Abstract
Background
Limitation in the activities of daily living (ADLs) is strongly prognostic for mortality. Current ADL assessments based on numbers of limitations (counts) obscure the particular activities limited, thus lacking clinical interpretability.
Objectives
To examine the independent association of 5 stages of ADL with mortality after accounting for known diagnostic and sociodemographic risk factors.
Design
For five stages (ADL 0 to IV), describing both the severity and pattern of ADLs limited, we estimated unadjusted life expectancies and adjusted associations with mortality using a Cox proportional hazards regression model.
Setting
Community
Participants
Included were 9,447 persons 70 years of age and older from the second Longitudinal Study of Aging.
Measurements
1-, 5-, and 10-year survival and time to death.
Results
For those with no ADL limitations, the median life expectancy was 10.6 years compared to 6.5, 5.1, 3.8, and 1.6 years for those at ADL I, II, III, and IV, respectively. The sociodemographic and diagnostic-adjusted hazard of death at 1 year was 5-fold greater at stage IV compared to stage 0 (hazard ratio=5.6; 95% confidence interval, 3.8–8.3). The associations of ADL stage with mortality declined over time, but remained statistically significant at 5 and 10 years.
Conclusion
ADL stage continued to explain mortality risk after adjusting for known risk factors including advanced age, stroke, and cancer. ADL stages might aid clinical care planning and policy as a powerful prognostic indicator particularly of short-term mortality, improving on current ADL measures by profiling activity limitations of relevance to determining community support needs.
Keywords: Activities of Daily Living, Staging, Mortality, Risk factors
INTRODUCTION
Dramatic increases in the United States (US) average life expectancy leave elderly individuals with increased exposure to the cumulative effects of chronic illnesses and subsequent limitations in activities of daily living (ADLs).1, 2 Although disability rates in the US are declining faster than mortality, the most severe ADL limitations are not declining and health expenses accumulate with onset of those limitations.3, 4 Estimates from the Medicare Current Beneficiary Survey (MCBS) indicate that in 2005, 18% of community-dwelling persons 65 years and older had 1–2, 5% had 3–4, and 3% had 5–6 ADL limitations.5 The number of older adults in the US with 3 or more ADL limitations is expected to more than double by 2040, to between 4.5 and 7.4 million.5
Mortality risk increases with ADL limitation.3, 6–8 Although greater numbers of limitations indicate greater severity, the specific pattern of activities limited remains obscured. Information about specific ADLs is essential for understanding the types of supportive care needed. For example, the care required by people with problems toileting differs from that required by those whose walking is limited. Consequently, we derived ADL stages recognizing that even for people with the same number of limitations, knowledge about the qualitative differences associated with alternative limitation patterns could substantially improve care planning.9
ADL stages established for elderly persons living in the community express activity limitation10 in a manner that profiles particular activities limited as well as severity. Stages define “thresholds of retained functioning” reflecting hierarchies consistent with theories of development, loss, recovery, and models and measures of disability.10–13 The thresholds specify the maximum difficulty individuals can experience for each of 6 self-care activities and still be at a stage. Similar stages predict nursing home use, functional improvements, and 6-month survival among rehabilitation patients.14–17 Using prospectively collected population-level data from the second Longitudinal Study of Aging (LSOA II), we apply ADL stages to study survival among elderly community-dwelling people.
Our primary hypothesis is that ADL stages will distinguish groups according to mortality risk over and above sociodemographic circumstances and medical conditions known to be associated with reduced survival.18–23 Stage-specific survival rates might help in screening patients and in projecting population care needs and prognosis with greater specificity.
METHODS
This study was approved by the Institutional Review Board at the University of Pennsylvania.
Study population
Baseline data from the second LSOA24 were merged with the disability supplement follow-back of the 1994 National Health Interview Survey (NHIS-D) to enhance information about disability. The LSOA II studied 9,447 community-dwelling people 70 years of age and older representative of the US population.25, 26 The overall response rate to the LSOA II was 87.4%.26 Overviews of sample design and survey methods can be obtained through the Center for Disease Control and Prevention website.24 The updated LSOA II Linked Mortality Public-use File provided mortality follow-up from the date of the LSOA II baseline interview through December 31, 2006,27 yielding follow-up ranging from 10 to 12 years depending on the baseline interview date.
Of the 9,447 persons at LSOA II baseline, 130 (1.4%) were missing one or more ADL and 45 (0.5%) were missing mortality, leaving 9,272 or 98.1% of the original sample for analysis. Although attempts were made to interview people themselves, close proxy respondents (living in the same household) provided information when targeted individuals could not be interviewed. Of the 9,272 individuals, 81.6% self-reported. Illness, sensory loss, cognitive impairment, communication difficulties, and other reasons were given as explanations for proxy use 24.1%, 26.3%, 27.9%, 11.0%, and 39.2% of the time, respectively, with some having multiple reasons.
Outcomes
All-cause mortality for up to 12 years after baseline interview was the outcome for analyses. Survival time was determined as the time between the baseline LSOA II interview and death. All Cox regression models were censored at death or the end of study, i.e., December 31, 2006. While all 12 years of available data were applied to estimate model parameters, mortality was reported at 1, 5, and 10 years.
