Abstract
Objective
Hearing loss is a common symptom in patients with cochleovestibular schwannoma (VS). Clinical and histological observations have suggested that the hearing loss may be caused by both retrocochlear and cochlear mechanisms. Our goal was to perform a detailed assessment of cochlear pathology in patients with VS.
Study Design
Retrospective analysis of temporal bone histopathology.
Setting
Multi-center study.
Material
Temporal bones from 32 patients with unilateral, sporadic VS within the internal auditory canal.
Main Outcome Measures
Sections through the cochleae on the VS side and opposite (control) ear were evaluated for loss of inner and outer hair cells, atrophy of the stria vascularis, loss of cochlear neurons, and for presence of endolymphatic hydrops and precipitate within endolymph or perilymph. Observed pathologies were correlated to nerve of origin, VS volume, and distance of VS from the cochlea. Hearing thresholds were also assessed.
Results
VS caused significantly more inner and outer hair cell loss, cochlear neuronal loss, precipitate in endolymph and perilymph, and decreased pure tone average, when compared to the opposite ear. Tumor size, distance from the cochlea, and nerve of origin did not correlate with structural changes in the cochlea or the hearing threshold.
Conclusions
There is significant degeneration of cochlear structures in ears with VS. Cochlear dysfunction may be an important contributor to the hearing loss caused by VS, and can explain certain clinically observed phenomena in patients with VS.
Keywords: cochlear changes, hair cell loss, hearing loss, temporal bone histopathology, vestibular schwannoma
INTRODUCTION
Sensorineural hearing loss (SNHL) is a common symptom caused by a schwannoma of the eighth nerve (acoustic neuroma, vestibular schwannoma [VS]) (1–3). In theory, the hearing loss may occur as a result of dysfunction of the cochlear nerve (a retrocochlear mechanism), or due to dysfunction of the cochlea (a cochlear mechanism), or both mechanisms may occur in a given case. Clinicians commonly think of VS-associated hearing loss as occurring due to dysfunction of the cochlear nerve; supporting evidence includes retrocochlear abnormalities on auditory brainstem response measurements (4,5) and histopathologic data demonstrating atrophy of the cochlear nerve (6,7). Less attention has focused on dysfunction of the cochlea caused by VS as a factor that contributes to the hearing loss. Evidence that such cochlear dysfunction can occur comes from clinical data showing abnormalities in otoacoustic emissions in VS (8–11), and histopathologic data in patients with VS showing pathological changes within the cochlea such as degeneration of the organ of Corti and the stria vascularis, occurrence of endolymphatic hydrops and an acidophilic-staining precipitate within inner ear fluid spaces. Such pathological changes have been reported by multiple authors in small case series ranging from 1 to 11 cases (12–17); in addition, Eckermeier et al. (6) described acidophilic-staining precipitate in a series of 21 sporadic VS cases.
Clinical considerations would also suggest that dysfunction of the cochlea might be common in patients who have hearing loss due to a VS. It has been well established that atrophy of the cochlear nerve (loss of cochlear neurons) disproportionately affects speech discrimination ability rather than pure tone hearing thresholds (18–20). Studies have shown that over 80% of cochlear neurons have to be lost before there is a significant shift in pure tone thresholds, and that such severe neuronal losses are associated with a severe drop in speech discrimination ability (21,22). Clinical data has demonstrated the occurrence of mild to moderate degrees of threshold shift with relatively preserved speech discrimination in patients with a VS: analysis of data pooled from 5 published studies (23–27) showed that a speech discrimination score (SDS) of > 65% was found in all patients (100%) with a preoperative pure tone average (PTA) between 20 – 40 dB HL, in 68% of the patients with a PTA between 40 – 60 dB HL, and in 50% with a PTA between 60 – 80 dB HL. Clinical studies have also shown that hearing loss does not correlate with the size of VS (28,29), indicating that compression of the cochlear nerve within the internal auditory canal may not be the only mechanism in hearing loss. In other words, it may be that the commonly observed hearing losses for pure tones (in the absence of poor speech scores) in patients with VS may be caused by cochlear mechanisms rather than retrocochlear dysfunction. If true, it is possible that cochlear dysfunction is more common in VS than is perceived in clinical practice.
The goal of our temporal bone histopathologic study was to assess the occurrence of cochlear pathology in a relatively large series of patients with VS, by combining cases contained with the temporal bone collections at the Massachusetts Eye and Ear Infirmary (MEEI), House Ear Institute (HEI) in Los Angeles, University of Minnesota at Minneapolis (UM) and University Hospital of Zurich (USZ). Our findings and results have implications for the diagnosis and management of VS.
MATERIAL AND METHODS
The archives of the temporal bone collections at MEEI, HEI, UM and USZ were searched to meet our inclusion criteria of unilateral, untreated VS. Both symptomatic (auditory or vestibular complaints) and occult cases of VS were included. Patients with tumors due to neurofibromatosis type 2 were excluded. The temporal bones had been processed for light microscopy using the standard method of fixation in formalin, decalcification in trichloracetic acid or ethylenediaminetetracetate, embedding in celloidin, serial sectioning in the axial plane at a thickness of 20 microns, and staining of every tenth section using hematoxylin and eosin (30). The respective institutional review boards approved the study.
