin Liver Dis. 1986;6:119–128. doi: 10.1055/s-2008-1040595. [DOI] [PubMed] [Google Scholar]
- Blei AT. Pathogenesis of brain edema in fulminant hepatic failure. Prog Liver Dis. 1995;13:311–330. [PubMed] [Google Scholar]
- Blei AT. Brain edema in acute liver failure: can it be prevented? Can it be treated? J Hepatol. 2007;46:564–569. doi: 10.1016/j.jhep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin TW, Krigman MR. Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res. 1975;99:444–448. doi: 10.1016/0006-8993(75)90053-0. [DOI] [PubMed] [Google Scholar]
- Brightman MW, Klatzo I, Olsson Y, Reese TS. The blood-brain barrier to proteins under normal and pathological conditions. J Neurol Sci. 1970;10:215–239. doi: 10.1016/0022-510x(70)90151-6. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O’Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar GJ, Humphries P. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10:930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- Castellote J, Llado L, Xiol X, Julia D, Ballester R, Ramos E, Fabregat J, Rafecas A. Successful reuse of liver grafts after death of the first recipient. Clin Transplant. 2006;20:604–608. doi: 10.1111/j.1399-0012.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- Cauli O, Lopez-Larrubia P, Rodrigo R, Agusti A, Boix J, Nieto-Charques L, Cerdan S, Felipo V. Brain region-selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology. 2011;140:638–645. doi: 10.1053/j.gastro.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Chan G, Taqi A, Marotta P, Levstik M, McAlister V, Wall W, Quan D. Long-term outcomes of emergency liver transplantation for acute liver failure. Liver Transpl. 2009;15:1696–1702. doi: 10.1002/lt.21931. [DOI] [PubMed] [Google Scholar]
- Chavarria L, Oria M, Romero-Gimenez J, Alonso J, Lope-Piedrafita S, Cordoba J. Diffusion tensor imaging supports the cytotoxic origin of brain edema in a rat model of acute liver failure. Gastroenterology. 2010;138:1566–1573. doi: 10.1053/j.gastro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Chen F, Hori T, Ohashi N, Baine AM, Eckman CB, Nguyen JH. Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure. Hepatology. 2011;53:1294–1305. doi: 10.1002/hep.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Ohashi N, Li W, Eckman C, Nguyen JH. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology. 2009;50:1914–1923. doi: 10.1002/hep.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ. 13N as a tracer for studying glutamate metabolism. Neurochem Int. 2011;59:456–464. doi: 10.1016/j.neuint.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P, Belanger M, Butterworth RF. Alterations in expression of genes coding for key astrocytic proteins in acute liver failure. J Neurosci Res. 2001;66:967–971. doi: 10.1002/jnr.10045. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Terrault NA, Ishitani M, Reddy KR, Sheiner P, Luketic V, Soldevila-Pico C, Fried M, Jensen D, Brown RS, Jr, Horwith G, Brundage R, Lok A. Protective antibody levels and dose requirements for IV 5% Nabi Hepatitis B immune globulin combined with lamivudine in liver transplantation for hepatitis B-induced end stage liver disease. Liver Transpl. 2006;12:124–133. doi: 10.1002/lt.20582. [DOI] [PubMed] [Google Scholar]
- Dixit V, Chang TM. Brain edema and the blood brain barrier in galactosamine-induced fulminant hepatic failure rats. An animal model for evaluation of liver support systems. ASAIO Trans. 1990;36:21–27. [PubMed] [Google Scholar]
- Ede RJ, Williams RW. Hepatic encephalopathy and cerebral edema. Semin Liver Dis. 1986;6:107–118. doi: 10.1055/s-2008-1040594. [DOI] [PubMed] [Google Scholar]
- Eefsen M, Jelnes P, Schmidt LE, Vainer B, Bisgaard HC, Larsen FS. Brain expression of the water channels Aquaporin-1 and -4 in mice with acute liver injury, hyperammonemia and brain edema. Metab Brain Dis. 2010;25:315–323. doi: 10.1007/s11011-010-9213-y. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- Gove CD, Hughes RD, Ede RJ, Williams R. Regional cerebral edema and chloride space in galactosamine-induced liver failure in rats. Hepatology. 1997;25:295–301. doi: 10.1002/hep.510250207. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Horowitz ME, Schafer DF, Molnar P, Jones EA, Blasberg RG, Patlak CS, Waggoner J, Fenstermacher JD. Increased blood-brain transfer in a rabbit model of acute liver failure. Gastroenterology. 1983;84:1003–1011. [PubMed] [Google Scholar]
