Abstract
What causes the positive, negative, and cognitive symptoms of schizophrenia? The importance of circuits is underscored by the finding that no single gene contributes strongly to the disease. Thus, some circuit abnormality to which many proteins can contribute is the likely cause. There are several major hypotheses regarding the circuitry involved: 1) a change in the balance of excitation/inhibition in the PFC; 2) abnormal EEG oscillations in the gamma range; 3) an increase in theta/delta EEG power related to changes in the thalamus (particularly midline nuclei); 4) hyperactivity in the hippocampus and consequent dopamine hyperfunction; or 5) deficits in corollary discharge. Evidence for these hypotheses will be reviewed.
Schizophrenia (SZ) involves delusions and hallucinations that collectively have been termed positive symptoms. The disease also has cognitive symptoms (e.g., memory processes and execute control), negative symptoms (e.g., anhedonia), and sensory deficits. Efforts are being made to develop physiological models that explain some or all of these symptoms and that are constrained by key findings bearing on the physiological basis of the disease. Among these findings are: 1) Disinhibition is suspected because of the reduction in the GABA-synthesizing enzyme, GAD-67, observed in postmortem tissue [1]. 2) Abnormalities in brain oscillations are observed, notably in the gamma and delta frequency bands [2,3]. 3) The most effective treatment of SZ is by drugs with D2 antagonism, suggesting dopamine hyperfunction [4]. 4) NMDAR antagonist, when given to normal subjects, produces many of the symptoms of SZ, suggesting that the disease involves NMDAR hypofunction [5,6]. Although no physiological model yet provides a clear explanation for all these findings, models with substantial explanatory power have been developed. These will now be reviewed.
Balance of excitation and inhibition in the prefrontal cortex
The prefrontal cortex (PFC) is implicated in SZ because of its involvement in executive and working memory functions that are abnormal in SZ. Furthermore, functional imaging indicates reduced activation of the PFC during cognitive tests of PFC function [7]. One basis for these abnormalities could be a change in the balance of excitation and inhibition. Disinhibition is suggested by the finding that GAD-67 is reduced in SZ, a reduction that would be expected to reduce inhibition [1]. This inference was tested in an animal model with reduced GAD-67 in parvalbumin-containing neurons of the PFC [8]. As expected, there was a reduction in functional inhibition, as indicated by the smaller size and lower frequency of mIPSCs. The reduction of GAD-67 (and other changes in gene expression) appears to be triggered by the reduction in Ca2+ entry through the NMDAR [**9]. This entry may be the mechanism by which these interneurons monitor pyramidal cell activity and try to stabilize it homeostatically. Thus, if NMDAR-mediated transmission is reduced, interneurons falsely sense less pyramidal cell activity and therefore reduce inhibition [10]. It would follow that GAD-67 would also be reduced if there were less pyramidal cell activity as a consequence of reduced excitatory input. This possibility is important to consider because in SZ there is reduced layer 3 spine density of PFC excitatory cells, a reduction that could be a consequence of the SZ risk genes, including DISC1 [11,12].
NMDAR antagonist also has acute effects by blocking the NMDAR-mediated EPSP that occurs in some interneurons and that results in reduced inhibition of pyramidal cells [*13]. Disinhibition of pyramidal cells due to a reduction of the firing of interneurons is seen in vivo [14] and can be counteracted by mGluR2/3 or mGluR5 agonists [15,16].
New optogenetic methods have made it possible to increase activity in excitatory cells by illumination of channel rhodopsin (related methods can fire parvalbumin-containing interneurons). An increase in the excitation/inhibition balance in mPFC produced a deficit in social interactions, as occurs in SZ [17]. Such methods open the door to critically testing the causation of deficits but will have to be combined with models that relate cellular function to behavioral function. Toward this goal, a computational model shows how recurrent excitation and inhibition can produce the persistent firing that underlies working memory function and how the effects of dopamine on synaptic and intrinsic conductances make this possible [18].
