Abstract
Background and Objectives
Nearly one-third of nursing home residents in the US receive antipsychotic medications, yet important questions remain concerning their safety. We sought to compare the risk of major medical events in residents newly initiated on conventional or atypical antipsychotics.
Design
Cohort study, using linked Medicaid, Medicare, Minimum Data Set and Online Survey Certification and Reporting data. Propensity score-adjusted proportional hazards models were used to compare risks for medical events at a class and individual drug level.
Setting
Nursing homes in 45 US states.
Participants
83,959 Medicaid eligible residents ≥65 who initiated antipsychotic treatment following nursing home admission in 2001-2005.
Interventions
Conventional and atypical antipsychotics.
Outcome measures
Hospitalization for myocardial infarction, cerebrovascular events, serious bacterial infections and hip fracture within 180 days of treatment initiation.
Results
Risks of bacterial infections (HR = 1.25, 95%CI 1.05-1.49) and possibly myocardial infarction (1.23, 95%CI 0.81-1.86) and hip fracture (1.29, 95%CI 0.95-1.76) were higher and risks of cerebrovascular events (0.82, 95%CI 0.65-1.02) were lower among patients initiating conventional compared to atypical agents. Little variation existed among individual atypical agents, except for a somewhat lower risk of cerebrovascular events with olanzapine (0.91, 95%CI 0.81-1.02) and quetiapine (0.89, 95%CI 0.79-1.02); a lower risk of bacterial infections (0.83, 95%CI 0.73-0.94) and possibly a higher risk of hip fracture (1.17, 95%CI 0.96-1.43) with quetiapine, all compared with risperidone. Dose-response relations were observed for all events (1.12, 95%CI 1.05-1.19 for high- vs low-dose for all events combined).
Conclusion
These associations underscore the importance of carefully selecting the specific antipsychotic agent and dose, and monitoring their safety, especially in nursing home residents who have an array of medical illnesses and receive complex medication regimens.
Keywords: Antipsychotics, nursing homes, safety, dementia
INTRODUCTION
Antipsychotic medication use is widespread in nursing homes (NHs),1, 2 especially among residents with behavioral disturbances. Up to one-third of all NH patients receive antipsychotic medications,3-7 mostly for the treatment of dementia-related behavioral disturbances. However, serious safety concerns surround use of antipsychotics in older adults with dementia-related psychoses. Based on randomized trials, the FDA issued warnings of increased risks of stroke and transient ischemic events for risperidone (2003), followed by olanzapine and aripiprazole (2004).8 In 2005, the FDA issued warnings of excess mortality associated with the use of atypical antipsychotics in older patients with dementia.9 Moreover, a National Institutes of Health-sponsored clinical trial concluded that the adverse effects of atypical antipsychotics offset their efficacy advantage in patients with Alzheimer's disease10 and that worsening of cognitive function is an additional risk of treatment with atypical antipsychotics.11 In June 2008, the FDA requested a similar boxed warning for conventional antipsychotics,12 based on non-randomized studies that used health care utilization databases.13-15 The proposed mechanisms for the higher mortality remain speculative and include metabolic dysregulation, cardiac conduction disturbances, changes in blood pressure or heart rate which may exacerbate pre-existing heart failure, and sedation leading to aspiration with secondary pneumonia.8, 16 Gait and movement disorders, confusion, delirium, excessive sedation, and orthostatic hypotension have also been associated with antipsychotic medication use and are well established risk factors for falls and hip fractures.17
An important next step in assessing the comparative safety of APMs is to examine the risk of these potential mediating cardiac and cerebrovascular events, infections, and hip fractures and to examine the extent to which risks differ between classes and across individual agents. We addressed these issues in a cohort of NH residents which represent the most vulnerable and most widely treated segment of the elderly population.
METHODS
Data Source
The study cohort was drawn from a merged dataset of Medicaid and Medicare claims, the Minimum Data Set (MDS) and the Online Survey Certification and Reporting (OSCAR) system in 45 US states (all except Arizona, Delaware, Nevada, Oregon, and Rhode Island) for 2001-2005. The claims data provided information on patient demographics, Medicaid eligibility, all physician services and hospitalizations, admissions to long-term care, and filled medication prescriptions. The MDS is a federally mandated health assessment tool used in NHs that captures information on physical, psychological and psychosocial functioning, active clinical diagnoses, health conditions, treatments and services, and as such provides information on both the patient's cognitive functioning and the presence of behavioral problems. OSCAR is a uniform database of NH regulatory reviews which is generated yearly for all CMS-certified NHs and includes operational and staffing characteristics and aggregate resident characteristics. Data are validated at onsite inspections that occur at least every 15 months.