ADL Stages
To assign ADL stages, respondents were asked questions about difficulties or inabilities eating, toileting (including getting to the toilet), dressing, transferring (getting in and out of bed or chairs), bathing, and walking as detailed previously.9 They rated limitations experienced when performing each activity as no difficulty (0), some difficulty (1), a lot of difficulty (2), and unable (3).28 Instructions for staging are shown in table 1. ADL stages were developed by observing item responses in the LSOA II baseline data using methods described previously.9, 14, 29 Those methods organized patterns of item difficulty into thresholds associated with increasing complexity.11,12, 30
Table 1.
Activity of Daily Living (ADL) Stage Threshold Definitions
| Stages for ADLs | Threshold Definitions for ADLStages |
|---|---|
| ADL 0 = NO Difficulty: None, absent, or negligible ADL limitation |
Is the individual able to eat, toilet, dress, transfer, bathe, and walk without difficulty (all=0)? |
| ADL I = MILD Difficulty: Slight or low level ADL limitation |
Is the individual able to eat and toilet without difficulty (=0), dress and transfer with no more than some difficulty (≤1), and bathe and walk with no more than a lot of difficulty (≤2)? |
| ADL II = MODERATE Difficulty: Medium or fair ADL limitation |
Is the individual able to eat without difficulty (=0), use the toilet, dress, and transfer with no more than a lot of difficulty (≤2), and possibly unable to bathe and walk (≤3)? |
| ADL III = SEVERE Difficulty: High or extreme ADL limitation |
Is the individual able to perform at least one ADL (i.e., eat, toilet, dress, transfer, bathe or walk) with or without assistance but does not meet the defined threshold for stage II? |
| ADL IV = COMPLETE Difficulty: Total ADL limitation |
Is the individual UNABLE to eat, toilet, dress, transfer, bathe and walk (all=3)? |
Demographic and Diagnostic Factors
Age was categorized as 70–74, 75–79, 80–84, and 85 years and older; race as white, black/African American, and other; and marital status as married and non-married (i.e., single, widowed, divorced, etc.). Educational status was dichotomized into those who did and did not graduate from high school.
Diagnoses were by self- or proxy-reported presence or absence of conditions diagnosed by a doctor including chronic bronchitis or emphysema, asthma, diabetes, cancer of any kind, coronary artery disease (angina, myocardial infarction), other heart disease, hypertension, a history of stroke, arthritis, and osteoporosis. A major mental illness was considered present if the respondent reported schizophrenia, bipolar disorder, major depression or paranoid disorder in the past 12 months.
The survey interviewer’s decision to rely on proxy-responses because of the sample person’s “poor memory, senility, confusion or Alzheimer’s disease,” was used to indicate the presence of cognitive impairment. Other reasons for proxy use, such as physical illness and/or disability, were not counted in the indicator of cognitive impairment.
Statistical Analysis
The LSOA II uses a multistage sample design. To obtain the correct point and variance estimates, we accounted for clustering, sample weights, and stratification in all analyses. Prevalence was calculated as weighted proportions. We reported unweighted sample sizes and weighted proportions. We estimated life expectancy for the community-dwelling elderly according to ADL stage from a Kaplan-Meier plot. We then reported 1-, 5-, and 10-year all-cause mortality by ADL stage without adjusting for other factors. The 1- and 5-year time horizons were selected for their clinical relevance, and the 10-year for its longer-term policy implications.
To understand the independent effects of ADL stage on prognosis, we reduced the influence of the sociodemographic and diagnostic conditions known to be most associated with mortality.18–23 We defined an indicator variable for all-cause mortality to calculate the 1-, 5-, and 10-year survival rates for the overall sample and for each group defined by all covariates. We then used Cox proportional hazards regression models to estimate the associations between the covariates and mortality. We included ADL stage and all sociodemographic and available diagnostic factors identified from the literature. Because our models were hypothesis driven, we performed an additional analysis to confirm that no important statistical associations were missed for those variables excluded from the model. We added all available variables not in our model (see table 2) and used backward selection to remove variables until the p-values for all variables were <0.05.