Sections through the cochleae on the VS side and opposite (control) ear were evaluated for loss of inner and outer hair cells, atrophy of the stria vascularis and loss of cochlear neurons. Visual estimates were noted for the severity of degeneration for each of these structures. The severity of degeneration for each of these structures was evaluated in a semi quantitative manner utilizing rating scales have been used by us and by others for similar assessments (17, 30). Degeneration of inner hair cells was noted as being none (no loss in all turns), mild (less than one-third missing), moderate (one-third to two-thirds missing) and severe (more than two-thirds missing). A similar scale was used for assessment of outer hair cells. The degree of atrophy of the stria vascularis was noted in terms of percentage loss averaged throughout the cochlea and was characterized as none (intact stria in all three turns), mild degeneration (less than 33% atrophy), moderate degeneration (34% to 66% atrophy) and severe degeneration (greater than 67% atrophy). Cell bodies of cochlear neurons in Rosenthal’s canal were compared to normal counts at birth, and degree of atrophy of neurons was characterized as none (no loss), mild degeneration (less than one-third missing compared to neonatal normal), moderate degeneration (one-third to two-thirds missing) and severe degeneration (greater than two-thirds missing). In each bone, the presence of hydrops was assessed by noting the position of Reissner’s membrane in the cochlea, and position of the labyrinthine membranes in the vestibular end organs. Convexity of Reissner’s membrane toward the scala vestibuli was indicative of hydrops in the cochlea. Similarly, distension of the membranous walls of the saccule, utricle or ampullae (compared to normal) was indicative of vestibular hydrops. Finally, the presence or absence of precipitate within the endolymphatic and perilymphatic spaces was assessed.
The nerve of origin of the tumor (vestibular nerve, superior division of vestibular nerve, inferior division of vestibular nerve, or cochlear nerve) was noted in each case. The maximum extent of each tumor in the horizontal plane (x and y dimensions) was measured. Similarly, the height (z dimension) was calculated from the number of vertical sections containing tumor. Tumor volume was then calculated from the x, y and z dimensions, assuming the VS to be an ellipsoid. In addition, the nearest distance of each tumor from the base of the modiolus was measured. Where available, air and bone conduction thresholds and speech discrimination scores were noted from the last audiogram before death. Statistical analyses were performed using Student’s t test, chi-squared test and regression analyses; p < 0.05 was considered as significant.
RESULTS
Our initial search of the temporal bone archives captured 49 patients with 50 sporadic, untreated tumors (17-MEEI, 15-HEI, 11-UM and 6-USZ). Assessment of cochlear pathology was not possible in 9 patients because of severe postmortem artifact, or confounding pathology such as postmeningitic ossification of the cochlea, cochlear implantation or congenital deafness. Seven tumors that were located within the cochlea or the vestibular labyrinth (intralabyrinthine schwannomas) were excluded. One patient had two tumors in the same ear, one within the internal auditory canal and one in the cochlea; this patient was excluded because it was unclear which tumor had caused the observed cochlear pathology. The final analysis consisted of a detailed examination of 32 schwannomas within the internal auditory canal from 32 patients (18 male, 14 female) with a mean age of 72 years (range 25–100 years).
Assessment of Cochlear Pathology
Table 1 shows details of cochlear pathology for each of the 32 patients. The data shown in Table 1 clearly indicate that the vast majority of patients with VS showed cochlear pathology including degeneration of inner hair cells (75%), outer hair cells (88%), stria vascularis (69%), or cochlear neurons (85%). Forty three percent showed a precipitate in the endolymphatic or perilymphatic spaces. Endolymphatic hydrops was seen in 25% (7 cases). The hydrops involved the cochlea in all 7 cases; the saccule was also distended in 4 of the 7 cases.
Table 1.
Details of Cochlear Pathology in IAC Schwannomas
| Case | Age | Side | Nerve of Origin | IHC | OHC | Stria | Neurons | Hydrops | Precipitate
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| EL | PL | |||||||||
| B1 | M / 81 | Tumor | SV | + | ++ | ++ | + | − | + | + |
| contralateral | − | − | + | − | − | − | − | |||
|
| ||||||||||
| B2 | M / 84 | Tumor | SV | + | + | ++ | + | − | − | − |
| contralateral | − | − | + | − | − | |||||
|
| ||||||||||
| B3 | M / 74 | Tumor | IV | + | ++ | + | +++ | − | − | + |
| contralateral | not available | |||||||||
|
| ||||||||||
| B4 | M / 75 | Tumor | VN | ++ | +++ | ++ | +++ | + | + | + |
| contralateral | + | + | ++ | − | − | − | ||||
|
| ||||||||||
| B5 | F / 100 | Tumor | VN | ++ | ++ | ++ | +++ | − | − | − |
| contralateral | − | − | + | ++ | − | − | − | |||
|
| ||||||||||
| B6 | F / 74 | Tumor | IV | + | + | − | − | − | − | − |
| contralateral | + | + | − | − | − | − | − | |||
|
| ||||||||||
| B7 | M / 79 | Tumor | SV | − | − | − | + | − | − | − |
| contralateral | not available | |||||||||
|
| ||||||||||
| B8 | M / 80 | Tumor | IV | − | − | + | − | − | − | − |