Gamma oscillations
A second line of work focuses on how an abnormal balance of excitation and inhibition might give rise to the abnormal gamma frequency oscillations observed in SZ. Oscillations in the gamma frequency range can be understood in terms of negative feedback in the local circuit [19–21]. Pyramidal cells having excitatory drive will fire and then excite interneurons. The fast-spiking interneurons that contain parvalbumin (basket and chandelier cells) produce perisomatic inhibition of pyramidal cells, which stops their firing (Fig.1). Once this inhibition decays, firing is reinitiated, thus starting the next oscillatory cycle. The high degree of convergence/divergence of the synaptic connections results in oscillatory synchrony among the cells involved. There are at least two known functions of gamma oscillations: 1) they allow only the most excitable cells to fire, thereby selecting the cells that form an ensemble [22]; and 2) they synchronize the cells of an ensemble, allowing the ensemble to be recognized by downstream networks using coincidence detection [23]. Multi-part messages can be coded by different ensembles firing in different gamma subcycles of slower theta oscillations [24]. Given the importance of gamma in neural coding, it would not be surprising that abnormalities in gamma would lead to confused messages. It has therefore been tempting to argue that disorganized thought in SZ might occur because of gamma abnormalities.
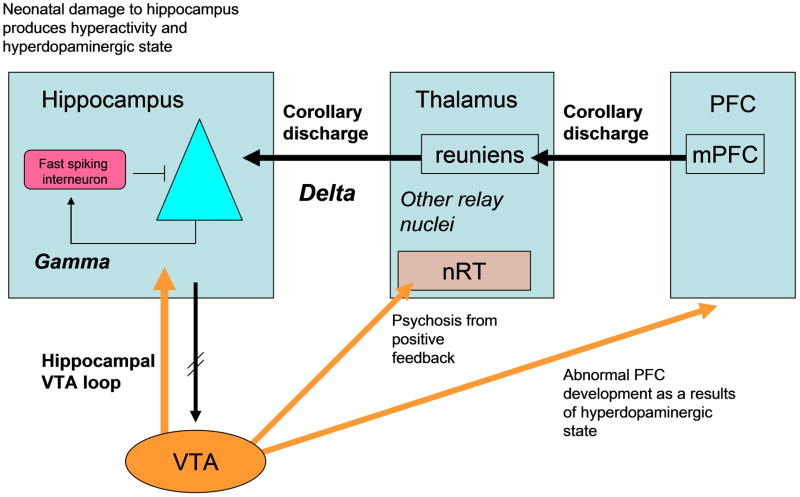
Fig. 1.
Interactions between brain regions proposed to produce symptoms of SZ.
Gamma EEG oscillations in humans can be stimulated by a task, induced by a stimulus, or evoked by repetitive inputs. In almost all cases, the amplitude of gamma is reduced in SZ [**25]. This has prompted efforts to understand the mechanism of this abnormality. Computational modeling is an important tool for this effort because gamma, as observed in the field potential, depends in complex ways on both the number of pyramidal cells that fire and on their synchronization. The gamma evoked by 40 Hz clicks is reduced in SZ, and this, as well as simultaneous changes in the 20 Hz response, can be accounted for by a model in which inhibition is slowed in SZ [26].
In another study [**27], inhibition was attenuated by genetic deletion of AMPARs that mediate input to fast-spiking interneurons. This was found to reduce gamma in the field potential. Both experiments and computational modeling indicated that the reduced gamma was due to desynchronization of pyramidal cells (even though they fired more). This study thus supports the idea that the reduced gamma in SZ could be due to reduced excitation of interneurons.