Table 2.
Survival at 1, 5, and 10 Years in a Cohort of 9,272 Community-dwelling Persons 70 Years and Older According to Sociodemographics, Diagnostic Category, and Activity of Daily Living (ADL) Stages.*
| Survival Numbers and Rates
|
||||
|---|---|---|---|---|
| Variable | Total Sample Size | 1 Year | 5 Years | 10 Years |
| no. (%) | no. (%) | no. (%) | no. (%) | |
| Sex | ||||
| Female | 5587 (59.8) | 5387 (96.3)a | 4260 (76.5)a | 2778 (49.8)a |
|
| ||||
| Male | 3685 (40.2) | 3481 (94.5)a | 2520 (68.7)a | 1499 (41.4)a |
|
| ||||
| Race | ||||
| White | 7926 (88.3) | 7586 (95.7) | 5823 (73.7) | 3693 (46.8)b |
|
| ||||
| Black | 986 (7.7) | 936 (94.5) | 694 (69.7) | 409 (41.0)b |
|
| ||||
| Other | 360 (4.0) | 346 (95.4) | 263 (72.4) | 175 (48.6)b |
|
| ||||
| Age | ||||
| 70–74 | 4265 (46.6) | 4137 (96.8)a | 3508 (82.3)a | 2629 (61.9)a |
|
| ||||
| 75–79 | 2502 (27.0) | 2407 (96.3)a | 1857 (74.3)a | 1105 (44.1)a |
|
| ||||
| 80–84 | 1557 (16.4) | 1473 (94.4)a | 995 (63.7)a | 436 (27.6)a |
|
| ||||
| ≥85 | 948 (9.9) | 851 (89.7)a | 420 (44.6)a | 107 (11.2)a |
|
| ||||
| High School Graduation | ||||
| No | 3906 (40.9) | 3716 (95.0)b | 2702 (69.2)a | 1570 (40.3)a |
|
| ||||
| Yes | 5366 (59.1) | 5152 (95.9)b | 4078 (76.2)a | 2707 (50.7)a |
|
| ||||
| Marital Status | ||||
| Not Married | 4352 (46.4) | 4156 (95.3) | 3069 (70.6)a | 1793 (40.9)a |
|
| ||||
| Married | 4920 (53.6) | 4712 (95.8) | 3711 (75.7)a | 2484 (51.2)a |
|
| ||||
| Chronic Obstructive Pulmonary Disease (COPD) | ||||
| No | 8404 (90.4) | 8071 (95.9)a | 6237 (74.4)a | 3986 (47.7)a |
|
| ||||
| Yes | 868 (9.6) | 797 (92.2)a | 543 (63.2)a | 291 (34.5)a |
|
| ||||
| Asthma | ||||
| No | 8734 (94.3) | 8361 (95.6) | 6420 (73.7)a | 4049 (46.7) |
|
| ||||
| Yes | 538 (5.7) | 507 (94.4) | 360 (67.2)a | 228 (41.8) |
|
| ||||
| Diabetes | ||||
| No | 8169 (88.4) | 7831 (95.8)b | 6100 (75.0)a | 3920 (48.3)a |
|
| ||||
| Yes | 1103 (11.6) | 1037 (94.1)b | 680 (60.8)a | 357 (32.1)a |
|
| ||||
| Cancer | ||||
| No | 7715 (82.8) | 7410 (96.0)a | 5738 (74.6)a | 3643 (47.5)a |
|
| ||||
| Yes | 1557 (17.2) | 1458 (93.4)a | 1042 (67.3)a | 634 (41.2)a |
|
| ||||
| Heart (Coronary Artery Disease [CAD]) | ||||
| No | 7400 (79.8) | 7130 (96.2)a | 5612 (76.1)a | 3653 (49.7)a |
|
| ||||
| Yes | 1872 (20.2) | 1738 (92.9)a | 1168 (62.6)a | 624 (33.3)a |
|
| ||||
| Heart other | ||||
| No | 8623 (93.0) | 8264 (95.8)a | 6388 (74.3)a | 4051 (47.4)a |
|
| ||||
| Yes | 649 (7.0) | 604 (92.8)a | 392 (60.2)a | 226 (34.1)a |
|
| ||||
| Hypertension | ||||
| No | 5225 (56.9) | 5013 (95.9) | 3874 (74.3)b | 2512 (48.5)a |
|
| ||||
| Yes | 4047 (43.1) | 3855 (95.1) | 2906 (72.0)b | 1765 (43.7)a |
|
| ||||
| Stroke | ||||
| No | 8505 (91.8) | 8169 (95.9)a | 6336 (74.7)a | 4090 (48.4)a |
|
| ||||
| Yes | 767 (8.2) | 699 (91.6)a | 444 (57.7)a | 187 (23.9)a |
|
| ||||
| Arthritis | ||||
| No | 4953 (53.3) | 4739 (95.6) | 3632 (73.7) | 2365 (48.5)a |
|
| ||||
| Yes | 4319 (46.7) | 4129 (95.5) | 3148 (73.0) | 1912 (44.1)a |
|
| ||||
| Osteoporosis | ||||
| No | 8618 (92.6) | 8236 (95.5) | 6296 (73.2) | 3978 (46.5) |
|
| ||||
| Yes | 654 (7.4) | 632 (96.7) | 484 (75.0) | 299 (45.2) |
|
| ||||
| Cognitive impairedc | ||||
| No | 8779 (94.9) | 8450 (96.2)a | 6594 (75.3)a | 4221 (48.4)a |
|
| ||||
| Yes | 493 (5.1) | 418 (84.3)a | 186 (36.9)a | 56 (10.9)a |
|
| ||||
| Mental Serious | ||||
| No | 9045 (97.5) | 8669 (95.8)a | 6661 (73.9)a | 4226 (47.1)a |
|
| ||||
| Yes | 227 (2.5) | 199 (85.7)a | 119 (51.2)a | 51 (21.6)a |
|
| ||||
| Stages | ||||
| ADL 0 | 6645 (72.1) | 6485 (97.5)a | 5314 (80.0)a | 3575 (54.2)a |
|
| ||||
| ADL I | 1505 (16.1) | 1402 (93.0)a | 934 (62.2)a | 480 (31.3)a |
|
| ||||
| ADL II | 662 (7.0) | 597 (89.7)a | 350 (53.2)a | 152 (22.5)a |
|
| ||||
| ADL III | 414 (4.3) | 355 (86.2)a | 177 (43.3)a | 68 (16.7)a |
|
| ||||
| ADL IV | 46 (0.5) | 29 (65.8)a | 5 (12.7)a | 2 (4.2)a |
Abbreviations: COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; ADL, activities of daily living; Heart (CAD) involves coronary heart disease, angina, heart attack or myocardial infarction; Heart other includes any heart disease other than in Heart (CAD)
All sample sizes were unweighted and all rates (%) were weighted according to the complex sample design.
P≤0.001
P<0.05
Approximated by reliance on proxy use because of “Alzheimer’s disease, poor memory, senility or confusion.”