Study Population
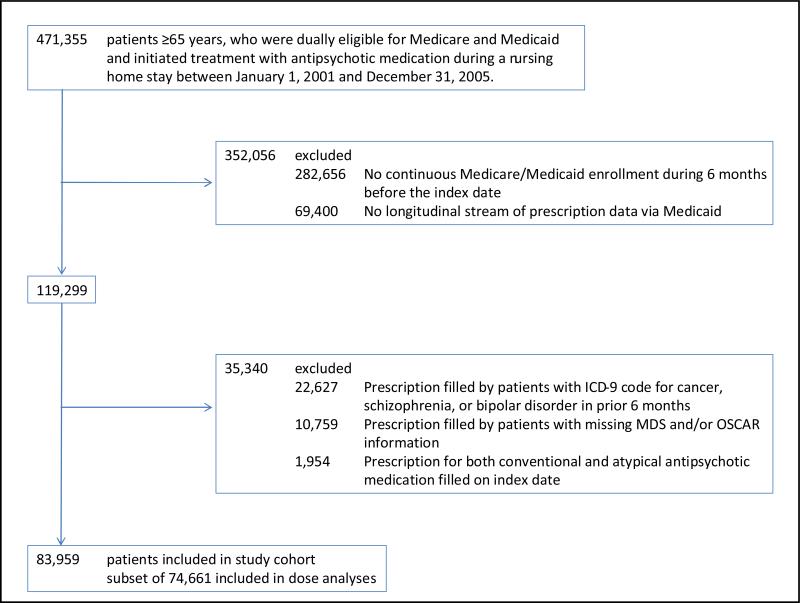
Our cohort consisted of patients ≥65 years who were dually eligible for Medicare and Medicaid, who began treatment with an APM during a NH stay, and who had 6 months of continuous Medicaid coverage preceding the antipsychotic drug initiation date (index date). Incident use required the absence of any filled APM prescription in the 6 months before the index date. We employed a new-user design to avoid under-ascertainment of events occurring soon after therapy begins,18 and to ensure baseline covariates at study entry were assessed before antipsychotic treatment initiation.19 Residents were excluded if they filled a prescription for both a conventional and an atypical APM on the index date and if they had a pre-existing diagnosis for schizophrenia, bipolar disorder or cancer since these residents may have been receiving antipsychotic medication for reasons other than dementia-related behavioral problems (e.g., reduce nausea associated with chemotherapy or potentiate pain medications) (Figure 1).
Figure 1.
Flowchart of Study Cohort ICD-9 indicates International Classification of Diseases, Ninth Revision; MDS: Minimum Data Set; OSCAR: Online Survey Certification And Reporting
Antipsychotic Medications
Atypical antipsychotic agents included risperidone, olanzapine, quetiapine, aripiprazole, and ziprasidone. Conventional agents included haloperidol, thioridazine, chlorpromazine, perphenazine, and fluphenazine. We converted daily doses of the index dispensing to chlorpromazine-equivalent milligrams,20-22 and used the median daily dose in the population (50 mg) as a cutoff to assess the effect in strata of higher and lower doses for patients using tablets or caplets. For analyses at the class level, atypical APMs were chosen as the referent. For analyses at the level of individual agents, the most commonly used agent in this population, risperidone, was chosen as the referent. Each subject was assigned to a specific antipsychotic agent and class based on the medication prescribed on the index date; exposure was considered discontinued if there was a treatment gap of ≥14 days.
Outcomes
Follow-up for endpoints extended up to 180 days. Outcomes considered included myocardial infarction (MI), cerebrovascular events (stroke or Transient Ischemic Attack [TIA]), serious bacterial infections (bacteremia/septicemia, encephalitis/meningitis, endocarditis/myocarditis, pyelonephritis, septic arthritis, osteomyelitis, pneumonia, or opportunistic infection), and hip fracture. Outcomes were defined based on a hospitalization with a relevant primary or secondary ICD-9 diagnostic or procedure code (eTable 1).
