Summary
The signals that guide neutrophils to sites of tissue injury or infection remain elusive. H2O2 has been implicated in neutrophil sensing of tissue injury and transformed cell, however its role in neutrophil recruitment to infection has not been explored. Here, using a pharmacological inhibitor of NADPH oxidases, diphenyleneiodonium (DPI), and genetic depletion of an epithelial specific NADPH oxidase, we show that H2O2 is not required for neutrophil detection of localized infection with the Gram-negative bacterium Pseudomonas aeruginosa. In contrast, PI3K signaling is required for neutrophil responses to both wounding and infection. In vivo imaging using a H2O2 probe detects dynamic H2O2 generation at wounds but not at infected tissue. Moreover, DPI no longer inhibits neutrophil wound attraction when Pseudomonas aeruginosa is present in the media. Finally, DPI also fails to inhibit neutrophil recruitment to localized infection with the Gram-positive bacterium, Streptococcus iniae. Our findings demonstrate that differential signals are involved in sensitizing neutrophils to pathogen versus non-pathogen induced tissue damage, providing a potential target to preferentially suppress non-specific immune damage without affecting the response to infection.
Introduction
Neutrophils are a key component of the innate immune system and the first responders to infection or tissue injury. Upon acute injury or infection, neutrophils transmigrate across the endothelium barrier, undergo interstitial migration to reach the inflamed site, where they engulf microorganisms, secrete granule contents, generate reactive oxygen species and/or release neutrophil extracellular traps to control infection and promote wound healing (Springer, 1994). Defects in neutrophil function can result in immunodeficiency, characterized by recurrent infections and poor wound healing. The inability of neutrophils to enter the circulation is seen in neutropenic patients with Warts, Hypogammaglobulinemia, Infections, and Myelokathexis (WHIM) syndrome (Zuelzer, 1964). Moreover, the trapping of neutrophils within the blood stream is a hallmark of leukocyte adhesion deficiency (Etzioni and Alon, 2004) and the failure of neutrophils to undergo oxidative burst is associated with chronic granulomatous disease (Baehner and Nathan, 1967). On the other hand, neutrophilic inflammation must be controlled to prevent non-specific damage to host tissues. Improper neutrophil infiltration is a hallmark of chronic inflammatory airway diseases (Gernez et al., 2010), inflammatory bowel disease (Seibold et al., 1992), autoimmunity (Peng, 2006) and other chronic inflammatory disorders. Attenuation of neutrophilic infiltration remains an attractive target to alleviate clinical symptoms or slow down disease progression. Therefore, understanding the mechanisms that regulate neutrophil trafficking to sites of infection or inflammation in vivo is fundamental to human health. Recently, a stepwise dissection of the signaling events that guide neutrophil recruitment to a site of sterile inflammation was reported (McDonald et al., 2010). However, the signaling events that guide neutrophil recruitment to bacterial infection are still largely unknown.
The zebrafish, Danio rerio, is gaining popularity as a model system to study innate immunity and leukocyte function. The innate immune system in the zebrafish larvae is highly conserved (Lieschke and Trede, 2009). Transparent larvae have made zebrafish an attractive model to observe leukocyte behavior in live animals. Ease of pharmacological and genetic manipulation has led to the discovery of novel signals that regulate rapid neutrophil recruitment in vivo. A gradient of hydrogen peroxide (H2O2) generated at injured epithelial cells has been shown to mediate rapid detection of wounds by neutrophils (Niethammer et al., 2009). Pharmacological inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes or depletion of a key oxidase in epithelial cells, dual oxidase (Duox), attenuated neutrophilic infiltration in the first 20 min post tissue injury. Recently, Lyn, a Src family kinase, was identified as a neutrophil sensor that detects H2O2 at injured tissue (Yoo et al., 2011). In addition, similar H2O2 signaling is generated by oncogene-transformed melanoblasts and goblet cells in zebrafish, which mediates attraction of both neutrophils and macrophages (Feng et al., 2010). A balanced role for reactive oxygen species in host defense against infection is implicated in Drosophila gut immunity. Depletion of dDuox from the intestine results in a marked increase in the mortality rate after natural gut infection (Ha et al., 2005a). Moreover, depletion of extracellular immune-regulated catalase, an enzyme that converts H2O2 to H2O, also shows a high mortality rate after similar gut infection (Ha et al., 2005b). It is speculated that H2O2 burstis a general primary response to various stressors that induce inflammatory feedback to leukocytes (Feng et al., 2010; Yoo and Huttenlocher, 2009). However, whether H2O2 signaling is involved in neutrophil detection of bacterial infection has not been determined.
Although a collection of bacterial infection models have been established in zebrafish, the majority of studies have focused on models of systemic infection with observations of direct pathogen-leukocyte interactions inside the vasculature (Brannon et al., 2009; Davis et al., 2002; Levraud et al., 2009; Phennicie et al., 2010; Stoop et al., 2011; Sullivan and Kim, 2008). A limited number of studies have investigated neutrophil recruitment to a localized infection (Brothers et al., 2011; Le Guyader et al., 2008; Lin et al., 2009; Phennicie et al., 2010). Here we adapted a localized Pseudomonas infection model that is optimal for monitoring pathogen-leukocyte interactions. We found that H2O2 was dispensable for neutrophil attraction to localized bacterial infections. Our results provide evidence that neutrophils detect tissue injury and infection by distinct signaling mechanisms and position tissue-generated H2O2 as an attractive target to differentially modulate neutrophil reaction to infection versus tissue damage.