We checked the proportional hazards assumption by adding all time-varying interactions between all predictors and the natural logarithm transformed time in the above models. Significance at p<0.05 indicated violation of the proportional hazards assumption. When the proportional hazards assumption is violated, it is then necessary to include interaction terms between these covariates and time to account for the non-constant associations between them and mortality over the follow-up time period. Considering the ease of interpretation and clinical relevance, we calculated hazard ratios (HRs) and 95% confidence intervals (95% CI) estimated for each discreet time point (1, 5, and 10 years) from models with these interaction terms. We also graphically displayed the HRs at 1, 5, and 10 years. All analyses used Stata 11.
RESULTS
The mean age of participants at study initiation was 77.4 years; 59.8% were female. At baseline, 72.1% of study participants (weighted percent) were at ADL 0 with no ADL limitations, 16.1% at ADL I (mild), 7.0% at ADL II (moderate), 4.3% at ADL III (severe), and 0.5% at ADL IV (complete limitation).
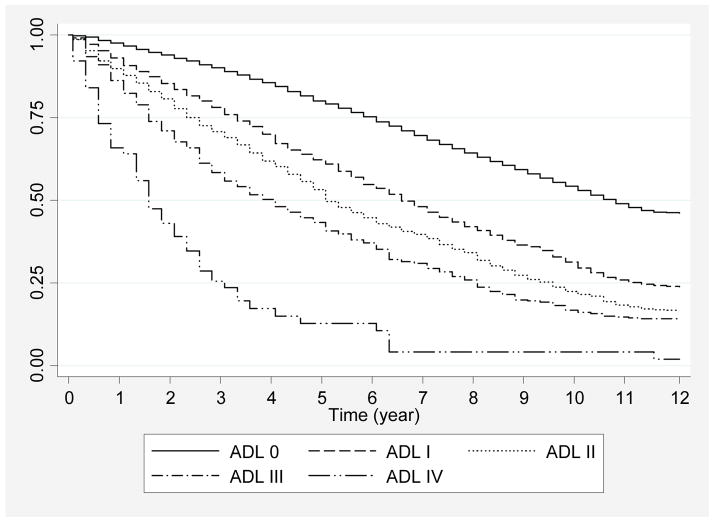
Overall 1-, 5-, and 10-year survival was 95.6%, 73.3%, and 46.4%, respectively. Kaplan-Meier survivor curves document that those at the least limited ADL stages had the most prolonged life expectancies (Figure 1). Those with no ADL limitations could be expected to live a median of 10.6 years, while median survival expectations for those at ADL I, II, III, and IV were 6.5, 5.1, 3.8, and 1.6 years, respectively. Survival rates for all available baseline covariates are shown (Table 2). By the 10th year, survival ranged from 54.2% to 4.2% for stages ADL 0 to IV. In comparison, survival at 10 years ranged from 45.2% for those with osteoporosis to 10.9% for those who required proxy use because of cognitive impairment.
Figure 1.
12-Year Kaplan-Meier Survival Estimates as a Function of Stages Based on Difficulty and Inability
RESULTS FROM COX REGRESSION MODELS
Table 3 shows unadjusted and sociodemographic and diagnoses adjusted HR estimates from the Cox regression models. The proportional hazards assumption of the Cox model was violated for stage, age, gender, stroke, cardiopulmonary, and cancer. For these covariates, HRs at 1, 5, and 10 years were shown for the adjusted models. For those covariates not violating the assumption, the HR stayed constant over time. . Results demonstrated that stage continued to explain risk of mortality after adjusting for sociodemographic and diagnostic factors showing a strong upward gradient with the hazard of death at 1 year increasing sharply with each higher stage. For example, being at ADL I was associated with a 2-fold increased risk of death at 1 year compared to ADL 0 (HR=2.0; 95% CI, 1.7–2.3), whereas the risk of death at ADL IV was increased more than 5-fold compared to ADL 0 (HR=5.2; 95% CI, 3.4–8.1).
Table 3.