| contralateral | − | − | − | − | − | − | − | |||
|
| ||||||||||
| B9 | F / 75 | Tumor | IV | − | − | + | + | − | − | − |
| contralateral | − | − | + | + | − | − | − | |||
|
| ||||||||||
| B10 | M / 80 | Tumor | SV | − | + | ++ | + | − | − | + |
| contralateral | not available | |||||||||
|
| ||||||||||
| B11 | F / 81 | Tumor | SV | +++ | +++ | + | + | + | − | − |
| contralateral | not available | |||||||||
|
| ||||||||||
| B12 | F / 74 | Tumor | VN | +++ | +++ | − | + | + | + | + |
| contralateral | +++ | +++ | − | − | + | − | − | |||
|
| ||||||||||
| Z1 | M / 25 | Tumor | VN | + | ++ | − | ++ | + | + | + |
| contralateral | + | + | + | + | − | + | + | |||
|
| ||||||||||
| Z2 | M / 87 | Tumor | VN | +++ | ++ | ++ | ++ | − | − | − |
| contralateral | not available | |||||||||
|
| ||||||||||
| Z3 | F / 57 | Tumor | SV | − | + | − | + | − | − | − |
| contralateral | not available | |||||||||
|
| ||||||||||
| Z4 | F / 53 | Tumor | VN | + | + | − | ++ | − | − | + |
| contralateral | not available | |||||||||
|
| ||||||||||
| Z5 | F / 72 | Tumor | VN | + | ++ | ++ | + | − | + | + |
| contralateral | not available | |||||||||
|
| ||||||||||
| Z6 | F / 39 | Tumor | VN | ++ | ++ | − | ++ | − | + | + |
| contralateral | − | + | − | + | − | − | + | |||
|
| ||||||||||
| M1 | M / 72 | Tumor | VN | +++ | +++ | − | + | − | − | − |
| contralateral | not available | |||||||||
|
| ||||||||||
| M2 | M / 77 | Tumor | SV | + | ++ | + | + | − | − | − |
| contralateral | + | ++ | + | + | − | − | − | |||
|
| ||||||||||
| M3 | M / 86 | Tumor | Co | +++ | +++ | +++ | +++ | + | + | + |
| contralateral | + | + | − | − | − | − | + | |||
|
| ||||||||||
| M4 | M / 68 | Tumor | SV | +++ | +++ | + | + | − | − | − |
| contralateral | +++ | +++ | + | + | + | − | − | |||
|
| ||||||||||
| M5 | M / 81 | Tumor | SV | ++ | ++ | − | +++ | − | + | + |
| contralateral | not available | |||||||||
|
| ||||||||||
| M6 | M / 67 | Tumor | SV | ++ | +++ | + | ++ | − | − | − |
| contralateral | ++ | +++ | + | + | − | − | − | |||
|
| ||||||||||
| M7 | M / 76 | Tumor | VN | + | +++ | − | ++ | + | + | + |
| contralateral | + | + | + | ++ | − | − | − | |||
|
| ||||||||||
| M8 | M / 52 | tumor | Co | − | + | + | + | − | − | − |
| contralateral | − | + | + | + | − | − | − | |||
|
| ||||||||||
| M9 | M / 44 | tumor | VN | + | + | + | − | − | + | + |
| contralateral | − | + | + | − | − | − | − | |||
|
| ||||||||||
| H1 | F / 69 | tumor | IV | +++ | +++ | ++ | + | − | − | + |
| contralateral | − | + | − | − | − | − | − | |||
|
| ||||||||||
| H2 | F / 79 | tumor | SV | − | + | ++ | + | − | − | − |
| contralateral | − | + | ++ | + | + | − | − | |||
|
| ||||||||||
| H3 | F / 76 | tumor | IV | + | ++ | + | + | − | − | − |
| contralateral | − | − | + | − | − | − | − | |||
|
| ||||||||||
| H4 | F / 71 | tumor | IV | − | − | + | − | − | − | − |
| contralateral | − | − | + | − | − | − | − | |||
|
| ||||||||||
| H5 | F / 94 | tumor | IV | + | +++ | ++ | − | + | − | − |
| contralateral | + | ++ | + | − | − | − | − | |||
IHC=inner hair cells, OHC=outer hair cells, Stria=strial atrophy, Neurons=cochlear neurons, EL=endolymph, PL=perilymph, IV=inferior division of vestibular nerve, SV=superior division of vestibular nerve, VN=vestibular nerve, Co=cochlear nerve.
Rating scale for IHC, OHC, Stria, and Neurons: +++ = 67 – 100% loss, ++ = 34 – 66% loss, + = 1 – 33% loss, − = 0% loss
For hydrops and precipitate: + indicates presence, − indicates absence
The contralateral ear was available for assessment in 22 out of the 32 cases, thus serving as a control. The severity of the cochlear pathology on the tumor side compared to the opposite ear in these 22 patients is shown in Table 2. The tumor side had significantly more inner hair cell loss (p=0.003), outer hair cell loss (p=0.0004), and cochlear neuronal loss (p=0.002) (t test). There was a trend toward greater severity of strial atrophy on the tumor side, although it did not reach the level of significance (p=0.07) (t test). Patients with tumors had significantly more precipitate in the endolymph (p=0.0001) and perilymph (p=0.0002) (chi-square analysis). There was also a trend for occurrence of endolymphatic hydrops on the tumor side, but it did not reach statistical significance (p=0.06) (chi-square test). Figures 1–3 show examples of VS in two patients, showing the occurrence of cochlear degeneration on the tumor side compared to the contralateral side.
Table 2.
Summary of Cochlear Pathology in IAC Schwannomas in 22 unilateral VS cases with available contralateral ears
| Pathology in cochlea | Severity of Change | Tumor Side (n) | Contralateral Side (n) |
|---|---|---|---|
| IHC Loss | none (−) | 5 | 12 |
| mild (+) | 9 | 7 | |
| moderate (++) | 4 | 1 | |
| severe (+++) | 4 | 2 | |
|
| |||
| OHC Loss | none (−) | 3 | 7 |
| mild (+) | 5 | 10 | |
| moderate (++) | 6 | 2 | |
| severe (+++) | 8 | 3 | |
|
| |||
| Strial Atrophy | none (−) | 5 | 6 |
| mild (+) | 9 | 14 | |
| moderate (++) | 7 | 2 | |
| severe (+++) | 1 | 0 | |
|
| |||
| Cochlear Neuronal Loss | none (−) | 5 | 12 |
| mild (+) | 10 | 8 | |
| moderate (++) | 4 | 2 | |
| severe (+++) | 3 | 0 | |
|
| |||
| Hydrops | no | 16 | 19 |
| yes | 6 | 3 | |
|
| |||
| Precipitate in Endolymph | no | 14 | 21 |
| yes | 8 | 1 | |
|
| |||
| Precipitate in Perilymph | no | 13 | 19 |
| yes | 9 | 3 | |
IHC=inner hair cell, OHC=outer hair cell
Figure 1.