Results
Generation of a localized infection model to monitor neutrophil recruitment
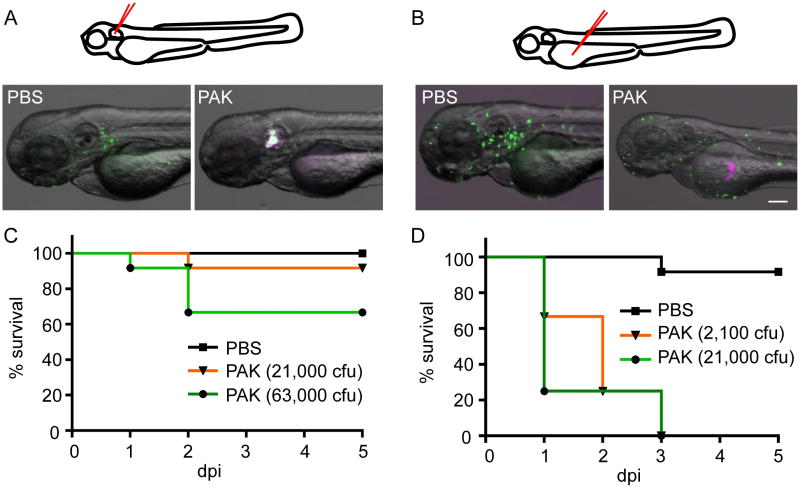
Many locations in zebrafish have been explored as sites for localized infections, including the pericardial cavity (Wiles et al., 2009), hindbrain ventricle (Brothers et al., 2011; Phennicie et al., 2010), the yolk sac (Lin et al., 2009; van der Sar et al., 2003), the left inner ear (Le Guyader et al., 2008) and the dorsal muscle (Lin et al., 2009). To select an infection model best suited to monitor neutrophil recruitment to a contained infection, we first injected Pseudomonas aeruginosa PAK strain in the left inner ear or the yolk sac. Bacteria expressing the red fluorescent protein mCherry (Brannon et al., 2009) in combination with transgenic fish expressing the green fluorescent protein Dendra2 specifically in neutrophils (Yoo and Huttenlocher, 2011) allows the direct visualization of neutrophil responses in real-time (Figure 1A,B). Four hours post infection, Dendra2 labeled neutrophils are recruited to the infected ear, demonstrated by their co-localization with mCherry-labeled PAK in the otic vesicle (Figure 1A). A mock injection with sterile PBS did not induce neutrophil infiltration, indicating that neutrophils respond to bacterial infection specifically. In contrast, neutrophils are not recruited to infection localized in the yolk sac (Figure 1B). Neutrophils, however, can sense the presence of bacteria, revealed by increased neutrophil presence on top of the yolk sac. These neutrophils are highly motile, constantly patrolling the yolk sac (data not shown), but fail to infiltrate into the infection site. Zebrafish larvae are highly resistant to localized ear infection (Figure 1C). Injection of ~63,000 cfu of PAK results in ~30% mortality of 3 dpf larvae. In contrast, when injected into the yolk sac, 2,100 cfu of PAK cause 100% mortality of 3 dpf larvae, which is probably due to the lack of neutrophil recruitment (Figure 1D). Taken together, the otic vesicle is the preferred site that allows direct observation of neutrophil recruitment to localized infection.
Figure 1. Localized Pseudomonas infection model.
A) PBS or PAK (pMKB1::mCherry) was injected into the otic vesicle of Tg(mpx:dendra2) at 3 day post fertilization (dpf). Images were taken 4 hours post infection (hpi).
B) PBS or PAK (pMKB1::mCherry) was injected into the yolk sac of Tg(mpx:dendra2) at 3 dpf. Images were taken 4 hpi.
C) Survival of wild-type AB zebrafish larvae infected at 3 dpf in the otic vesicle with PBS or PAK at the indicated number of colony forming units (cfu).
D) Survival of wild-type AB zebrafish larvae infected at 3 dpf in the yolk sac with PBS or PAK at the indicated number of cfu.
Results are representative of 3 independent experiments. Scale bar: 100 μm.
Phagocytosis of Pseudomonas aeruginosa by neutrophils and resolution of inflammation
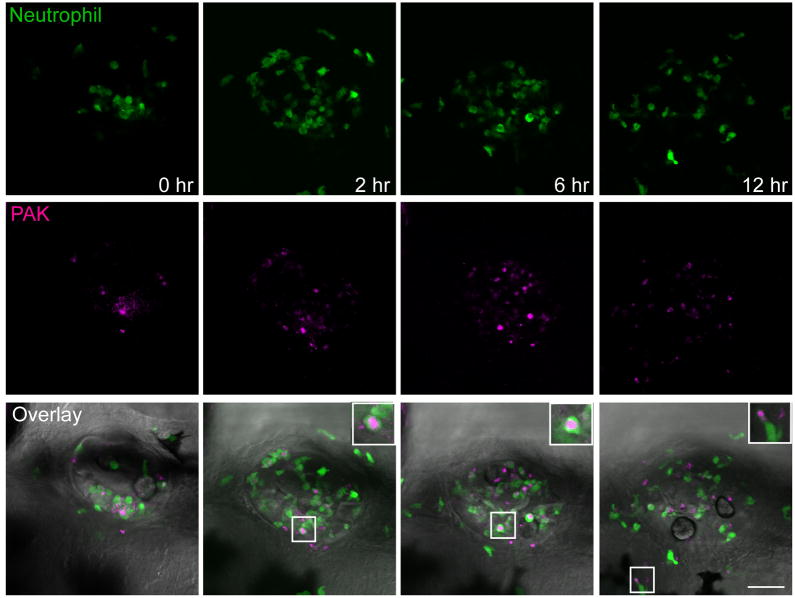
To better observe neutrophil behavior at the infected ear, a confocal movie was taken immediately after bacterial inoculation into the otic vesicle (Figure 2). Neutrophils infiltrate into the ear immediately after infection and their number in the ear peaks ~6 h post infection. Approximately 65% of neutrophils recruited to the infected ear contain detectable phagocytosed bacteria throughout the infection process. Neutrophilic inflammation slowly resolves and by 12 h post infection, the neutrophil number at the infected ear significantly decreases. Several neutrophils containing phagocytosed bacteria are visible outside the ear, indicating that neutrophils migrate away from the site of infection, contributing to the resolution of inflammation.
Figure 2.
Phagocytosis of Pseudomonas aeruginosa (PAK) by neutrophils. PAK (pMKB1::mCherry) at ~12,000 cfu was injected into the otic vesicle of Tg(mpx:dendra2). Still images at indicated time points from a representative confocal movie started immediately after infection are shown. Boxed regions are enlarged to show bacteria inside neutrophils. Scale bar: 50 μm.