Adjusted 1-, 5-, and 10-Year Mortality According to Sociodemographics, Diagnostic Category, and ADL Stages
| Variable | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | ||
|---|---|---|---|---|
| 1 Year | 5 Years | 10 Years | ||
| Sex | ||||
| Male | 1.3 (1.2–1.4)a | 1.9 (1.7–2.1)a | 1.6 (1.5–1.7)a | 1.5 (1.3–1.6)a |
|
| ||||
| Race | ||||
| Black | 1.2 (1.1–1.3)a | 1.1 (1.0–1.2) | No Change¶ | No Change¶ |
|
| ||||
| Other | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | No Change¶ | No Change¶ |
|
| ||||
| Age | ||||
| 75–79 | 1.7 (1.6–1.8)a | 1.3 (1.1–1.5)a | 1.7 (1.6–1.8)a | 1.9 (1.7–2.1)a |
|
| ||||
| 80–84 | 2.6 (2.4–2.8)a | 1.8 (1.5–2.1)a | 2.5 (2.3–2.7)a | 2.9 (2.6–3.3)a |
|
| ||||
| ≥85 | 4.3 (4.0–4.8)a | 2.8 (2.4–3.2)a | 4.0 (3.6–4.4)a | 4.7 (4.0–5.4)a |
|
| ||||
| Educational Level | ||||
| High School Grad | 0.7 (0.7–0.8)a | 1.0 (0.9–1.0) | No Change¶ | No Change¶ |
|
| ||||
| Marital Status | ||||
| Married | 0.8 (0.7–0.8)a | 0.9 (0.8–0.9)a | No Change¶ | No Change¶ |
|
| ||||
| Stroke | 1.9 (1.8–2.1)a | 1.1 (1.0–1.3)b | 1.3 (1.2–1.5)a | 1.4 (1.2–1.7)a |
|
| ||||
| COPD | 1.5 (1.3–1.6)a | 1.4 (1.3–1.6)a | No Change¶ | No Change¶ |
|
| ||||
| Diabetes | 1.6 (1.5–1.8)a | 1.5 (1.3–1.6)a | No Change¶ | No Change¶ |
|
| ||||
| Cancer | 1.2 (1.1–1.3)a | 1.4 (1.2–1.6)a | 1.1 (1.0–1.2) | 1.0 (0.9–1.1) |
|
| ||||
| CAD | 1.6 (1.5–1.7)a | 1.3 (1.2–1.4)a | No Change¶ | No Change¶ |
|
| ||||
| Cognitive Impairedc | 3.3 (3.0–3.7)a | 1.7 (1.5–1.9)a | No Change¶ | No Change¶ |
|
| ||||
| Stages | ||||
| ADL I | 1.9 (1.8–2.1)a | 2.0 (1.7–2.3)a | 1.5 (1.4–1.7)a | 1.4 (1.2–1.5)a |
|
| ||||
| ADL II | 2.5 (2.3–2.8)a | 2.4 (2.1–2.8)a | 1.7 (1.5–1.9)a | 1.4 (1.2–1.7)a |
|
| ||||
| ADL III | 3.2 (2.9–3.7)a | 3.1 (2.6–3.7)a | 1.8 (1.6–2.1)a | 1.5 (1.2–1.8)a |
|
| ||||
| ADL IV | 8.5 (5.7–12.7)a | 5.2 (3.4–8.1)a | 1.9 (0.8–4.9) | 1.3 (0.4–4.1) |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease
The table shows unadjusted and adjusted hazard ratios for 1-, 5-, and 10-year mortality applying a Cox regression model estimated using up to 12 years of follow-up data. ADL stages consist of hierarchical sets of thresholds defining the maximum amount of difficulty experienced for each of 6 activities with higher numbers indicating greater limitation. They are ranked from mild (ADL I) to complete limitation (ADL IV). The omitted stage is ADL 0 indicating no difficulty with any of the 6 ADLs. The omitted category for sex is female, race white, age 70–74, education level did not graduate from high school, marital status not married and each diagnostic condition absence of the condition.
No change indicates that the hazards ratio was the same over time, i.e., the proportional hazards assumption was not violated.
P≤0.001
P<0.05
Approximated by reliance on proxy use because of “Alzheimer’s disease, poor memory, senility or confusion”.
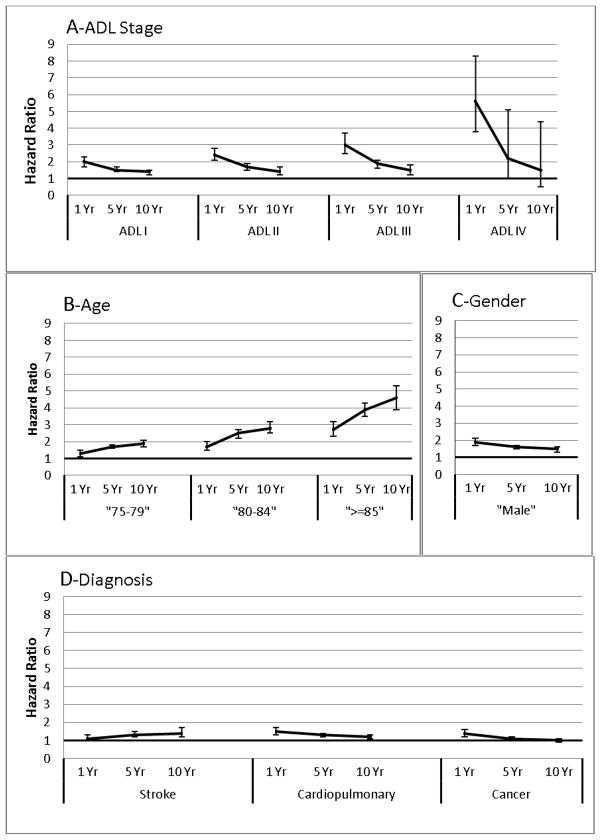
Figure 2 demonstrates graphically the dynamic evolution of association over time for each stage relative to the no limitation reference ADL 0 after adjusting for all other covariates in the statistical model (panel A). The slope was steepest for 1-year mortality, indicating that there was a decrement in the strength of association between stage and mortality risk over time. This decrement in association is strongest at the most severe stages of disability. The plots show that while at 1 year the adjusted hazard ratio for ADL IV compared to ADL 0 was very high (i.e., HR=5.1; 95% CI, 3.1–8.1), the hazard ratio for mortality decreased strikingly over the 5 and 10 year time points and became insignificant when compared to those with no disability (i.e., the 95% CI included 1). While at 1 year the hazard ratios for those with less severe disabilities, i.e., at ADL I, II, and III compared to ADL 0 were smaller than for ADL IV, the hazard ratios of mortality decreased relatively less over the 5 and 10 year time points and remained statistically significant when compared to those with no disability.
Figure 2. Plots of Hazards Ratios for Key Variables Violating the Proportional Hazards Assumption of the Cox Regression Model for All-cause Mortality at 1, 5, and 10 Years.