Given the ability of NMDARs to induce many of the symptoms of SZ, it has been of interest to determine whether gamma oscillations are affected by block of these channels. Here the results have been somewhat confusing. NMDAR antagonist in slices increases gamma in some brain regions, but decreases it others [**28]. Experiments done in vivo show an increase in gamma power in response to NMDAR antagonist [29]. Several studies have knocked out NMDARs from fast-spiking interneurons [**30–32], producing an enhancement of baseline gamma oscillations and deficits in various behavioral paradigms. The increase in gamma is consistent with most pharmacological studies, but is difficult to reconcile with the decrease in gamma found in SZ.
Party of the difficulty in understanding changes in gamma amplitude is the multitude of factors that can affect it. In elucidating the mechanisms that produce changes in gamma in SZ, it will be important to differentiate the local mechanisms (e.g., changes in the efficacy of feedback inhibition) from external task-dependent effectors (e.g., neuromodulatory inputs that depend on motivation and behavioral state). One method that may minimize the influence of task-dependent effectors is the use of a pulse of transcranial magnetic stimulation in the absence of a task. With this method, gamma frequency oscillations are induced and their power is found to be reduced in SZ [33]. There are many methods for inducing gamma in the slice, but gamma induced by a pulse-like tetanus [34] may be particularly useful because it allows comparison to the human results using a pulse of transcranial magnetic stimulation.
Is the deficit in SZ global or due to specific brain circuits?
Is SZ a general disease of the brain, or is there some specific circuit involved? Cognitive testing has shown that there are very few spared functions in SZ. Consistent with this, examination of arrays of genes related to inhibition (GAD-67 being only one) show abnormal expression not just in PFC, but in all regions examined, including visual cortex [35]. Such changes in visual cortex may well account for deficits in early visual function in SZ [36]. Thus, one view is that global abnormalities in cellular function change the balance of excitation and inhibition (and gamma). These diffuse changes might then account for the large number of functional deficits in SZ. On the other hand, the highly unusual positive symptoms of the disease suggest that some very specific brain processes are affected. The following sections deal with specific brain circuits that might account for such symptoms.
Hippocampus-dependent upregulation of the dopamine system
The hippocampus has been implicated in schizophrenia because there are changes in its functional activation and because there are memory deficits associated with the disease [37,38]. However, changes in hippocampus may have their largest effect not by changing hippocampal memory function, but by affecting down-stream targets of the hippocampus. Experiments show that direct excitation of the hippocampal region can, through a polysynaptic pathway, excite dopamine cells of the VTA [39,40] (Fig.1). This pathway is part of a learning circuit [41] by which a novelty signal computed in the hippocampus leads to excitation of the VTA. This, in turn, leads to release of dopamine in the hippocampus that is vital for stable LTP and memory [42].
The link between the hippocampus and the dopamine system may be very important in SZ. Damage to the neonatal ventral hippocampus produces symptoms of SZ in adult animals [43]. Physiological analysis shows that the hippocampus is hyperactive, probably because of loss of interneuron function [44]. This hyperactivity leads to elevation in the firing of dopamine neurons in the VTA. Importantly, procedures that normalize hippocampal activity normalize the firing of dopamine cells and behavior [*45].
Remarkably, the effects of neonatal hippocampal lesion can lead, in completely other regions, to some of the cardinal symptoms of SZ: a reduction of GAD-67 in the PFC and a reduction of gamma oscillations [44]. This spread to other regions may be mediated by hippocampal-induced abnormalities in the dopamine system. Notably, dopaminergic modulation of PFC interneurons develops abnormally, leading to PFC disinhibition [46].
Delta/theta oscillations originating from the thalamus via an NR2C-dependent mechanism
As noted above, there are EEG abnormalities in the gamma frequency band in SZ, but abnormalities have also been found in low-frequency (theta (4–8Hz) and delta (1–4Hz) ranges. Indeed, two recent meta-analyses conclude that enhanced low-frequency oscillations in the delta/theta frequency band of the EEG or MEG is a highly replicable finding in SZ [3,47]. The abnormality is seen in non-medicated patients and is not seen in unaffected relatives, making it a good marker for the disease.