Neutrophil recruitment to bacterial infection is not dependent on tissue generated H2O2
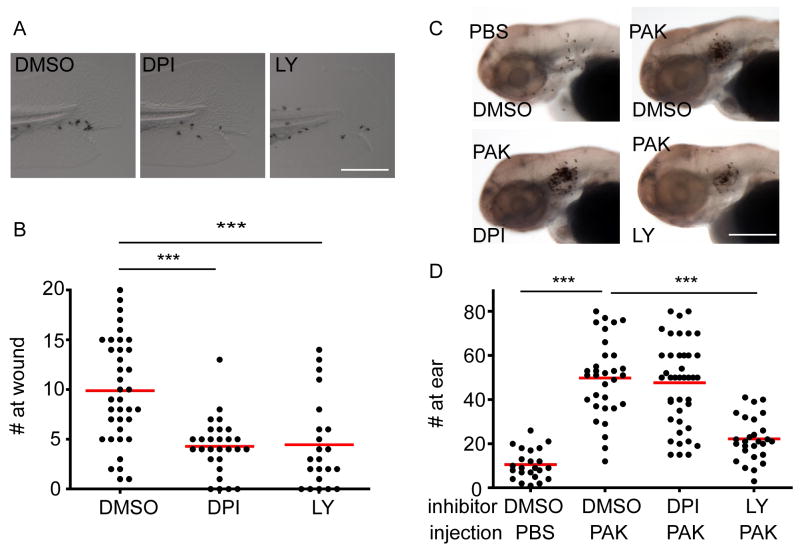
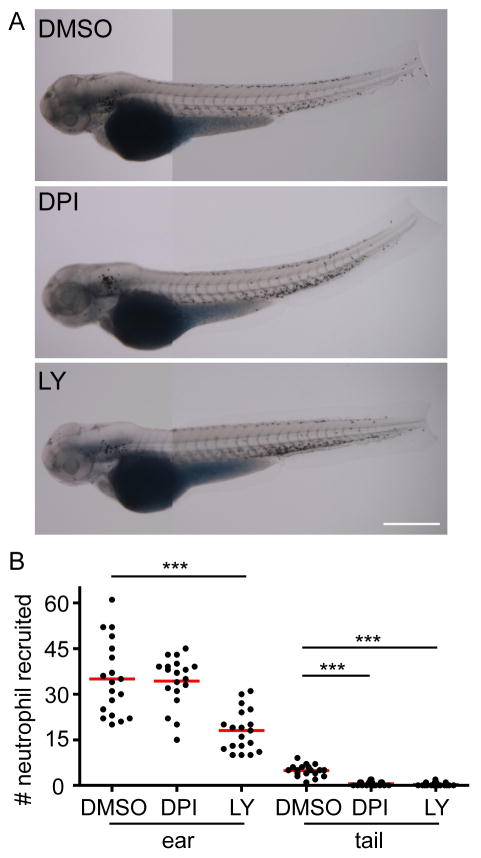
The signals that mediate rapid neutrophil detection of bacterial infection are still obscure. H2O2 is generated by wounded epithelium (Niethammer et al., 2009) or oncogene-transformed cells (Feng et al., 2010) to mediate neutrophil recruitment to tissue injury or transformed cells, respectively. We next investigated the role of H2O2 in mediating neutrophil recruitment to PAK infection. Diphenyleneiodonium (DPI), an inhibitor for the NADPH complex that generates reactive oxygen species (ROS), results in a significant inhibition of neutrophil recruitment to tissue injury compared with vehicle control (Figure 3A, B). Injecting PAK into the left ear leads to significant neutrophil recruitment compared with injecting sterile PBS alone. However, DPI does not inhibit PAK-induced neutrophil recruitment (Figure 3C, D). LY294002, a pan-phosphatidylinositol 3-kinase (PI3K) inhibitor previously shown to reduce neutrophil motility in vivo (Yoo et al., 2010), inhibits neutrophil responses to both tissue injury and PAK infection (Figure 3).
Figure 3. NADPH oxidase activity is required for neutrophil recruitment to wounds but not to infected tissue.
A)Wild-type AB zebrafish larvae at 3 dpf were pretreated with DMSO, the NADPH oxidase inhibitor, diphenyleneiodonium (DPI) or the PI3K inhibitor, LY294002 (LY) for 1 h followed by needle wounding at the tail fin. Larvae were fixed 1 hour post wounding (hpw) and neutrophils were visualized by Sudan Black staining. Representative images of neutrophils recruited to the tail fin are shown.
B) Quantification of A. n= 37 (DMSO), 28 (DPI), 22 (LY). ***, p<0.001, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
C) Wild-type AB zebrafish larvae at 3 dpf were pretreated with DMSO, diphenyleneiodonium (DPI) or LY294002 (LY) for 1 h followed by otic infection with ~ 1, 200 cfu of PAK (pMKB1::mCherry). Larvae were fixed 1 hpi and neutrophils were visualized by Sudan Black staining. PBS was also injected into DMSO treated larvae as a control. Representative images of neutrophils recruited to the ear are shown.
D) Quantification of C. n= 23 (DMSO/PBS), 32 (DMSO/PAK), 43 (DPI/PAK), 27 (LY/PAK). ***, p<0.001, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
Results were pooled from 3 independent experiments. Scale bar: 50 μm.
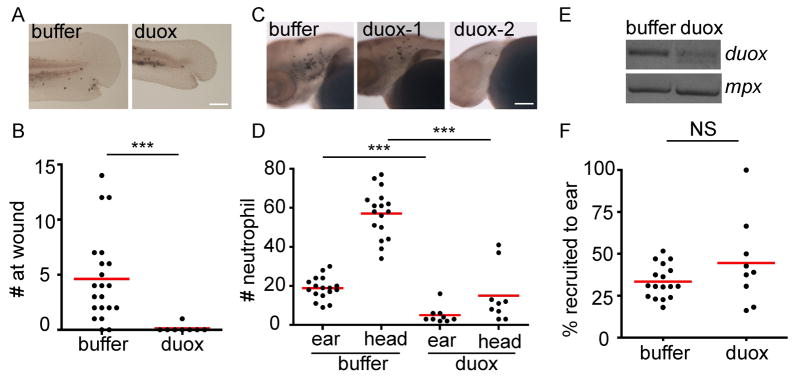
To complement the DPI results with genetic approaches, a key component of the tissue-specific NADPH complex, Duox, was depleted from zebrafish larvae using morpholino oligonucleotide (MO) (Niethammer et al., 2009). RT-PCR of mRNA extracted from 3dpf morphants shows a partial inhibition of duox pre-mRNA splicing, indicated by a smaller band corresponding to exclusion of the target exon (Figure 4E). Neutrophil recruitment to wounds is abolished in the duox morphant, suggesting functional inhibition of Duox (Figure 4A, B). Although reduced numbers of neutrophils are present in the rostral mesenchymal tissues in duox morphants (Figure 4C, D), there is no significant difference in the percentage of head resident neutrophils that are recruited to ear infection between control and duox morphants (Figure 4F), indicating that Duox knockdown does not specifically inhibit neutrophil responses to localized PAK infection. The smaller fin and reduced neutrophil numbers in the duox morphants may be due to non-specific toxicity of the duox MO. Taken together, our data suggest that H2O2 is dispensable for rapid neutrophil detection of PAK infection.