Key for figure 2:
The plots show separate hazard ratios and 95% confidence limits (y-axes) of all-cause mortality as determined at 1, 5, and 10 years follow-up (x-axes) for those variables that violated the proportional hazards assumption. The variables are ADL stage (panel A), age (panel B), gender (panel C), and various diagnostic condition categories (panel D). These estimates were generated by Cox regressions implemented in the full sample (N=9,272) and adjusted for all variables in the final model. The HRs for ADL I, ADL II, ADL III, and ADL IV (in panel A) are referenced to ADL 0. The HRs for the 3 age categories (in panel B) are referenced to those 70 to 74 years of age. The HRs for male (in panel C) are relative to female. The HRs for each diagnostic condition (in panel D) are referenced to those without the specific condition.
The plots suggest that the impact of having an ADL limitation on the likelihood of mortality is much greater short-term than long-term. This effect was most prominent at ADL IV. This means that those at stage IV unable to perform any ADLs who survived had relatively less marked reductions in the likelihood of mortality over time compared to those in the reference group without any limitation in ADLs at baseline. Conversely, the contribution of advanced age strengthened over time (Panel B). The figure shows that looking at the age 85 or older cohort compared to those between 70 and 74 years of age (reference), the hazard ratios of mortality increased. This means that relative to the 70 to 74 years age group, the oldest old group over the 1, 5, and 10 year periods had the sharpest increases in the likelihood of mortality compared to the younger groups where the time-related increases in mortality were less remarkable. This means that when referenced to the youngest, the oldest old group (≥85 years of age) had the sharpest time-related increases in mortality. Panel C illustrates that the survival disadvantage of being male compared to female is projected to decrease over time. Panel D indicates that while the likelihood of mortality is projected to increase for those with a previous stroke compared to those without, the effects of cardiopulmonary disorders and cancer on mortality appears to attenuate over 1, 5, and 10 years.
Cognitive impairment, diabetes, chronic obstructive pulmonary disease (COPD) or emphysema, cancer, coronary artery disease, and stroke raised the hazard of 1-year mortality by approximately 70%, 50%, 40%, 40%, 30%, and 10%, respectively, after adjusting for stage and sociodemographics. The hazard of mortality associated with cancer was no longer significant at 5 years. Additional analysis showed that while all of the diagnoses originally hypothesized to be important risk factors elevated mortality risk, none of the diagnoses or other variables not originally hypothesized to be important significantly elevated risk.
DISCUSSION
Numerous staging systems in medicine express both the severity and qualitative nature of pathology. The Tumor, Nodes, Metastases (TNM) system, stimulated remarkable advances in oncology, with sustained declines in death rates attributed to risk factor reduction, better screening, and improved treatments.31 We hope that disability staging, which characterizes patients by function rather than pathology, will provide a similar stimulus to advancing disability management. Similar to TNM stages, results confirm that ADL stages are capable of distinguishing among groups of elderly community-dwelling people according to 1-, 5-, and 10-year survival. Anticipated depletion of the Medicare trust fund by 201932 as the number of baby boomers eligible for Medicare benefits swells, along with increasing prevalence of ADL limitations with advanced age, high associated costs of long-term care, and looming shortages of caregivers33 all highlight the importance of being able to project trends in longevity among groups of older adults according to qualities of supportive care need. ADL stages simultaneously distinguish among groups of older adults according to the qualities of supportive care need and prognosis for survival.
The self-care tasks people can be expected to perform become clear at each stage. People at ADL 0 have no difficulty performing self-care tasks and expected survival is high. Expected 1-, 5-, and 10-year survivals decrease directly with stage. People at ADL I with “mild” ADL limitations can still perform all tasks, but are expected to have a lot of difficulty with bathing and/or walking. At ADL II, people with “moderate” limitations can no longer be expected to perform these most complex ADLs themselves. Those at ADL III have “severe” disability patterns that fall outside the typical hierarchy. They experience difficulties with the usually easiest and most fundamental self-care tasks of eating or using the toilet while retaining abilities to perform more challenging functions such as walking. At “complete” limitation ADL IV, people are unable to perform any ADLs, all self-care ability is lost, care burden is maximized, and expected survival is lowest. Our study differs from previous reports on ADL where typical counts or summation methods only characterize severity and the pattern of retained abilities is not transparent.34, 35
The 5 ADL stages continue to distinguish groups in the population by an ordered gradient of mortality after controlling for known risk factors.18–23 ADL stage remained strongly associated with death at all three time periods. Therefore, ADL stage might be applied to indicate mortality risk over and above sociodemographic factors and diagnoses. Our findings are consistent with numerous reports documenting strong associations between ADL limitations and mortality.34, 36–39 The study most similar to ours was a prognostic index developed by Carey and coworkers.36 Because it included instrumental ADLs in addition to basic ADLs, it would be expected to produce a broader gradient. Their 2-year mortality was 5% for the lowest-risk group and 36% for the highest-risk group.36 In our study, 1-year mortality ranged from 2.5% for ADL 0 to 34.2% for ADL IV.