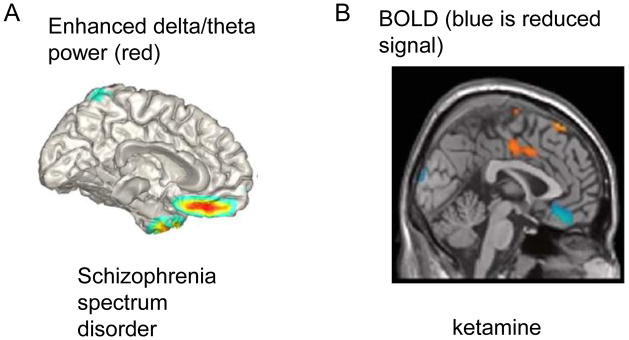
Delta oscillations are normally present during slow-wave sleep, so their presence in the awake state in SZ suggests that part of the brain may be in an inactive sleep-like state [48]. MEG localizes these slow oscillations to the medial ventral PFC [**49] (Fig.2A). Remarkably, the same region is one of the few locations where NMDAR antagonist lowers metabolism [**50] (Fig.2B). This correspondence makes sense given studies of slow wave sleep showing that delta oscillations lower metabolism, probably because cell firing and gamma oscillations occur during only a small fraction of the delta cycle [51]. Indeed in this way, the presence of delta might account for the lowered average gamma power in SZ.
Fig. 2.
Evidence for medial ventral prefrontal cortex involvement in SZ and response to NMDAR antagonist. A. Regions of enhanced delta/theta power in the resting awake state in schizophrenia spectrum disorders, as measured by MEG. (Reproduced with permission from authors as originally published in [**49]). B. Regions of decreased BOLD signal (blue) in response to ketamine. (Reproduced with permission from [**50]).
NMDAR antagonist can induce many of the symptoms of SZ, and recent work shows that this is also true for delta oscillations [**52]. Insight has been gained into the mechanism by which NMDAR antagonist induces delta: antagonist acts on NR2C receptors in the nucleus reticularis of the thalamus (Fig.1), a region that contains PV-containing GABAergic cells and that is implicated in the control of thalamic relay cells and their interactions with cortex [53], in the sensitivity to NMDAR antagonist, and in SZ [54–58]. NR2C lacks Mg2+ block and can thus be partially activated at resting potential by ambient glutamate. Blocking these channels therefore produces hyperpolarization, and this removes inactivation from T-type Ca channels, leading them to produce bursting at delta frequency. This bursting can spread to thalamic relay nuclei and from there to hippocampus and parts of cortex.
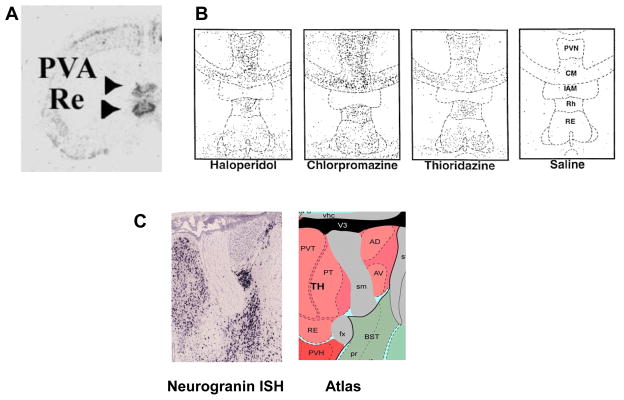
All regions of cortex have thalamic innervations, but SZ is unlikely to result from a uniform deficit in neuronal function. It is therefore of interest that c-fos studies show that thalamic midline nuclei are particularly activated by NMDAR antagonist (Fig.3A). Among these is the nucleus reuniens [59], which receives input from the mPFC and sends output to the entorhinal cortex and CA1 region of the hippocampus [60]. The delta oscillations in the reuniens leads to excitation of the CA1 region [61]. Such excitatory input might explain the selective activation of CA1 in SZ [62].