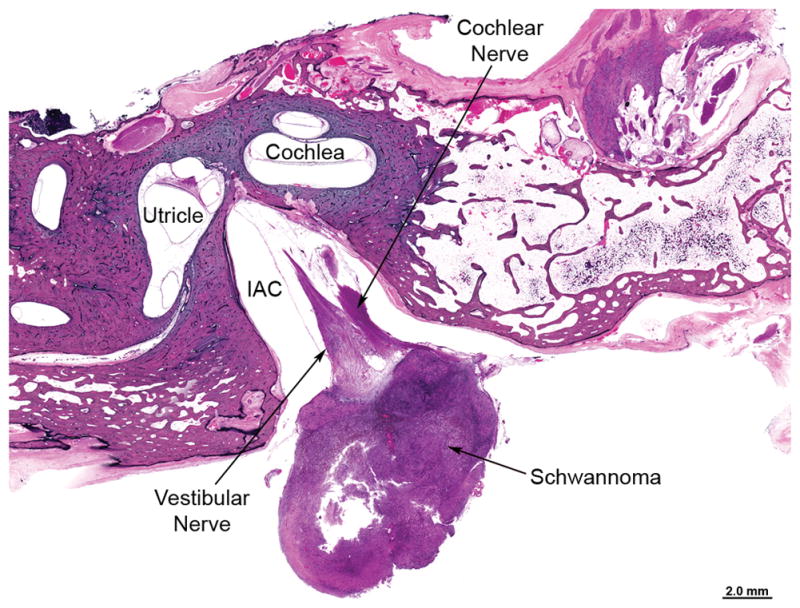
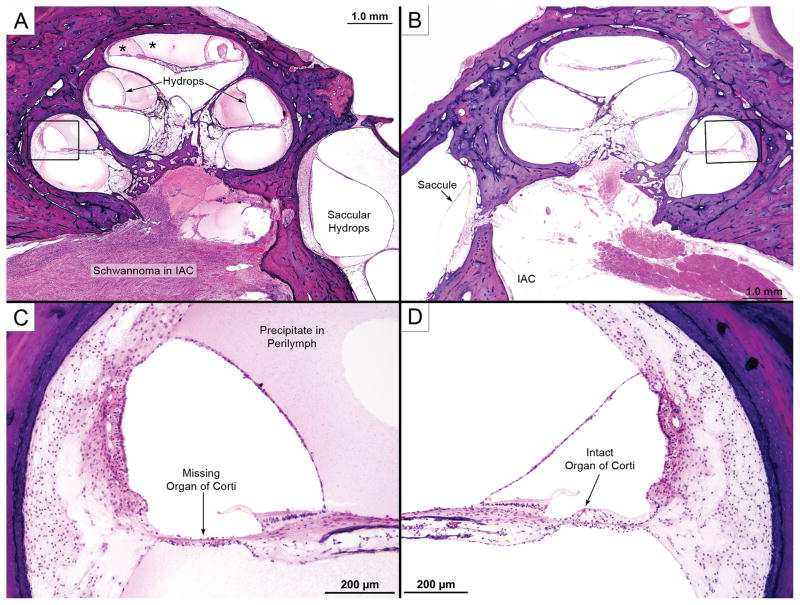
100-year old woman (Case B5 in Table 1) with untreated solitary vestibular schwannoma (VS) approximately 11×8×8 cm in size located at the entrance of the internal auditory canal (IAC) in the left temporal bone. The VS was located mainly in the cerebellopontine angle and did not come close to the cochlea in any section.
Figure 3.
Views of the distal part of the internal auditory canal (IAC), saccule and cochlea from a 75-year old man (Case B4 in Table 1). (A) shows a VS in the IAC that reached the fundus but did not invade the inner ear. The cochlea showed acidophilic precipitate within both the endolymphatic and perilymphatic spaces (*) as well as endolymphatic hydrops in all turns. There was also hydrops of the saccule. (B) shows comparable view from the contralateral ear showing lack of hydrops and lack of precipitate within inner ear fluids. Higher power views of boxed areas are shown in (C) and (D): there was hair cell loss on the VS side (C), with an intact organ of Corti in the opposite ear (D).
Of the 22 patients with both ears available, 8 (36%) had occult tumors, 11 (15%) had symptomatic tumors, while the existence of symptoms was indeterminate in 3 (14%) patients because an accurate history was not available. Symptomatic patients showed significantly more inner hair cells loss (p=0.03), outer hair cell loss (p=0.01), and cochlear neuronal loss (p=0.01), but not more strial atrophy (p=0.19) (t test). Symptomatic ears also showed significantly more hydrops (p=0.002) and significantly more precipitate in the endolymph (p=0.0001) and perilymph (p=0.007) (chi-square analysis). Patients with occult neoplasms did not reveal significant differences for any of the cochlear structures between the two sides.
Correlation of Cochlear Pathology with Tumor Morphology
We also performed analyses to determine if the observed cochlear pathology was correlated with morphologic aspects of the tumor such as its volume, distance from the cochlea and nerve of origin.
Tumor volume
Mean tumor volume was 76 mm3 (range, 0.06 – 502.65 mm3). No significant correlations were observed between tumor volume and degree of cochlear degeneration for all 32 cases, except for precipitate in the endolymph, which was more common in large tumors (p=0.045). A t test comparing tumor volume across different categories for degeneration of inner hair cells, outer hair cells, strial atrophy, neuronal loss, presence of hydrops and precipitate found only one significant difference: large tumors showed significant inner ear hair cell loss (p=0.009).
Distance of tumor from cochlea
The nearest distance from each tumor to the modiolus was measured and compared to degeneration within the cochlear structures. The mean distance was 1.94 mm (range, 0 to 3.8 mm). No significant correlations were observed between the measured distance and cochlear degeneration.
Nerve of origin
There were 11 tumors arising from the vestibular nerve, 11 from the superior division of the vestibular nerve and 8 from the inferior division. There were only 2 schwannomas arising from the cochlear nerve and they were excluded from this analysis. There were no significant differences between nerve of origin and occurrence of inner hair cell loss, outer hair cell loss, strial atrophy and cochlear neuronal loss (t test). Tumors originating from the vestibular nerve showed significantly more hydrops (p=0.002) and more precipitate in the endolymph/perilymph (p=0.006) (chi-squared test).