- Jalan R. Intracranial hypertension in acute liver failure: pathophysiological basis of rational management. Semin Liver Dis. 2003;23:271–282. doi: 10.1055/s-2003-42645. [DOI] [PubMed] [Google Scholar]
- Jiang W, Desjardins P, Butterworth RF. Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem. 2009a;109:485–493. doi: 10.1111/j.1471-4159.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- Jiang W, Desjardins P, Butterworth RF. Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J Cereb Blood Flow Metab. 2009b;29:944–952. doi: 10.1038/jcbfm.2009.18. [DOI] [PubMed] [Google Scholar]
- Jin Y, Uchida I, Eto K, Kitano T, Abe S. Size-selective junctional barrier and Ca(2+)-independent cell adhesion in the testis of Cynops pyrrhogaster: expression and function of occludin. Mol Reprod Dev. 2008;75:202–216. doi: 10.1002/mrd.20662. [DOI] [PubMed] [Google Scholar]
- Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, Ranjan P, Pandey CM, Narayana PA. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- Kato M, Hughes RD, Keays RT, Williams R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology. 1992;15:1060–1066. doi: 10.1002/hep.1840150615. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004;129:851–860. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Kiptoo P, Sinaga E, Calcagno AM, Zhao H, Kobayashi N, Tambunan US, Siahaan TJ. Enhancement of drug absorption through the blood-brain barrier and inhibition of intercellular tight junction resealing by E-cadherin peptides. Mol Pharm. 2011;8:239–249. doi: 10.1021/mp100293m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzo I. Presidental address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967;26:1–14. doi: 10.1097/00005072-196701000-00001. [DOI] [PubMed] [Google Scholar]
- Knecht K, Michalak A, Rose C, Rothstein JD, Butterworth RF. Decreased glutamate transporter (GLT-1) expression in frontal cortex of rats with acute liver failure. Neurosci Lett. 1997;229:201–203. doi: 10.1016/s0304-3940(97)00444-8. [DOI] [PubMed] [Google Scholar]
- Kristiansen RG, Lindal S, Myreng K, Revhaug A, Ytrebo LM, Rose CF. Neuropathological changes in the brain of pigs with acute liver failure. Scand J Gastroenterol. 2010;45:935–943. doi: 10.3109/00365521003675047. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone AS, Potvin M, Goresky CA, Finlayson MH, Hinchey EJ. Changes in the blood-brain barrier in hepatic coma after hepatectomy in the rat. Gastroenterology. 1977;73:697–704. [PubMed] [Google Scholar]
- Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010;30:1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- Mussack T, Huber SM, Ladurner R, Hummel T, Mutschler W. Bilateral decompressive craniectomy due to intracranial hypertension during acute posttraumatic liver dysfunction. J Trauma. 2005;58:1061–1065. doi: 10.1097/01.ta.0000171989.63817.8c. [DOI] [PubMed] [Google Scholar]
- Nafidi O, Letourneau R, Willems BE, Lapointe RW. Reuse of liver graft from a brain dead recipient. Clin Transplant. 2007;21:773–776. doi: 10.1111/j.1399-0012.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- Nguyen JH, Yamamoto S, Steers J, Sevlever D, Lin W, Shimojima N, Castanedes-Casey M, Genco P, Golde T, Richelson E, Dickson D, McKinney M, Eckman CB. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006;44:1105–1114. doi: 10.1016/j.jhep.2005.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldhafer KJ, Bornscheuer A, Fruhauf NR, Frerker MK, Schlitt HJ, Ringe B, Raab R, Pichlmayr R. Rescue hepatectomy for initial graft non-function after liver transplantation. Transplantation. 1999;67:1024–1028. doi: 10.1097/00007890-199904150-00015. [DOI] [PubMed] [Google Scholar]