Fig. 3.
Evidence of special involvement of midline thalamic nuclei in SZ-related assays. A. c-fos activation in response to systemic NMDAR antagonist (Reproduced with permission from [59]). B. c-fos activation in response to neuroleptics used to treat SZ. Response is in local interneurons. (Reproduced with permission from [*74]). C. The midline group of the dorsal thalamus is labeled in this neurogranin RNA in situ hybridization image (left) from the Allen Brain Atlas. In the atlas key (right), thalamic nuclei are pink. The midline group consists of the paraventricular (PVT (PVN in B)) nucleus, the parataenial nucleus, and nucleus of reuniens (RE). Also shown in these images are the anteromedial (AM) and anteroventral (AV) thalamic nuclei. Note that A,B,C are at different magnification and at different sections through the thalamus.
As noted earlier, activity in the hippocampus can produce dopamine release. Among the regions of dopamine release is the nucleus reticularis of the thalamus, where dopamine produces hyperpolarization that promotes delta [**52]. Thus, the thalamo-hippocampal-VTA loop has the potential for positive feedback (the thalamus excites the hippocampus, which stimulates the VTA, which promotes the bursting in the thalamus). One proposal is that genetic risk factors or prenatal insults move this loop closer to the threshold for positive feedback [*63]. Conditions that stimulate strong dopamine release, such as stress, might push the loop above the threshold for positive feedback, thereby accounting for the sudden transition to the psychotic state. This, in turn, could activate the multiple targets of the dopamine system, including the PFC (Fig.1) and striatum. Schizophrenia generally does not occur until adolescence. Interestingly, positive feedback depends on NR2C in the thalamus, the expression of which does not occur until adolescence in the rat [64]. It would be important to determine the temporal expression of NR2C in humans.
Deficits in corollary discharge might underlie first-rank symptoms
The “first-rank symptoms” of schizophrenia are delusions, hallucinations, and the feeling that one is not controlling one’s own actions. It is this cluster of symptoms that seems most difficult to explain, but one class of models has led to progress [65,66]. The idea is that self-initiated action (e.g., speech) is identified as self by a comparison of the resulting sensation to the prediction of that sensation. The prediction arrives from motor-planning regions and is called corollary discharge. If the comparison produces a “match,” it provides the feeling that the speech was self-generated; if the comparison produces a “mismatch,” as would occur if the speech was due to someone else, there is the appropriate feeling of otherness. It is posited that this system fails in SZ by producing a mismatch signal to self-generated speech, leading the affected person to feel inappropriately that the speech was generated by “other.” In the absence of seeing someone else, it would be logical to conclude that the speech was “implanted by the FBI.”
In support of this class of model, theta frequency synchronization between frontal areas of motor control and temporal areas of sensory processing is poor when recorded speech is heard, but high during self-generated speech, perhaps reflecting the synchronizing ability of corollary discharge. If corollary discharge is defective in SZ, synchronization should be poor in both conditions. Experiments have shown this to be case [**67]. The pathways that carry corollary discharge are generally not known, but in the one case that has been extensively studied, the corollary discharge about eye movements is sent from the superior colliculus to frontal eye fields via a thalamic pathway [68]. Thus, the corollary discharge for speech might also involve thalamic nuclei and possibly the nucleus reuniens (Fig.1). The delta state of sleep is known to interfere with normal information transmission by the thalamus. Thus, delta in SZ might interfere with corollary discharge. If this could be confirmed, it would provide a mechanistic understanding of some of the first-rank symptoms.