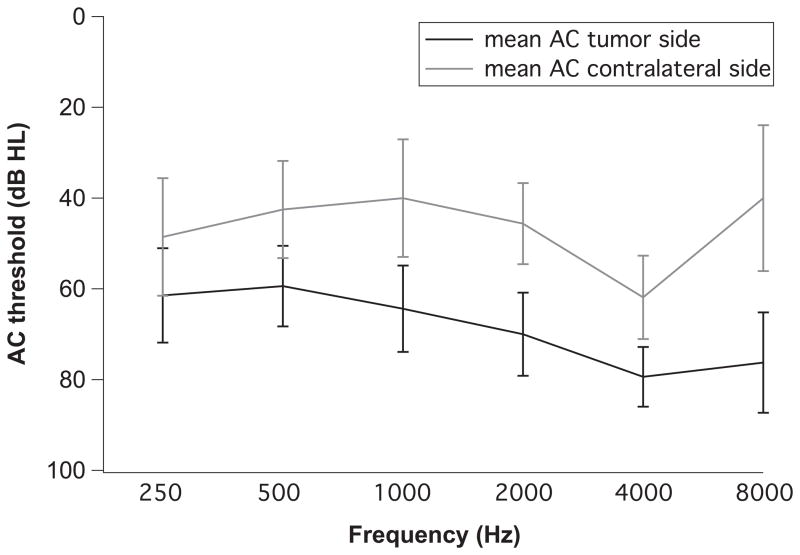
Audiometric Analysis
Eight patients had an audiogram recorded within six years of death (mean 2 years, range 1 month to 6 years). Figure 4 shows the mean and standard deviation of the air conduction (AC) averages at various pure tones for the tumor side compared to the contralateral side. The AC threshold averaged for 0.25, 0.5, 1, 2, 4 and 8 kHz was 70.5 dB on the tumor side and 50.6 dB on the contralateral side (a significant difference, p=0.04, t test).
Figure 4.
Comparison of pure tone average for air conduction (AC) thresholds for the tumor side and the contralateral side. Standard deviation is indicated by error bars.
Bone conduction (BC) thresholds for frequencies 0.5, 1, 2 and 4 kHz were available in 7 patients. The average for these frequencies ranged from 19 dB to 70 dB with a mean of 49 dB for patients with tumors, and from 21 dB to 54 dB with a mean of 35 dB for the non-tumor side. The difference was not significant (p = 0.09).
Speech discrimination scores were available in 5 patients, ranging from 0 to 96% with a mean of 49 % on the tumor side, and a range of 52 to 100% and a mean of 82% for the contralateral side. Due to the small N and the large variance, this rather large difference in the mean score between the tumor and the contralateral side was not significant (p=0.15).
No significant correlations were observed between tumor volumes and AC thresholds, BC thresholds and speech discrimination scores. Similarly, no significant correlations were noted between the distance of the tumor to the cochlea and AC thresholds, BC thresholds and speech scores. Statistical analysis of audiometric data verses nerve of origin was not possible because of low number in each group.
DISCUSSION
By using the contralateral side as a control (which allows one to eliminate confounding variables such as effects of age and genetics in the causation of the observed cochlear pathology), we found significantly more inner hair cell loss, outer hair cell loss, cochlear neuronal loss and precipitate in the inner ear fluid on the tumor side in the 22 VS cases. In addition, ears with VS showed significantly more hydrops when the analysis was restricted to symptomatic tumors. Audiometric data was available in 8 of 22 patients, and significantly elevated AC thresholds were observed on the tumor side. Patients with VS also showed worse BC thresholds and speech discrimination scores, but these trends were not significant, probably because of small numbers and large variance in the audiometric data. We also attempted to ascertain if cochlear pathology could be correlated to certain morphological aspects of the schwannomas. With a few exceptions, we did not find significant correlations between the occurrence of cochlear pathology and tumor volume, distance of tumor to the modiolus and nerve of origin of tumor.
What might be the mechanisms responsible for the cochlear abnormalities? Direct invasion of the modiolus or the cochlea by schwannoma was not observed in any case, and therefore, is unlikely to be a mechanism. One possibility is vascular compromise (11,15). We evaluated specimens in the MEEI collection and did not find evidence for occlusion of cochlear blood vessels on the VS side, nor did we find a decrease in the number of vessels within the cochlea compared to the contralateral side. These observations notwithstanding, ischemia of the cochlea caused by compression of the labyrinthine artery by a VS remains a possible mechanism.
Tumors are known to produce a large number of cytokines (31,32) and VS is probably no exception. It has been shown that cytokine balance within the cochlea is critical for its homeostasis (33). It is possible that cytokines produced by a VS might be responsible for inducing the observed degenerative changes within the labyrinth.
The acidophilic precipitate within the endolymphatic and perilymphatic fluids seen in many patients with VS deserves mention. This abnormality was described in temporal bone specimens many decades ago (12,14,16). Silverstein et al. (34) showed that the perilymph of patients with VS has a very high concentration of protein, and it has been assumed that the acidophilic precipitate represents such protein. In fact, before the advent of imaging, high protein concentration in a perilymphatic ‘tap’ was used as a diagnostic test to differentiate between hearing loss caused by VS verses Meniere’s syndrome (35,36). More recently, proteomic analysis of inner ear fluids has shown a higher total protein content in VS patients compared to controls (37).
The occurrence of cochlear pathology in patients with VS has several implications that are of clinical relevance. It is possible, even likely, that some of the audiometric hearing loss for pure tones in patients with VS is caused by cochlear pathology. A retrocochlear mechanism alone cannot explain adequately pure tone losses in many patients with VS, since studies have shown that pure tone threshold shifts occur only after losses of over 80–90% of cochlear nerve fibers (21). Speech discrimination is affected disproportionately with atrophy of the cochlear nerve (18–20); thus, a purely retrocochlear mechanism of hearing loss would mean that pure tone threshold shifts would almost always be accompanied by severe decrement in speech discrimination. Clinical experience has clearly shown that many patients with VS have relative preservation of speech discrimination along with pure tone hearing loss (23–27). Therefore, it is logical to conclude that cochlear pathology of the type observed in this study contributes to the pure tone threshold shifts in VS. The lack of correlation between tumor size and hearing loss reported in the present study as well as in previous clinical reports (28,29) also suggests that neural mechanisms caused by compression of the cochlear nerve are not alone responsible for hearing loss in patients with VS.