Figure 4. Duox activity is required for neutrophil recruitment to wounds, but not to Pseudomonas aeruginosa (PAK) infection.
Wild-type AB zebrafish larvae were injected with buffer or Duox morpholino oligonucleotide both supplemented with p53 morpholino oligonucleotide at 1 cell stage.
A) 3 dpf control or duox morphants were fixed 1 hpw. Representative images are shown.
B) Quantification of A. n= 21 (buffer), 7 (duox). ***, P<0.001, two-tailed Mann-Whitney test.
C) Three dpf control or duox morphants were fixed 1 hpi with ~ 1,200 cfu of PAK (pMKB1::mCherry). Representative images of neutrophils recruited to ear are shown. Two images of duox morphants are included to show the variation in developmental defects.
D) Quantification of C. n= 17 (buffer), 9 (duox). ***, P<0.001, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
E) RT-PCR of duox and mpx from mRNA extracted from control or duox morphants at 3 dpf.
F) Percentage of neutrophils recruited to PAK ear infection relative to neutrophil numbers in the head region in 3 dpf control or duox morphants. n= 17 (buffer), 9 (duox). NS, non significant, two-tailed Mann-Whitney test.
Results are representative of 3 independent experiments. ***, P<0.001, two-tailed Mann-Whitney test. Scale bar: 30 μm.
Differential H2O2 generation at wounds and localized PAK infections
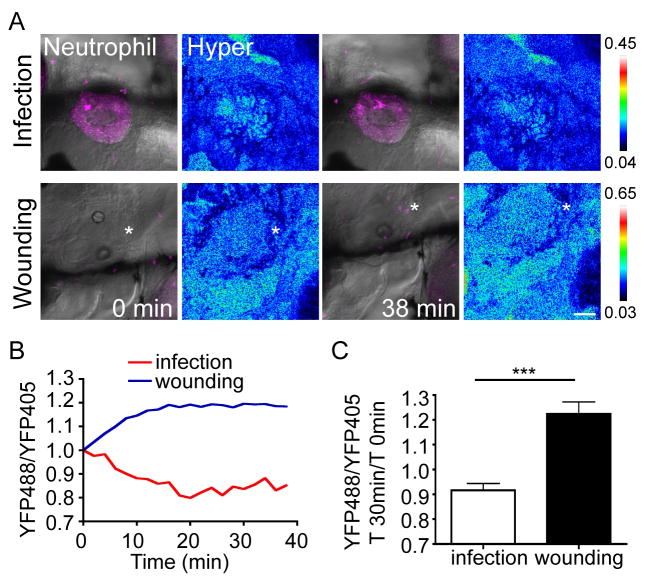
After the characterization that the H2O2 signal is not required for neutrophil recruitment to a localized PAK infection (Figure 3), we next determined whether this is due to an absence of H2O2 at a PAK infected ear, or a dispensable role for H2O2 (if generated) to attract neutrophils. Using a genetically-encoded H2O2 probe, HyPer (Belousov et al., 2006; Niethammer et al., 2009), we observed inhibition of H2O2 generation at wounds either by DPI or in the duox morphant (Figure S1), consistent with previous reports (Niethammer et al., 2009). A slight increase in H2O2 signal in the infected ear compared with the surrounding tissue is observed, which could be due to the injection process that causes a very small wound or an imaging artifact from fluid inside the ear, since mock infection with sterile PBS results in a similar H2O2 signal (data not shown). However, H2O2 signal within the infected ear does not propagate into the surrounding tissue and decreases over time (Figure 5 and Movie S1). In contrast, wounding of the other ear of the same fish results in a burst of H2O2 in the surrounding tissue which accompanies rapid neutrophil infiltration (Figure 5 and Movie S1). Taken together, localized PAK infection does not induce a dynamic H2O2 generation in the surrounding tissue, consistent with the dispensable role of H2O2 in mediating neutrophil attraction to infection.
Figure 5. H2O2 production in zebrafish ear with wounding or localized infection.
A) Wild-type AB zebrafish larvae were injected with HyPer mRNA at 1 cell stage. Larvae at 3 dpf were infected with ~ 1,400 cfu of PAK (pMKB1::mCherry) in the left ear and real-time ratiometric imaging was performed to measure the relative amount of H2O2 generated in the tissue. The right ear of the same fish was then wounded and generation of H2O2 was monitored by YFP488/YFP405 ratiometric imaging under the same conditions. Still images at the indicated times after infection or wounding from a representative movie are shown. * indicates wound site. Scale bar: 50 μm.
B) Quantification of A. YFP488/YFP405 ratiometric fluorescence intensity in the ear at indicated time after wounding or infection. Initial YFP488/YFP405 values were normalized to 1.
C) Quantification of fold changes in YFP488/YFP405 in the ear 30 min post wounding/infection compared with YFP488/YFP405 at time 0. Six fish infected and wounded on each side were quantified. Values shown are mean+SEM. ***, P<0.001, two-tailed paired t test.