Conclusions
This brief review has highlighted areas of recent progress in understanding how specific circuit deficits might underlie SZ. Several important topics, including sensory gating and latent inhibition, could not be dealt with because of space limitations. A large number of susceptibility genes for SZ have been identified and can be found at: http://www.szgene.org/. Many of these are involved in glutamatergic transmission [10], while others (notably the ErbB4, a binding partner of the SZ risk gene, neuregulin) have a special role in interneurons [69–71]. Recent work extends the study of neuregulin-1 by showing that it affects the hippocampal-striatal pathway [72]. In terms of the certainty of linkage to SZ, neuregulin ranks 26th. The highest ranking gene (3d), and the only of the top 3 with known neuronal function, is the calmodulin binding protein, neurogranin. As noted earlier, midline thalamic nuclei are preferentially activated by NMDAR antagonist (Fig.3A). These same nuclei have local interneurons that are preferentially activated by neuroleptics [73] (Fig.3B). It was thus of interest to ask whether neurogranin is especially concentrated in midline nuclei. Fig.3C shows that this is the case. There are thus three quite independent lines of evidence pointing to the importance of midline thalamic nuclei in SZ.
The understanding of how brain circuits interact to perform function is an important goal for SZ research. The fact that no single gene strongly affects schizophrenia and that many genes have small effects makes it unlikely that a unique molecular process is involved. Similarly, it seems unlikely that any single brain region is involved; indeed, the abnormalities in GABA-related proteins are widespread. But still, the very unique and unusual properties of SZ suggest that something very specific must be involved. One resolution is that the changes in interneuron GAD-67 and the resulting changes in local circuitry lead to altered gamma. These local circuit changes are, however, quite general, leading to widespread modest cognitive deficits, many of which precede the psychotic break. These problems may arise from multiple genetic or developmental risk factors. At the same time, the reduced inhibition leads to enhanced brain activity that biases a specific brain circuit toward a sudden transition to abnormal function during the psychotic break. The thalamic-hippocampal-VTA loop discussed above is such a possibility. The specific large-scale loop would be commonly affected by a variety of risk factors. The identification of such a malfunctioning loop offers hope for therapeutic intervention; rather than having to treat the myriad gene abnormalities and perinatal insults that are risk factors for the disease (with different combinations in each individual), agents that normalize the function of the large-scale loop would have more general efficacy.
Highlights.
Analysis of postmortem tissue shows a reduction in the GABA synthesizing enzyme, GAD-67, in schizophrenia. Experiments in which this reduction is mimicked show a reduction in functional inhibition.
Gamma oscillations during a task are reduced in schizophrenia, perhaps because of reduction in feedback inhibition onto pyramidal cells. Procedures that reduce gamma lead to memory and cognitive deficits.
Delta oscillations in parts of the thalamo-cortical system are enhanced in the awake state in schizophrenia, a symptom that has been mimicked by application of NMDAR antagonist to the thalamus. The sleep-like delta oscillations in specifically affected thalamic nuclei may interfere with normal function. Three lines of evidence point to a particular role of the midline thalamic nuclei.
The best explanation for psychosis is the interference with corollary discharge, a process that for speech involves theta frequency synchronization between frontal and temporal cortex. This synchronization is abnormal in schizophrenia, perhaps because of thalamic delta oscillations.