The occurrence of cochlear pathology has implications regarding the accuracy of otoacoustic emissions (OAE) and auditory brain stem response (ABR) testing as diagnostic tools for VS. If one assumes that VS affects retrocochlear function and has little effect on the cochlea itself (4,5), then VS is expected to result in pathologic ABR with preservation of OAEs. Clinical experience has not borne out this prediction, and it has been observed that OAEs are often absent or compromised in VS (11,38,39). The occurrence of cochlear pathology (e.g. losses of outer hair cells) as shown in the present study can explain why emissions may be absent in VS. Clinical experience has also shown that a small number of tumors with audiometrically documented hearing loss demonstrate a normal ABR (40), an observation that can be explained on the basis of a schwannoma causing hearing loss by purely cochlear mechanisms.
Sudden loss of hearing is occasionally observed in patients with VS (41–43). The clinical presentation is similar to that seen in the syndrome of idiopathic, sudden sensorineural hearing loss. Furthermore, clinical experience has also shown that sudden hearing loss in VS is usually quite responsive to steroid therapy (44,45). The mechanism of sudden deafness in a patient with a VS is not known, and there have been no temporal bone case reports (to our knowledge) where a patient with VS had sudden deafness during life. On theoretical grounds, it is difficult to conceive of a retrocochlear mechanism of hearing loss that would be sudden in onset and responsive to steroids. On the other hand, a VS could induce biochemical changes in the cochlea which might result in sudden deafness, and which might also be reversed by steroid therapy. We hypothesize that cochlear pathology is responsible for the sudden deafness type of presentation seen in a small number of patients with VS.
The occurrence of cochlear pathology has implications for hearing outcomes after treatment of VS. Clinical experience has shown that it is rare for a pure tone hearing loss to recover after complete surgical removal of a VS, but it is possible to preserve hearing at the preoperative level (46,47). If the hearing loss were caused by a retrocochlear mechanism, one might expect that tumor removal could result in hearing improvement in at least some cases. On the other hand, cochlear pathology such as loss of hair cells or strial atrophy (induced by VS) is unlikely to reverse, which may explain why successful tumor removal does not result in hearing improvement.
Treatment of VS with stereotactic radiation or gamma knife radiosurgery often results in cessation of further growth of the tumor. Studies that have assessed long-term hearing outcomes have shown that there is a deterioration of hearing in a small number of patients, at a rate greater than that expected for age (48). The reason(s) for such hearing deterioration is not known. One explanation may be continued progression of cochlear pathology of the type observed in our study. Such progression of pathology may be caused by continued production of cytokines by the tumor causing interference with cochlear homeostasis, or continued ischemic compromise of the vascular supply to the cochlea, or by other (unknown) mechanisms. Continued progression of cochlear pathology may also be one of the reasons why hearing preservation is often unsuccessful when the internal auditory canal is simply decompressed or when a VS is partially removed (49).
With the advent of molecular biology, targeted therapies are being increasingly developed for treatment of VS. For example, avastin and other drugs have been shown to be beneficial in patients with neurofibromatosis type 2 (50,51), and efforts are ongoing to develop similar therapies for patients with sporadic VS (52). The ultimate goals of such targeted therapy are cessation of tumor growth and preservation of hearing function. In order to achieve both goals, it will be important to not only stop VS growth, but also to counteract degenerative changes in the cochlea of the type that we observed in our study. Research is needed to understand the mechanisms by which VS induce cochlear pathology to ensure that these new molecular therapies achieve their promise.
CONCLUSIONS
There is significant degeneration of cochlear structures in ears with VS. Cochlear dysfunction may be an important contributor to the hearing loss caused by a VS, and can explain certain clinically observed phenomena in patients with VS.
Figure 2.
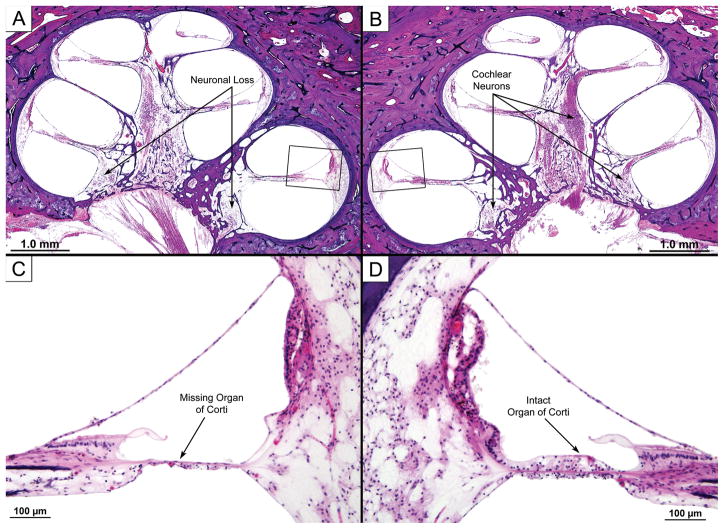
Same case as Fig. 1. Photomicrographs of cochlea on VS side are shown along with similar views from the contralateral ear. The low power views demonstrate loss of neuronal cells within the modiolus on the VS side (A) compared to the opposite ear (B). High power views of boxed areas show that the organ of Corti in the basal turn was missing (C), while it was preserved on the contralateral side (D). The hair cell and neuronal losses were likely to have been caused by the VS.
Acknowledgments
We thank Jon Pack for the photomicrographs. Supported by NIH (NIDCD) grant U24 DC011943, and the “Nachwuchsförderungskredit” of the University of Zurich.