DPI selectively impairs neutrophil attraction to wounds in the presence of infection
A signal hierarchy has been described in isolated human neutrophils where neutrophils preferentially migrate to end target chemoattractants (e.g., fMLP and C5a) emanating from the site of infection over intermediary endogenous chemoattractants (e.g., IL-8 and LTB4) (Heit et al., 2002). To determine whether signals generated at infection sites compete with wound signals, we performed infection and wounding in the same larvae. Efficient neutrophil recruitment to both wounds and infections are observed (Figure 6 and Movie S2). The result is not affected by performing either wounding or PAK infection first (data not shown). Therefore, no obvious competition between infection and wound generated signals is observed in this whole fish setting. However, it is possible that individual neutrophils are not exposed to competing signals due to the physical distance between the ear and the tail. Nevertheless, DPI attenuates neutrophil attraction to tail transection, but not to PAK infection in the same larvae. The PI3K inhibitor LY294002 reduces neutrophil motility and impairs neutrophil infiltration both to sites of tissue injury and PAK infection (Figure 6 and Movie S2). These findings confirm a differential role of H2O2 in mediating neutrophil recruitment to tissue injury and bacterial infection.
Figure 6. Differential requirement of NADPH oxidase activity in neutrophil response to simultaneous wounding and infection.
A) Wild-type AB zebrafish larvae at 3 dpf were treated with DMSO, diphenyleneiodonium (DPI) or LY294002 (LY) for 1 h, then infected with ~ 1,200 cfu of PAK (pMKB1::mCherry) followed by tail transection. Representative images of neutrophils recruited to either the infection or the wound are shown.
B) Quantification of A. n= 19 for all. ***, P<0.001, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
Results are representative of 3 independent experiments. Scale bar: 100 μm.
DPI does not inhibit neutrophil attraction to PAK wound-infection
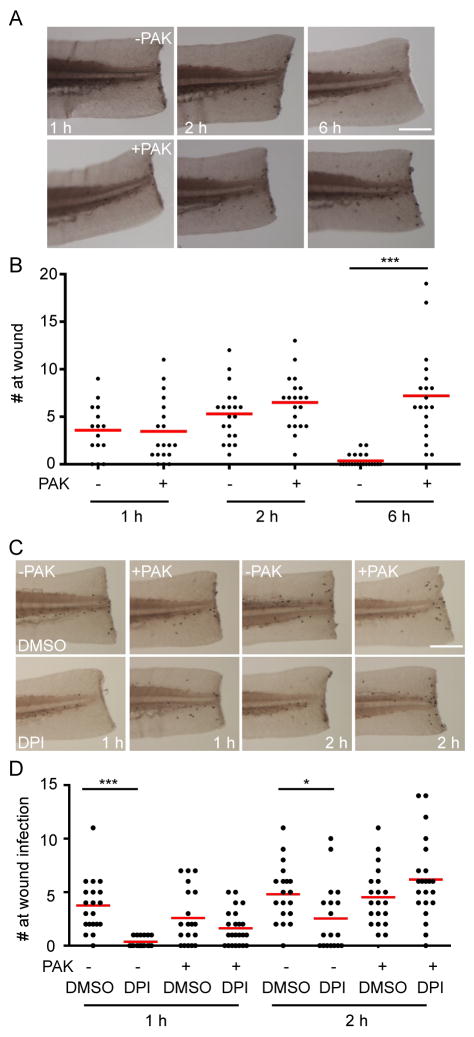
To complement our results in localized infections, we next determined the requirement of H2O2 in neutrophil recruitment in a wound-infection model with the addition of PAK to the embryo medium during the wound response (Brown et al., 2007). A typical neutrophil wound responseis characterized by initial recruitment of neutrophils during the first several hours post wounding, followed by a resolution phase. The presence of PAK in embryo medium does not affect initial neutrophil recruitment, but delays the resolution, as indicated by increased numbers of neutrophils at the tail wound 6 hours post wounding (Figure 7A, B), suggesting that the presence of PAK alters the neutrophil response. DPI attenuates neutrophil attraction (during the first two hour post wounding) to tissue injury. However, with the presence of PAK in the media, neutrophil recruitment is not significantly affected by DPI (Figure 7C, D), indicating that PAK induces neutrophil recruitment to the wounded tail independent of H2O2 generation. Taken together, our results indicate that zebrafish neutrophils detect PAK in a H2O2 independent manner.
Figure 7. NADPH oxidase activity is not required for neutrophil recruitment to wounds in the presence of infection.
A) Time course of neutrophil recruitment to wound-infection. Wild-type AB zebrafish larvae at 3 dpf were treated with DMSO for 1 h, followed by tail transection. Larvae were incubated in the presence or absence of PAK in the water (pMKB1::mCherry) and fixed after the indicated times. Representative images of neutrophils recruited within close proximity of tail transection are shown.
B) Quantification of A. n=15 (1h/−), 19 (1h/+), 20 (2h/+), 20 (2h/−), 21 (6h/+), 19 (6h/−). ***, p<0.001; Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
C) DPI does not affect neutrophil recruitment to wounds in the presence of PAK. Wild-type AB zebrafish larvae at 3 dpf were treated with DMSO or diphenyleneiodonium (DPI) for 1 h, followed by tail transection. Larvae were incubated in the presence or absence of PAK (pMKB1::mCherry) at a final OD600 of 0.005 and fixed after indicated time. Representative images of neutrophils recruited within close proximity to tail transection are shown.
D) Quantification of C. n=21, 19, 20, 24, 21, 19, 21, 22, respectively. ***, p<0.001; *, p<0.05, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
Results are representative of 3 independent experiments. Scale bar: 30 μm.
DPI does not inhibit neutrophil attraction to localized infection with Streptococcus iniae
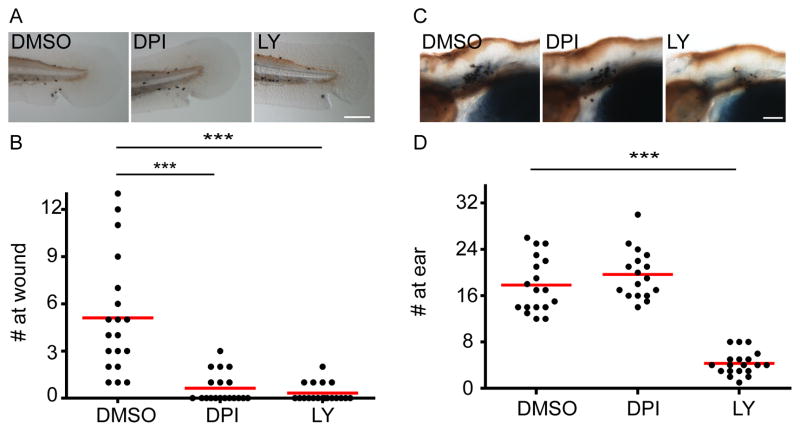
Since most of the experiments were performed with a Gram-negative bacterium, we next tested the effect of DPI on neutrophil recruitment to Streptococcus iniae, a Gram-positive bacterium and a natural zebrafish pathogen (Neely et al., 2002). Similarly, under conditions that attenuate neutrophil attraction to tissue injury, neutrophil recruitment to localized S. iniae infection is not significantly affected by DPI treatment (Figure 8), indicating that detection of Gram-positive bacteria by neutrophil is also independent of H2O2.