Acknowledgments
This work was supported by NIH/NIMH Conte Center Grant 5P50 MH060450.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiology & behavior. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- 2.Spencer KM. Visual gamma oscillations in schizophrenia: implications for understanding neural circuitry abnormalities. Clinical EEG and neuroscience : official journal of the EEG and Clinical Neuroscience Society. 2008;39:65–68. doi: 10.1177/155005940803900208. [DOI] [PubMed] [Google Scholar]
- 3.Galderisi S, Mucci A, Volpe U, Boutros N. Evidence-based medicine and electrophysiology in schizophrenia. Clinical EEG and neuroscience : official journal of the EEG and Clinical Neuroscience Society. 2009;40:62–77. doi: 10.1177/155005940904000206. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson ML, Carlsson A, Nilsson M. Schizophrenia: from dopamine to glutamate and back. Current medicinal chemistry. 2004;11:267–277. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- 5.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cellular and molecular neurobiology. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, Ell PJ. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Molecular psychiatry. 2006;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 7.Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. Journal of psychopharmacology. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. Journal of neurophysiology. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. It is remarkable that the main GABA phenotype seen in postmortem tissue can be reproduced in a cell culture system; this is schizophrenia in a dish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nature neuroscience. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Molecular psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 13*.Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. This is the classic paper showing the suprisingly strong effect of NMDAR antagonists on interneuron function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27 :11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biological psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. Journal of neurophysiology. 2005;93 :1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- 17.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011 doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biological psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Mann EO, Radcliffe CA, Paulsen O. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. The Journal of physiology. 2005;562:55–63. doi: 10.1113/jphysiol.2004.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews Neuroscience. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 21.Borgers C, Kopell N. Effects of noisy drive on rhythms in networks of excitatory and inhibitory neurons. Neural computation. 2005;17:557–608. doi: 10.1162/0899766053019908. [DOI] [PubMed] [Google Scholar]
- 22.de Almeida L, Idiart M, Lisman JE. A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7497–7503. doi: 10.1523/JNEUROSCI.6044-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer W. Distributed processing and temporal codes in neuronal networks. Cognitive neurodynamics. 2009;3:189–196. doi: 10.1007/s11571-009-9087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophrenia bulletin. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews. Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. An excellent review of issues relevant to the role of gamma in schizophrenia. [DOI] [PubMed] [Google Scholar]
- 26.Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. Journal of neurophysiology. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. This paper uses a combination of genetic, electrophysiological and computational methods to provide a solid understand of the role of parvalbumin interneurons in the generation of gamma oscillations. This paper stands as a model of excellence for how this type of research should proceed. [DOI] [PubMed] [Google Scholar]
- 28**.Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophrenia bulletin. 2008;34:962–973. doi: 10.1093/schbul/sbn059. For connecting NMDAR's to gamma oscillations, this is the key paper. Unfortunately, the situation is complicated by regional differences and various ways of inducing gamma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biological psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 30**.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature neuroscience. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]; neuroscience. 13(1):76–83. This paper critically tests the role of NMDA channels in fast-spiking inteneurons. The results confirm that there are schizophrenia-like consequences provided the inhibition occurs at a young age. Surprisingly, there is little effect if the inhibition is done in adults. [Google Scholar]
- 31.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. The American journal of psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- 34.Traub RD, Whittington MA, Buhl EH, Jefferys JG, Faulkner HJ. On the mechanism of the gamma --> beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1088–1105. doi: 10.1523/JNEUROSCI.19-03-01088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. The American journal of psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28 :7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. The American journal of psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 38.Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The neural basis of relational memory deficits in schizophrenia. Archives of general psychiatry. 2006;63:356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 39.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in Neurosciences. 2011 doi: 10.1016/j.tins.2011.07.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in pharmacological sciences. 2011 doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. For those who have doubts about the role of the hippocampus in schizophrenia, this is a good paper to review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30 :17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010;27:179–190. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends in Neurosciences. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 49**.Schulman JJ, Cancro R, Lowe S, Lu F, Walton KD, Llinas RR. Imaging of thalamocortical dysrhythmia in neuropsychiatry. Frontiers in Human Neuroscience. 2011;5:69. doi: 10.3389/fnhum.2011.00069. This is best work so far on the localization of the enhanced low frequency (delta/theta) oscillations in schizophrenia and other mental disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Archives of general psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. What's most interesting here is that some structures in the human brain increase their metabolism in response to ketamine, while others decrease their metabolism. The fact that only a small number of structures decrease their metabolism makes these of special interest. Presumably these are structures that have turned off. [DOI] [PubMed] [Google Scholar]
- 51.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 52*.Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the Nucleus Reticularis of the Thalamus Produces Delta Frequency Bursting. Frontiers in neural circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. This paper provides the key linkage between the effects of NMDAR antagonist in the thalamus and the generation of the delta frequency abnormality seen in schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiological reviews. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 54.Kargieman L, Santana N, Mengod G, Celada P, Artigas F. NMDA antagonist and antipsychotic actions in cortico-subcortical circuits. Neurotoxicity research. 2008;14:129–140. doi: 10.1007/BF03033805. [DOI] [PubMed] [Google Scholar]
- 55.Kiss T, Hoffmann WE, Scott L, Kawabe TT, Milici AJ, Nilsen EA, Hajos M. Role of Thalamic Projection in NMDA Receptor-Induced Disruption of Cortical Slow Oscillation and Short-Term Plasticity. Frontiers in psychiatry / Frontiers Research Foundation. 2011;2:14. doi: 10.3389/fpsyt.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meador-Woodruff JH, Clinton SM, Beneyto M, McCullumsmith RE. Molecular abnormalities of the glutamate synapse in the thalamus in schizophrenia. Annals of the New York Academy of Sciences. 2003;1003:75–93. doi: 10.1196/annals.1300.005. [DOI] [PubMed] [Google Scholar]
- 57.Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S. Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends in neurosciences. 2001;24:330–334. doi: 10.1016/s0166-2236(00)01817-8. [DOI] [PubMed] [Google Scholar]
- 58.Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophrenia bulletin. 2011;37:306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaisanen J, Ihalainen J, Tanila H, Castren E. Effects of NMDA-receptor antagonist treatment on c-fos expression in rat brain areas implicated in schizophrenia. Cellular and molecular neurobiology. 2004;24:769–780. doi: 10.1007/s10571-004-6918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoover WB, Vertes RP. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain structure & function. 2011 doi: 10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Lisman J Neuroscience Sf: The Society for Neuroscience. Ketamine enhances delta and gamma oscillations in hippocampal field potentials. Society for Neuroscience; San Diego, CA: 2010. [Google Scholar]
- 62.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biological psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. This is a speculative model based on substantial physiological results. The model shows a dynamic system in which the key requirements of a predisposition for schizophrenia are specified. It is further specified what happens in the sudden transition to the psychotic state. Understanding the former is of particular importance given the large number of predispositions (genetic and otherwise) for schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Molecular and cellular neurosciences. 2007;34:468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychological medicine. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- 66.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophrenia bulletin. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 67**.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. This is a key paper towards identifying an abnormality is schizophrenia that could directly underly psychosis. [DOI] [PubMed] [Google Scholar]
- 68.Wurtz RH, McAlonan K, Cavanaugh J, Berman RA. Thalamic pathways for active vision. Trends in cognitive sciences. 2011;15:177–184. doi: 10.1016/j.tics.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends in neurosciences. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marenco S, Geramita M, van der Veen JW, Barnett AS, Kolachana B, Shen J, Weinberger DR, Law AJ. Genetic Association of ErbB4 and Human Cortical GABA Levels In Vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11628–11632. doi: 10.1523/JNEUROSCI.1529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, Lewis DA, McBain CJ, Buonanno A. Conserved Interneuron-Specific ErbB4 Expression in Frontal Cortex of Rodents, Monkeys, and Humans: Implications for Schizophrenia. Biological psychiatry. 2011 doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nason MW, Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:488–496. doi: 10.1038/npp.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen BM, Cherkerzian S, Ma J, Ye N, Wager C, Lange N. Cells in midline thalamus, central amygdala, and nucleus accumbens responding specifically to antipsychotic drugs. Psychopharmacology. 2003;167:403–410. doi: 10.1007/s00213-003-1423-0. [DOI] [PubMed] [Google Scholar]
- 74*.Cohen BM, Wan W, Froimowitz MP, Ennulat DJ, Cherkerzian S, Konieczna H. Activation of midline thalamic nuclei by antipsychotic drugs. Psychopharmacology. 1998;135:37–43. doi: 10.1007/s002130050483. Worth a look because the results are so surprising. [DOI] [PubMed] [Google Scholar]