Certain predictive factors become weaker or stronger with respect to impact on short-versus longer-term mortality. Differing time-related associations were particularly notable for people at differing ADL stages. The impact of having complete ADL limitations on likelihood of mortality when compared to those with no limitations was far greater for short-than long-term suggesting that the few people at ADL IV who survived 5 or 10 years were a unique sub-group who tended to continue to survive. Time-related decrements in the association between stage and mortality also present at ADL I, II, and III compared to ADL 0, but less remarkable than for ADL IV, suggest that ADL stage, like diagnoses such as cancer, better predict shorter-than longer-term mortality. This likely relates to high short-term mortality at higher stages of limitation and to the dynamic nature of function.34, 35, 40 Intervening factors like cognitive decline determine whether people can be expected to get better, stay the same, or get worse. Stage transitions as they occur over time could reduce the strength of associations with mortality, highlighting the need for periodic reassessment.
Our study applied self-reported diagnoses and functioning, which may differ from provider assessments. Also, the patterns of mortality and survival found are only generalizable to community-dwelling elderly people; they do not necessarily reflect patterns among those in institutions. Further, observational studies are always vulnerable to unmeasured confounding factors. The data did not include a standard measure of cognition. Nevertheless, simple self- and proxy-reported diagnostic and functional status information has long been recognized as effective for stratifying community-dwelling persons according to varying mortality risk.36, 41 Moreover, all questions were extensively field tested.42 Also, people already in institutions represent a different population with needs that are distinct from those still living in the community. Finally, we adjusted for many known characteristics most associated with mortality.18–23 Future work should address stage transitions and associations with other outcomes.
Stages might be applied to project 1-, 5-, and 10-year survival in the aging US population. Constructed to reflect the profile of care needs of survivors, stages are easily determined, requiring only individuals’ (or, when necessary, close proxy) answers to simple questions about difficulties experienced in performing each activity. Application of stages to patient screening during outpatient visits or homecare might capture early ADL problems while still treatable. Moreover, stratification by stage could aid researchers or policy makers in testing alternative disability management strategies and projecting needs for rehabilitative, supportive, and long-term care in the aging population. The impact of those strategies might be assessed relative to stage-specific survival. ADL stages, by placing a ceiling on the maximum amount of difficulty an individual can experience with each of the 6 ADLs along with estimated survival time, could prove beneficial in planning for the functional assistance and supportive aspects of care for the population.
Acknowledgments
Sponsor’s Role: The National Institute on Aging nor the National Center for Health Statistics (which is only responsible for the initial data) played a role in the design or conduct of the study, in the analysis, and interpretation of the data or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: There are no personal conflicts of interest of any of the authors.
Author Contributions: Margaret Stineman wrote the manuscript and contributed to the study concept and design, analysis and statistical interpretation of the data, and acquisition of the data. Dawei Xie contributed to the study concept and design, analysis and statistical interpretation of the data, and critical revision of the manuscript for important intellectual content. Qiang Pan contributed to the analysis and statistical interpretation of the data. Jibby E. Kurichi contributed to the critical revision of the manuscript for important intellectual content and acquisition of the data. John Henry-Sánchez and Zi Zhang contributed to the critical revision of the manuscript. Debra Saliba contributed to the critical revision of the manuscript for important intellectual content. Joel Streim contributed to the critical revision of the manuscript for important intellectual content and acquisition of the data.
Financial Disclosure: The research for this manuscript was supported by the National Institutes of Health (AG032420-01A1) and by a Post-Doctoral Fellowship for Dr. Henry-Sánchez (T32-HD-007425) awarded to the University of Pennsylvania from the National Institute of Child Health and Human Development (NICHD) National Center for Medical Rehabilitation Research (NCMRR).
There are no personal conflicts of interest of any of the authors, and no authors reported disclosures beyond the funding source. The opinions and conclusions of the authors are not necessarily those of the sponsoring agency or of the NCHS who is responsible only for provision of the data.
References
- 1.U.S. Environmental Protection Agency. Remarks for Benjamin H. Grumbles, Assistant Administrator for Water, U.S. Environmental Protection Agency at the Carolina Environmental Program 2006 Symposium Safe Drinking Water: Where Science Meets Policy; Chapel Hill, North Carolina. [Accessed January 1, 2011]. http://www.epa.gov/ow/speeches/031606bg.html. [Google Scholar]
- 2.Fries JF. Successful aging--an emerging paradigm of gerontology. Clin Geriatr Med. 2002;18:371–382. doi: 10.1016/s0749-0690(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 3.Lubitz J, Cai L, Kramarow E, et al. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 4.Schoeni RF, Freedman VA, Wallace RB. Persistent, consistent, widespread, and robust? Another look at recent trends in old-age disability. J Gerontol B Psychol Sci Soc Sci. 2001;56:S206–S218. doi: 10.1093/geronb/56.4.s206. [DOI] [PubMed] [Google Scholar]
- 5.Federal Interagency Forum on Aging Related Statistics. [Accessed March 22, 2011];Older Americans 2008: Key indicators of well-being (online) http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/2008_Documents/Health_Status.pdf.