Figure 8. NADPH oxidase activity is not required for neutrophil recruitment to S.iniae infection.
A)Wild-type AB zebrafish larvae at 3 dpf were pretreated with DMSO, diphenyleneiodonium (DPI) or LY294002 (LY) for 1 h followed by needle wounding at the tail fin. Larvae were fixed 1 hour post wounding (hpw) and neutrophils were visualized by Sudan Black staining. Representative images of neutrophils recruited to the tail fin are shown.
B) Quantification of A. n= 19 for all. ***, p<0.001, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
C) Wild-type AB zebrafish larvae at 3 dpf were pretreated with DMSO, diphenyleneiodonium (DPI) or LY294002 (LY) for 1 h followed by ear infection with ~100 cfu of S. iniae. Larvae were fixed 1 hpi and neutrophils were visualized by Sudan Black staining. Neutrophils recruited to the infected ear were quantified and representative images are shown.
D) Quantification of C. n= 18 (DMSO), 17 (DPI), 19(LY). ***, p<0.001, Kruskal-Wallis test followed by Dunn’s Multiple Comparison test.
Results are representative of 3 independent experiments. Scale bar: 30 μm.
Discussion
Here we report that zebrafish localized otic infection is suited for real-time observation of neutrophil infiltration and identification of host signaling pathways that sensitize neutrophils to bacterial infection. Neutrophils are recruited immediately to the site of infection (Movie S2) and pathogen-leukocyte interactions are readily observed at the onset of the infection (Figure 2). In contrast, neutrophils respond 90 min post infection with another strain of Pseudomonas, PA14, injected into the hindbrain ventricle (HBV) in 2 dpf larvae (Phennicie et al., 2010). The potential difference in the dynamics of neutrophil infiltration into the ear or HBV may be due to the developmental stage of the larvae, dose and type of bacterial strains used, or the immune-privileged nature of the brain, which is well known in humans and other higher vertebrates. Although direct comparison of neutrophil recruitment in the ear and HBV infection is technically difficult (Levraud et al., 2008), our study indicates that neutrophil responses to infection can be readily observed with the ear infection model in 3 dpf larvae. The yolk sac, on the other hand, is not a good site to investigate pathogen-leukocyte interactions since neutrophils fail to infiltrate into the yolk sac, making the larvae more susceptible to infection, consistent with a previous report (van der Sar et al., 2003). Nonetheless, neutrophils are able to sense the presence of bacteria inside the yolk, indicated by the increased numbers and motility of neutrophils patrolling on top of the yolk in response to infection (Figure 1 and data not shown). Taken together, our findings identify the localized otic infection as a powerful model system to characterize mechanisms that mediate leukocyte recruitment to sites of localized bacterial infection using real-time imaging.
Since the identification of the requirement of H2O2 in mediating leukocyte detection of wounds and transformed cells, the enhanced production of H2O2 has been speculated to be a universal mechanism used by the host to sense the destruction of tissue homeostasis by various insults and to activate the appropriate innate immune response (Feng et al., 2010; Yoo and Huttenlocher, 2009). In animal cells, H2O2 is known to serveas a second messenger that regulates transcription, proliferation or enzyme activity (Bedard and Krause, 2007). Surprisingly, we visualize minimal H2O2 generation upon localized Pseudomonas aeruginosa infection (Figure 5). Accordingly, neutrophil recruitment to localized infection with both Gram-negative and Gram-positive bacteria is independent of tissue generated H2O2 (Figures 3, 4, 6, 7, 8), suggesting a context dependent requirement of H2O2 signaling in sensitizing professional phagocytes. However, whether tissue generated H2O2 is required for efficient clearance of bacterial infection in zebrafish has not been determined (due to the limitation of current tools).
Although H2O2 is considered an antiseptic and is routinely used for treating minor wounds, it remains questionable whether physiologically generated H2O2 could directly kill microbes since H2O2 is only microbicidal at high concentrations (Hampton et al., 1998). Secondary oxidants with a higher destructive capacity, such as HOCl generated by neutrophil-derived myeloperoxidase, are more likely to conduct direct microbicidal activities (Klebanoff, 1974; Rosen et al., 2002). Therefore, tissue generated H2O2 may primarily modulate the local environment, which can mediate leukocyte infiltration and inflammation. Leukocytes, on the other hand, are the major effectors that clear microbes by phagocytosis, releasing ROS and neutrophil extracellular traps.
The identities of the signals that mediate neutrophil recognition of bacterial infection and recruitment in vivo still remain elusive. The neutrophil chemoattractant could be bacterially-derived peptides with formylated N-terminal methionine groups, which are recognized by the host N-formyl peptide receptor (FPR) and mediate neutrophil chemotaxis (Migeotte et al., 2006). However, fMLP, a synthetic peptide that mimics the activity of bacterially-derived peptides, fails to attract neutrophils when injected into the ear (Figure S2A, B). In addition, treatment with cyclosporin H, an FPR inhibitor, does not affect neutrophil recruitment to infection (Figure S2C, D). Collectively, these results suggest that N-formyl peptides are not likely to be involved in rapid neutrophil detection of infection in zebrafish. Other candidate signaling molecules include those in the complement system, such as C5a. The complement system has been extensively studied in zebrafish (Sun et al., 2010) and multiple mannose binding lectin loci have been identified (Jackson et al., 2007). However, it is not clear whether the complement system mediates rapid neutrophil detection of pathogens.