References
- 1.Harner SG, Fabry DA, Beatty CW. Audiometric findings in patients with acoustic neuroma. Am J Otol. 2000;21:405–11. doi: 10.1016/s0196-0709(00)80052-6. [DOI] [PubMed] [Google Scholar]
- 2.Saliba I, Martineau G, Chagnon M. Asymmetric hearing loss: rule 3,000 for screening vestibular schwannoma. Otol Neurotol. 2009;30:515–21. doi: 10.1097/MAO.0b013e3181a5297a. [DOI] [PubMed] [Google Scholar]
- 3.Gimsing S. Vestibular schwannoma: when to look for it? J Laryngol Otol. 2010;124:258–64. doi: 10.1017/S0022215109991423. [DOI] [PubMed] [Google Scholar]
- 4.Selters WA, Brackmann DE. Acoustic tumor detection with brainstem electric response audiometry. Arch Otolaryngol. 1977;103:181–7. doi: 10.1001/archotol.1977.00780210037001. [DOI] [PubMed] [Google Scholar]
- 5.Bozorg Grayeli A, Refass A, Smail M, et al. Diagnostic value of auditory brainstem responses in cerebellopontine angle tumours. Acta Otolaryngol. 2008;128:1096–100. doi: 10.1080/00016480701881803. [DOI] [PubMed] [Google Scholar]
- 6.Eckermeier L, Pirsig W, Mueller D. Histopathology of 30 non-operated acoustic schwannomas. Arch Otorhinolaryngol. 1979;222:1–9. doi: 10.1007/BF00456332. [DOI] [PubMed] [Google Scholar]
- 7.Johnsson LG, Hawkins JE, Jr, Rouse RC. Sensorineural and vascular changes in an ear with acoustic neurinoma. Am J Otolaryngol. 1984;5(1):49–59. doi: 10.1016/s0196-0709(84)80020-4. [DOI] [PubMed] [Google Scholar]
- 8.Bonfils P, Uziel A. Evoked otoacoustic emissions in patients with acoustic neuromas. Am J Otol. 1988;9:412–17. [PubMed] [Google Scholar]
- 9.Prasher DK, Tun T, Brookes GB, et al. Mechanisms of hearing loss in acoustic neuroma: an otoacoustic emission study. Acta Otolaryngol. 1995;115:375–81. doi: 10.3109/00016489509139332. [DOI] [PubMed] [Google Scholar]
- 10.Telischi F. An objective method of analyzing cochlear versus noncochlear patterns of distortion-product otoacoustic emissions in patients with acoustic neuromas. Laryngoscope. 2000;110:553–62. doi: 10.1097/00005537-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Gouveris HT, Victor A, Mann WJ. Cochlear origin of early hearing loss in vestibular schwannoma. Laryngoscope. 2007;117:680–3. doi: 10.1097/MLG.0b013e31803146c5. [DOI] [PubMed] [Google Scholar]
- 12.Dix MR, Hallpike CS. Observations on the Pathological Mechanism of Conductive Deafness in Certain Cases of Neuroma of the VIII Nerve. Proc R Soc Med. 1950;43:291–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Perez de Moura LF. Inner ear pathology in acoustic neuroma. Arch Otolaryngol. 1967;85:125–33. doi: 10.1001/archotol.1967.00760040127002. [DOI] [PubMed] [Google Scholar]
- 14.Schuknecht HF, McNeill RA. Light microscopic observations on the pathology of endolymph. J Laryngol Otol. 1966;80:1–10. doi: 10.1017/s0022215100064902. [DOI] [PubMed] [Google Scholar]
- 15.Suga F, Lindsay JR. Inner ear degeneration in acoustic neurinoma. Ann Otol Rhinol Laryngol. 1976;85:343–58. doi: 10.1177/000348947608500305. [DOI] [PubMed] [Google Scholar]
- 16.Nager GT. Acoustic schwannomas. In: Nager GT, Hyams VJ, editors. Pathology of the Ear and Temporal Bone. Baltimore: Williams and Wilkins; 1993. pp. 515–67. [Google Scholar]
- 17.Mahmud MR, Kahn AM, Nadol JB., Jr Histopathology of the inner ear in unoperated acoustic neuroma. Ann Otol Rhinol Laryngol. 2003;112:979–86. doi: 10.1177/000348940311201111. [DOI] [PubMed] [Google Scholar]
- 18.Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–46. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Pauler M, Schuknecht HF, Thornton AR. Correlative studies of cochlear neuronal loss with speech discrimination and pure-tone thresholds. Arch Otorhinolaryngol. 1986;243:200–6. doi: 10.1007/BF00470622. [DOI] [PubMed] [Google Scholar]
- 20.Schuknecht HF. Auditory and cytocochlear correlates of inner ear disorders. Otolaryngol Head Neck Surg. 1994;110:530–8. doi: 10.1177/019459989411000610. [DOI] [PubMed] [Google Scholar]
- 21.Schuknecht HF, Woellner RC. An experimental and clinical study of deafness from lesions of the cochlear nerve. J Laryngol Otol. 1955;69:75–97. doi: 10.1017/s0022215100050465. [DOI] [PubMed] [Google Scholar]
- 22.Jerger J. Audiological manifestations of lesions in the auditory nervous system. Laryngoscope. 1960;70:417–25. doi: 10.1288/00005537-196004000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Nadol JB, Jr, Levine R, Ojemann RG, et al. Preservation of hearing in surgical removal of acoustic neuromas of the internal auditory canal and cerebellar pontine angle. Laryngoscope. 1987;97:1287–94. doi: 10.1288/00005537-198711000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Chee GH, Nedzelski JM, Rowed D. Acoustic neuroma surgery: the results of long-term hearing preservation. Otol Neurotol. 2003;24:672–6. doi: 10.1097/00129492-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Gjuric M, Mitrecic MZ, Greess H, et al. Vestibular schwannoma volume as a predictor of hearing outcome after surgery. Otol Neurotol. 2007;28:822–7. doi: 10.1097/MAO.0b013e318068b2b0. [DOI] [PubMed] [Google Scholar]
- 26.Kim CH, Chung KW, Kong DS, et al. Prognostic factors of hearing preservation after gamma knife radiosurgery for vestibular schwannoma. J Clin Neurosci. 2010;17:214–8. doi: 10.1016/j.jocn.2009.07.087. [DOI] [PubMed] [Google Scholar]