- Ott P, Larsen FS. Blood-brain barrier permeability to ammonia in liver failure: a critical reappraisal. Neurochem Int. 2004;44:185–198. doi: 10.1016/s0197-0186(03)00153-0. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lim YS, Hwang S, Heo NY, Lee HC, Suh DJ, Yu E, Lee SG. Emergency adult-to-adult living-donor liver transplantation for acute liver failure in a hepatitis B virus endemic area. Hepatology. 2010;51:903–911. doi: 10.1002/hep.23369. [DOI] [PubMed] [Google Scholar]
- Rai V, Nath K, Saraswat VA, Purwar A, Rathore RK, Gupta RK. Measurement of cytotoxic and interstitial components of cerebral edema in acute hepatic failure by diffusion tensor imaging. J Magn Reson Imaging. 2008;28:334–341. doi: 10.1002/jmri.21438. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Tong X, Curtis KM, Norenberg MD. Brain aquaporin-4 in experimental acute liver failure. J Neuropathol Exp Neurol. 2010;69:869–879. doi: 10.1097/NEN.0b013e3181ebe581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Rentsch M, Meyer J, Andrassy J, Fischer-Frohlich CL, Rust C, Mueller S, Angele M, Lohe F, Jauch KW, Graeb C. Late reuse of liver allografts from brain-dead graft recipients: the Munich experience and a review of the literature. Liver Transpl. 2010;16:701–704. doi: 10.1002/lt.22053. [DOI] [PubMed] [Google Scholar]
- Ringe B, Lubbe N, Kuse E, Frei U, Pichlmayr R. Total hepatectomy and liver transplantation as two-stage procedure. Ann Surg. 1993;218:3–9. doi: 10.1097/00000658-199307000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sanovich E, Bartus RT, Friden PM, Dean RL, Le HQ, Brightman MW. Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7. Brain Res. 1995;705:125–135. doi: 10.1016/0006-8993(95)01143-9. [DOI] [PubMed] [Google Scholar]
- Sawara K, Desjardins P, Chatauret N, Kato A, Suzuki K, Butterworth RF. Alterations in expression of genes coding for proteins of the neurovascular unit in ischemic liver failure. Neurochem Int. 2009;55:119–123. doi: 10.1016/j.neuint.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Shimojima N, Eckman CB, McKinney M, Sevlever D, Yamamoto S, Lin W, Dickson DW, Nguyen JH. Altered expression of zonula occludens-2 precedes increased blood-brain barrier permeability in a murine model of fulminant hepatic failure. J Invest Surg. 2008;21:101–108. doi: 10.1080/08941930802043565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk DB, Williams R. Acute liver failure. Br J Hosp Med. 1979;22:437–446. [PubMed] [Google Scholar]
- Tantawi B, Cherqui D, Duvoux C, Dhumeaux D, Fagniez PL. Reuse of a liver graft five days after initial transplantation. Transplantation. 1996;62:868–869. doi: 10.1097/00007890-199609270-00029. [DOI] [PubMed] [Google Scholar]
- Traber PG, Dal Canto M, Ganger DR, Blei AT. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology. 1987;7:1272–1277. doi: 10.1002/hep.1840070616. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Chung C, Blei AT. Brain edema in acute liver failure. A window to the pathogenesis of hepatic encephalopathy. Ann Hepatol. 2003;2:12–22. [PubMed] [Google Scholar]
- Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist’s view. Brain Res Brain Res Rev. 2003;42:221–242. doi: 10.1016/s0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, Libert C. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lee WM, Cheng CY. Targeted and reversible disruption of the blood-testis barrier by an FSH mutant-occludin peptide conjugate. Faseb J. 2007;21:438–448. doi: 10.1096/fj.05-4144com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, Williams R, Davies C, Moore KP, Jalan R. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007a;45:1517–1526. doi: 10.1002/hep.21599. [DOI] [PubMed] [Google Scholar]
- Wright G, Shawcross D, Olde Damink SW, Jalan R. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis. 2007b;22:375–388. doi: 10.1007/s11011-007-9071-4. [DOI] [PubMed] [Google Scholar]
- Wright G, Soper R, Brooks HF, Stadlbauer V, Vairappan B, Davies NA, Andreola F, Hodges S, Moss RF, Davies DC, Jalan R. Role of aquaporin-4 in the development of brain oedema in liver failure. J Hepatol. 2010;53:91–97. doi: 10.1016/j.jhep.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Nguyen JH. TIMP-1/MMP-9 imbalance in brain edema in rats with fulminant hepatic failure. J Surg Res. 2006;134:307–314. doi: 10.1016/j.jss.2005.11.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]