The responses of neutrophils can also be driven by a diverse array of pattern recognition receptors (PRRs) that bind pathogen-associated molecular patterns (PAMPs). The toll-like receptor (TLR) family of cell surface receptors recognizes different foreign biomolecules including those exposed on the cell surface of both Gram-negative and Gram-positive bacteria, such as lipopeptides, lipopolysaccharide (LPS) and flagellin. TLRs signal through several intracellular adaptor molecules, among which, MyD88 plays a pivotal role. TLRs and MyD88 are well conserved in the zebrafish (van der Sar et al., 2006). However, depleting endogenous MyD88 with two separate morpholino oligonucleotides indicates a dispensable role for MyD88 in mediating neutrophil recruitment to PAK infection (Figure S3). It is possible that MyD88 plays roles in later sustained neutrophil recruitment or activation, but not in the initial rapid neutrophil response. It is also possible that another adaptor protein, such as TRAM can function in place of MyD88 and mediate functional PRR signaling (Yamamoto et al., 2003). In contrast, we find that MyD88 is involved in neutrophil wound detection (Figure S3), which is not entirely surprising since TLRs are implicated in recognition ofendogenous ligands that are derived from necrotic cells, such as HMGB1 (Apetoh et al., 2007; Scaffidi et al., 2002) or extracellular matrix components that are generated as a result of tissue injury (Jiang et al., 2006). A challenge for future investigation will be to identify the specific signaling mechanisms that mediate leukocyte attraction to infected tissues in live animals.
In summary, we have identified a differential requirement for tissue generated H2O2 in mediating neutrophil recruitment to tissue injury and bacterial infection. Our results indicate that signals mediating leukocyte response to infection or acute injury are indeed unique and suggest the potential of identifying novel signaling events that are differentially involved in sensitizing immune cells to non-pathogen driven versus pathogen driven inflammation.
Experimental Procedures
Zebrafish maintenance and drug treatment
Adult fish were maintained in accordance with the University of Wisconsin-Madison Research Animal Resources Center (Madison, WI, USA). For live imaging or wounding assays, larvae were anesthetized in E3 containing 0.2 mg/ml Tricaine (ethyl 3-aminobenzoate; Sigma-Aldrich, St. Louis, MO, USA). To prevent pigment formation, some larvae were maintained in E3 containing 0.2 mM N-phenylthiourea (Sigma-Aldrich). Where indicated, larvae were pretreated with 32.5 μM LY294002 (Calbiochem) (Yoo et al., 2010), 20 μM Cyclosporin H (Enzo Life Sciences) or 100 μM DPI (Sigma) (Niethammer et al., 2009) in 1% DMSO in embryo medium (E3) to facilitate drug delivery for 1 hour. The following experiments were performed in E3 supplemented with the indicated drug for the indicated period of time.
Bacterial strains and culture methods
P. aeruginosa strain PAK carrying a constitutive plasmid-encoded red fluorescent protein mCherry (pMKB1::mCherry) was kindly provided by S. Moskowitz, University of Washington, Seattle, WA, USA (Brannon et al., 2009). The S. iniae strain was kindly provided by M. Caparon, Washington University, St Louis, MO, USA (Neely et al., 2002). S. iniae for infection was prepared as described (Neely et al., 2002). Frozen stock obtained with single colony of PAK (pMKB1::mCherry) was streaked freshly on Vogel-Bonner minimal (VBM) plate supplemented with 200 μg/mL carbenicillin the night before infection. Bacteria were resuspended in phosphate-buffered saline (PBS) and the optical density at 600 nm was measured with a Nanodrop spectrophotometer. To prepare the final inoculum, the bacteria suspension was diluted or pelleted by centrifugation at 1500 g for 5 min followed by resuspension to achieve the desired bacterial density. Phenol red tracking dye was added to the bacterial aliquots prior to injection for a final concentration of 0.1%. After infection, the inoculum was injected into PBS, diluted when necessary and plated to quantify colony forming units.
Bacterial infection, ear injection, tailfin wounding and Sudan Black staining
Bacterial infection in the otic vesicle was performed as described (Levraud et al., 2008). For yolk infection, larvae were prepared as for otic infection with slight modifications: dorsal side (instead of ventral side for otic infection) was positioned against the injection groove. For fMLP injection, one nl of 10 μM or 1 μM fMLP (Sigma) in PBS was injected into the otic vesicle. The tail fins of larvae at 3 dpf were either wounded with a needle or transected with a sterile razor blade. For wound-infection, PAK(pMKB1::mCherry) was added to E3 medium at a final OD600 of 0.005 (Brown et al., 2007). Larvae were fixed at the indicated time post wounding, and neutrophils were stained with Sudan Black as previously described (Le Guyader et al., 2008).
Morpholino oligonucleotides (MO) microinjection and RT-PCR
All MOs were purchased from GeneTools, LLC, resuspended in distilled water and stored at RT at a stock concentration of 1 mM. Three nanoliters of MOs were injected into the yolk of 1 cell-stage embryos at the concentrations indicated below: 100 μM Duox MO (Niethammer et al., 2009) was injected in combination with 300 μM p53 MO (Niethammer et al., 2009) to minimize non-specific MO toxicity. MyD88 MO e2i2 (van der Sar et al., 2006) and e1i1 (5′-CACATGGAATTTAGA TGACGTACCT-3′) were injected at 1 mM. Efficient MO mediated knockdown was confirmed with RT-PCR using mRNA extract from 3dpf morphants. Primers to amplify duox (Niethammer et al., 2009), myd88 (van der Sar et al., 2006) and ef1 α as a house keeping gene (Walters et al., 2010) were previously described. Primers for the neutrophil specific marker mpx were as follows: mpx forward: 5′-ACCAGTGAGCCTGAGACACG CA-3′; mpx reverse: 5′-TGCAGACACCGCTGGCAGTT-3′.
Live imaging and HyPer imaging
Larvae at 3 dpf were settled on a custom-made, glass-bottom dish. Time-lapse fluorescence images were acquired with a confocal microscope (FluoView FV1000, Olympus, Center Valley, PA, USA) using a numeric aperture 0.75/20x objective or Nikon SMZ-1500 zoom microscope (Nikon, Melville, NY, USA). For confocal imaging, each fluorescence channel (488 nm and 543 nm) and DIC images were acquired by sequential line-scanning. Z-series was acquired using a 200- to 300-μm pinhole and 2–10 μm step sizes. Z-stacked fluorescence images were overlayed with a single DIC plane. For imaging of H2O2, 1-cell stage zebrafish embryos from Tg(mpx:mCherry) were injected with HyPer mRNA. At 3 dpf, larvae were infected with PAK (pMKB1::mCherry) in the left ear. The HyPer fluorescence was excited with 405 and 488 laser and corresponding YFP emission was acquired every 2 min after infection. Emission at 510–525 was collected for YFP488 emission and 505–510 was collected for YFP405 emission. The right ear of the same fish was wounded immediately after the acquisition of the movie on the infected side and the same conditions were used to acquire the movie after wounding. For calculating HyPer ratio images, stacked, smoothed YFP488 and YFP405 images were divided.