- 27.Woodson EA, Dempewolf RD, Gubbels SP, et al. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol. 2010;31:1144–52. doi: 10.1097/MAO.0b013e3181edb8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arriaga MA, Long S, Nelson R. Clinical correlates of acoustic neuroma volume. Am J Otol. 1993;14:465–8. doi: 10.1097/00129492-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Nadol JB, Jr, Diamond PF, Thornton AR. Correlation of hearing loss and radiologic dimensions of vestibular schwannomas (acoustic neuromas) Am J Otol. 1996;17:312–6. [PubMed] [Google Scholar]
- 30.Merchant SN. Methods of removal, preparation and study. In: Merchant SN, Nadol JB, editors. Schuknecht’s pathology of the ear. Shelton: PMPH; 2010. pp. 3–51. [Google Scholar]
- 31.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams JC. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002;23:316–22. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein H. Labyrinthine tap as a diagnostic test for acoustic neurinoma. Otolaryngol Clin North Am. 1973;6:229–44. [PubMed] [Google Scholar]
- 35.Silverstein H. A rapid protein test for acoustic neurinoma. Arch Otolaryngol. 1972;95:202–4. doi: 10.1001/archotol.1972.00770080344003. [DOI] [PubMed] [Google Scholar]
- 36.Silverstein H, Schuknecht HF. Biochemical studies of inner ear fluid in man. Changes in otosclerosis, meniere’s disease, and acoustic neuroma. Arch Otolayngol. 1966;84:395–402. doi: 10.1001/archotol.1966.00760030397003. [DOI] [PubMed] [Google Scholar]
- 37.Lysaght AC, Kao SY, Paulo JA, et al. Proteome of Human Perilymph. J Proteome Res. 2011;10:3845–51. doi: 10.1021/pr200346q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telischi FF, Roth J, Stagner BB, et al. Patterns of evoked otoacoustic emissions associated with acoustic neuromas. Laryngoscope. 1995;105:675–82. doi: 10.1288/00005537-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Ferri GG, Modugno GC, Calbucci F, et al. Hearing loss in vestibular schwannomas: analysis of cochlear function by means of distortion-product otoacoustic emissions. Auris Nasus Larynx. 2009;36:644–8. doi: 10.1016/j.anl.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Murofushi T, Takehisa M. Vestibular schwannoma with absent vestibular evoked myogenic potentials to clicks but normal ABR, caloric responses and vestibular evoked myogenic potentials to 500 Hz tone bursts. Acta Otolaryngol. 2010;130:525–8. doi: 10.3109/00016480903258016. [DOI] [PubMed] [Google Scholar]
- 41.Chaimoff M, Nageris BI, Sulkes J, et al. Sudden hearing loss as a presenting symptom of acoustic neuroma. Am J Otolaryngol. 1999;20:157–60. doi: 10.1016/s0196-0709(99)90063-7. [DOI] [PubMed] [Google Scholar]
- 42.Nageris BI, Popovtzer A. Acoustic neuroma in patients with completely resolved sudden hearing loss. Ann Otol Rhinol Laryngol. 2003;112:395–7. doi: 10.1177/000348940311200501. [DOI] [PubMed] [Google Scholar]
- 43.Sauvaget E, Kici S, Kania R, et al. Sudden sensorineural hearing loss as a revealing symptom of vestibular schwannoma. Acta Otolaryngol. 2005;125:592–5. doi: 10.1080/00016480510030246. [DOI] [PubMed] [Google Scholar]
- 44.Berenholz LP, Eriksen C, Hirsh FA. Recovery from repeated sudden hearing loss with corticosteroid use in the presence of an acoustic neuroma. Ann Otol Rhinol Laryngol. 1992;101:827–31. doi: 10.1177/000348949210101005. [DOI] [PubMed] [Google Scholar]
- 45.Aronzon A, Ruckenstein MJ, Bigelow DC. The efficacy of corticosteroids in restoring hearing in patients undergoing conservative management of acoustic neuromas. Otol Neurotol. 2003;24:465–8. doi: 10.1097/00129492-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Tringali S, Ferber-Viart C, Fuchsmann C, et al. Hearing preservation in retrosigmoid approach of small vestibular schwannomas: prognostic value of the degree of internal auditory canal filling. Otol Neurotol. 2010;31:1469–72. [PubMed] [Google Scholar]
- 47.Rabelo de Freitas M, Russo A, Sequino G, et al. Analysis of Hearing Preservation and Facial Nerve Function for Patients Undergoing Vestibular Schwannoma Surgery: The Middle Cranial Fossa Approach versus the Retrosigmoid Approach - Personal Experience and Literature Review. Audiol Neurootol. 2011;17:71–81. doi: 10.1159/000329362. [DOI] [PubMed] [Google Scholar]
- 48.Roos DE, Potter AE, Brophy BP. Stereotactic Radiosurgery for Acoustic Neuromas: What Happens Long Term? Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.04.068. (in press) [DOI] [PubMed] [Google Scholar]
- 49.Gadre AK, Kwartler JA, Brackmann DE, et al. Middle fossa decompression of the internal auditory canal in acoustic neuroma surgery: a therapeutic alternative. Laryngoscope. 1990;100:948–52. doi: 10.1288/00005537-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361:358–67. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12:14–8. doi: 10.1093/neuonc/nop010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blair KJ, Kiang A, Wang-Rodriguez J, et al. EGF and bFGF promote invasion that is modulated by PI3/Akt kinase and Erk in vestibular schwannoma. Otol Neurotol. 2011;32:308–14. doi: 10.1097/MAO.0b013e318206fc3d. [DOI] [PubMed] [Google Scholar]