- 6.Covinsky KE, Eng C, Lui LY, et al. The last 2 years of life: Functional trajectories of frail older people. J Am Geriatr Soci. 2003;51:492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 8.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 9.Stineman MG Xie D, Pan Q, et al. Activity of Daily Living staging, chronic health conditions and perceived lack of home accessibility features among elderly people living in the community. J Am Geriatr Soc. 2011;59:454–462. doi: 10.1111/j.1532-5415.2010.03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 11.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The Index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 12.Saliba D, Orlando M, Wenger NS, et al. Identifying a short functional disability screen for older persons. J Gerontol Biol Sci Med Sci. 2000;55:M750–M756. doi: 10.1093/gerona/55.12.m750. [DOI] [PubMed] [Google Scholar]
- 13.Stineman MG, Streim JE. The biopsycho-ecological paradigm: A foundational theory for medicine. PM&R. 2010;2:1035–1045. doi: 10.1016/j.pmrj.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stineman MG, Ross RN, Fiedler R, et al. Staging functional independence validity and applications. Arch Phys Med Rehabil. 2003;84:38–45. doi: 10.1053/apmr.2003.50060. [DOI] [PubMed] [Google Scholar]
- 15.Stineman MG, Ross RN, Granger CV, et al. Predicting the achievement of 6 grades of physical independence from data routinely collected at admission to rehabilitation. Arch Phys Med Rehabil. 2003;84:1647–1656. doi: 10.1053/s0003-9993(03)00317-4. [DOI] [PubMed] [Google Scholar]
- 16.Stineman MG, Kurichi JE, Kwong PL, et al. Survival analysis among amputees based on physical independence grade achievement. Arch Surg. 2009;144:543–551. doi: 10.1001/archsurg.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jette DU, Warren RL, Wirtalla C. The relation between therapy intensity and outcomes of rehabilitation in skilled nursing facilities. Arch Phys Med Rehabil. 2005;86:373–379. doi: 10.1016/j.apmr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Croxson SC, Price DE, Burden M, et al. The mortality of elderly people with diabetes. Diabet Med. 1994;11:250–252. doi: 10.1111/j.1464-5491.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 19.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–1166. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Kochanek KD, Murphy S, et al. [Accessed December 1, 2010];Deaths: Final Data for 2007: National Vital Statistics. 58(19) http://www.cdc.gov/NCHS/data/nvsr/nvsr58/nvsr58_19.pdf. [PubMed]
- 21.Manzoli L, Villari P, Pironi MG, et al. Marital status and mortality in the elderly: A systematic review and meta-analysis. Soc Sci Med. 2007;64:77–94. doi: 10.1016/j.socscimed.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Bassuk SS, Berkman LF, Amick BC., 3rd Socioeconomic status and mortality among the elderly: Findings from four US communities. Am J Epidemiol. 2002;155:520–533. doi: 10.1093/aje/155.6.520. [DOI] [PubMed] [Google Scholar]
- 23.Ganguli M, Dodge HH, Shen C, et al. Alzheimer disease and mortality: A15-year epidemiological study. Arch Neurol. 2005;62:779–784. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention. [Accessed January 27, 2012];Longitudinal Study on Aging II (LSOA II): Survey Description. http://www.cdc.gov/nchs/lsoa/lsoa2.htm.
- 25.National Center for Health Statistics. Data File Documentation, National Health Interview Survey on Disability, Phase I, Adult File 1994. Hayattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 26.National Health Interview Survey on Disability, Phase II, Adult File 1995. Hayattsville, MD: Center for Disease Control and Prevention; 1998. National Center for Health Statistics: Data file Documentation. [Google Scholar]
- 27.National Center for Health Statistics. [Accessed December 5, 2010];Office of Analysis and Epidemiology. Public-use Second Longitudinal Study of Aging (LSOA II) Linked Mortality File. http://www.cdc.gov/nchs/data_access/data_linkage/mortality/lsoaii_linkage_public_use.htm.
- 28.Stineman M, Ross R, Maislin G. Functional status measures for integrating medical and social care. Int J Integr Care. 2005;5:e07. doi: 10.5334/ijic.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stineman MG, Ross RN, Fiedler R, Granger CV, Maislin G. Functional independence staging: conceptual foundation, face validity, and empirical derivation. Archives of Physical Medicine and Rehabilitation. 2003 Jan;84(1):29–37. doi: 10.1053/apmr.2003.50061. [DOI] [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 31.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Board of Trustees. 2007 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds: Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. Washington, DC: Center for Medicare & Medicaid Services; 2007. [Google Scholar]
- 33.Institute of Medicine of the National Academies. Retooling for an Aging America: Building the Care Work Force April 11, 2008. 2008. [Google Scholar]
- 34.Covinsky KE, Justice AC, Rosenthal GE, Palmer RM, Landefeld CS. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997 Apr;12(4):203–208. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J Epidemiol Community Health. 2000 Feb;54(2):123–129. doi: 10.1136/jech.54.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey EC, Walter LC, Lindquist K, et al. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: Results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. doi: 10.1186/1471-2318-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller BK, Potter JF. Predictors of mortality in outpatient geriatric evaluation and management clinic patients. J Gerontol. 1994 Nov;49(6):M246–251. doi: 10.1093/geronj/49.6.m246. [DOI] [PubMed] [Google Scholar]
- 39.Mayo NE, Nadeau L, Levesque L, Miller S, Poissant L, Tamblyn R. Does the addition of functional status indicators to case-mix adjustment indices improve prediction of hospitalization, institutionalization, and death in the elderly? Med Care. 2005 Dec;43(12):1194–1202. doi: 10.1097/01.mlr.0000185749.04875.cb. [DOI] [PubMed] [Google Scholar]
- 40.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan VS, Au D, Heagerty P, Deyo RA, McDonell MB, Fihn SD. Validation of case-mix measures derived from self-reports of diagnoses and health. J Clin Epidemiol. 2002 Apr;55(4):371–380. doi: 10.1016/s0895-4356(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 42.United States Bureau of Census. [Accessed January 21, 2011];National Health Interview Survey 1994 Supplement Booklet. http://www.cdc.gov/nchs/data/nhis/dis_ph1.pdf.