Supplementary Material
Acknowledgments
The authors would like to thank Sa Kan Yoo for critical reading of the manuscript, lab members for zebrafish maintenance. This work is supported by grants from the National Institute of Health to A.H. (GM074827), National Science Foundation to N.K. and A.H. (EFRI-1136903) and National Institute of Health - Microbes in Health and Disease Training Grant to E.A.H. (5T32AI055397-09).
References
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Baehner RL, Nathan DG. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967;155:835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Brannon MK, Davis JM, Mathias JR, Hall CJ, Emerson JC, Crosier PS, Huttenlocher A, Ramakrishnan L, Moskowitz SM. Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell Microbiol. 2009;11:755–768. doi: 10.1111/j.1462-5822.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KM, Newman ZR, Wheeler RT. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell. 2011;10:932–944. doi: 10.1128/EC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, Tucker CS, Ford C, Lee Y, Dunbar DR, Mullins JJ. Class III antiarrhythmic methanesulfonanilides inhibit leukocyte recruitment in zebrafish. J Leukoc Biol. 2007;82:79–84. doi: 10.1189/jlb.0107030. [DOI] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Etzioni A, Alon R. Leukocyte adhesion deficiency III: a group of integrin activation defects in hematopoietic lineage cells. Curr Opin Allergy Clin Immunol. 2004;4:485–490. doi: 10.1097/00130832-200412000-00003. [DOI] [PubMed] [Google Scholar]
- Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernez Y, Tirouvanziam R, Chanez P. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J. 2010;35:467–469. doi: 10.1183/09031936.00186109. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005a;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, Lee WJ. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005b;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AN, McLure CA, Dawkins RL, Keating PJ. Mannose binding lectin (MBL) copy number polymorphism in Zebrafish (D. rerio) and identification of haplotypes resistant to L. anguillarum. Immunogenetics. 2007;59:861–872. doi: 10.1007/s00251-007-0251-5. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974;249:3724–3728. [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, Mordelet E, Zapata A, Shinomiya H, Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- Levraud JP, Colucci-Guyon E, Redd MJ, Lutfalla G, Herbomel P. In vivo analysis of zebrafish innate immunity. Methods Mol Biol. 2008;415:337–363. doi: 10.1007/978-1-59745-570-1_20. [DOI] [PubMed] [Google Scholar]
- Levraud JP, Disson O, Kissa K, Bonne I, Cossart P, Herbomel P, Lecuit M. Real-time observation of listeria monocytogenes-phagocyte interactions in living zebrafish larvae. Infect Immun. 2009;77:3651–3660. doi: 10.1128/IAI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Trede NS. Fish immunology. Curr Biol. 2009;19:R678–682. doi: 10.1016/j.cub.2009.06.068. [DOI] [PubMed] [Google Scholar]
- Lin A, Loughman JA, Zinselmeyer BH, Miller MJ, Caparon MG. Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection. Infect Immun. 2009;77:5190–5201. doi: 10.1128/IAI.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Neely MN, Pfeifer JD, Caparon M. Streptococcus-zebrafish model of bacterial pathogenesis. Infect Immun. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL. Neutrophil apoptosis in autoimmunity. J Mol Med (Berl) 2006;84:122–125. doi: 10.1007/s00109-005-0007-3. [DOI] [PubMed] [Google Scholar]
- Phennicie RT, Sullivan MJ, Singer JT, Yoder JA, Kim CH. Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2010;78:4542–4550. doi: 10.1128/IAI.00302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Crowley JR, Heinecke JW. Human neutrophils use the myeloperoxidase-hydrogen peroxide-chloride system to chlorinate but not nitrate bacterial proteins during phagocytosis. J Biol Chem. 2002;277:30463–30468. doi: 10.1074/jbc.M202331200. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Seibold F, Weber P, Klein R, Berg PA, Wiedmann KH. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657–662. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stoop EJ, Schipper T, Rosendahl Huber SK, Nezhinsky AE, Verbeek FJ, Gurcha SS, Besra GS, Vandenbroucke-Grauls CM, Bitter W, van der Sar AM. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis Model Mech. 2011;4:526–536. doi: 10.1242/dmm.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C, Kim CH. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Sun G, Li H, Wang Y, Zhang B, Zhang S. Zebrafish complement factor H and its related genes: identification, evolution, and expression. Funct Integr Genomics. 2010;10:577–587. doi: 10.1007/s10142-010-0182-3. [DOI] [PubMed] [Google Scholar]
- van der Sar AM, Musters RJ, van Eeden FJ, Appelmelk BJ, Vandenbroucke-Grauls CM, Bitter W. Zebrafish embryos as a model host for the real time analysis of Salmonella typhimurium infections. Cell Microbiol. 2003;5:601–611. doi: 10.1046/j.1462-5822.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- van der Sar AM, Stockhammer OW, van der Laan C, Spaink HP, Bitter W, Meijer AH. MyD88 innate immune function in a zebrafish embryo infection model. Infect Immun. 2006;74:2436–2441. doi: 10.1128/IAI.74.4.2436-2441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles TJ, Bower JM, Redd MJ, Mulvey MA. Use of zebrafish to probe the divergent virulence potentials and toxin requirements of extraintestinal pathogenic Escherichia coli. PLoS Pathog. 2009;5:e1000697. doi: 10.1371/journal.ppat.1000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Huttenlocher A. Innate immunity: wounds burst H2O2 signals to leukocytes. Curr Biol. 2009;19:R553–555. doi: 10.1016/j.cub.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Huttenlocher A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J Leukoc Biol. 2011;89:661–667. doi: 10.1189/jlb.1010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011 doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelzer WW. “Myelokathexis”--a New Form of Chronic Granulocytopenia. Report of a Case. N Engl J Med. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.