Patient and Nursing Home Characteristics
Patient characteristics were assessed during the 6 months preceding cohort entry. Socio-demographic characteristics included age, sex, race, education, and geographic region (state). Clinical characteristics were determined based on the most recent MDS assessment before treatment initiation, ICD-9 diagnostic and procedure codes associated with hospitalizations and physician visits, as well as medication use. Variables considered included psychiatric morbidity, cardiovascular morbidity, cerebrovascular disease, Parkinson's disease, epilepsy, diabetes, obesity, functional impairment, Charlson index,23 and the use of health care services potentially predictive of adverse health outcomes in the short-term (number of days hospitalized for any reason, number of distinct prescription drugs excluding APMs).24 NH characteristics, which may be correlated with care processes and risk of adverse health outcomes, were obtained from OSCAR. These included variables such facility size, occupancy rate, availability of special care units, staffing levels, ownership, resident characteristics (proportion with dementia, depression) and quality indicators (e.g., proportion of residents bed- or chair-bound).
Data Analysis
We calculated rates of the various outcomes during follow-up, with follow-up censored at the time of treatment discontinuation or switch to a medication belonging to a different APM class (class-level analysis) or a switch to a different medication (individual-agent analysis). Other censoring events included death and hospitalizations of ≥10 days for reasons other than the outcomes of interest.
We fit unadjusted; age-, sex- and calendar year-adjusted; and multivariate-adjusted proportional hazards models for pairwise comparisons against atypical APMs (class-level analysis) and risperidone (individual-agent analysis). We detected no violations of the assumption of proportional hazards based on graphical approaches and goodness-of-fit tests._For more efficient estimation, we used propensity score (PS) adjustment to balance measured risk factors for the outcomes between drug user groups.25 PS at treatment initiation were derived from predicted probabilities estimated in logistic regression models which contained all covariates listed earlier, and Cox models were stratified across deciles of the PS. Models were run separately in strata defined by diagnoses of dementia, behavioral disturbances and delirium, and by dose dividing each drug user group into those taking ≤50mg or >50mg chlorpromazine equivalents.
In secondary analyses, we used high-dimensional PS (hdPS) adjustment.26 A limitation of standard approaches to confounding adjustment which hdPS attempt to overcome is their reliance on the investigator being able to specify all factors that may confound a causal drug-outcome association. The hdPS algorithm evaluates thousands of diagnoses, procedures, and pharmacy claim codes, as well as clinical (MDS) and facility (OSCAR) characteristics (referred to as data dimensions) to identify and prioritize those covariates that serve as proxies for unmeasured confounders. Specifically, the 200 most prevalent codes in each data dimension were identified and from these a total of 500 likely confounders were selected based on their prevalence and potential for confounding in the study population. These empirically identified confounders are combined with investigator-identified covariates (i.e., socio-demographic variables and general indicators of comorbidity) to improve confounding adjustment. A dose-response analysis was conducted by comparing the risk of the various event types in residents treated with high- versus low-dose APMs, for all agents combined.
Adjustments for multiple comparisons were not considered.27 Rather than interpreting results qualitatively in terms of what is or is not statistically significant (through use of the p-value or use of the confidence interval as a pseudo significance test), results were interpreted quantitatively to enhance the assessment of competing explanations, such as uncontrolled confounding. This approach keeps separate the two components of estimation – effect size and precision – instead of confusing them in a single qualitative assessment, namely significance testing.28-34
RESULTS
Of the 83,959 NH residents included in our study cohort, 8.9% were prescribed a conventional antipsychotic and 91.1% an atypical drug. Subjects in the conventional drug group were more likely to be male and non-white than those in the atypical drug group. They were more likely to have cardiovascular disease and less likely to have psychiatric comorbidities. Both groups were comparable in terms of the severity of cognitive and functional impairment and the presence of diagnosed behavioral problems. Patients using conventional antipsychotics had lower rates of antidepressant and dementia medication use, but higher rates of hypnotics. They also had a higher Charlson index indicating multiple general medical comorbidities. Patients treated with conventional drugs were less likely than patients treated with atypical drugs to reside in a NH located in the northeast region or in an urban setting (Table 1).
Table 1.
Selected characteristics for residents initiating antipsychotics during a nursing home stay
| Conventional APMs | Haloperidol | Atypical APMs | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | |
|---|---|---|---|---|---|---|---|---|
| (N=7,463) % or mean | (N=6,477) % or mean | (N=76,496) % or mean | (N=1,989) % or mean | (N=25,068) % or mean | (N=17,336) % or mean | (N=30,945) % or mean | (N=1,158) % or mean | |
| High-dose* | 55.1% | 58.4% | 34.2% | 86.6% | 45.2% | 50.8% | 10.5% | 68.8% |
| Region | ||||||||
| Northeast | 10.8% | 11.0% | 16.7% | 18.3% | 16.7% | 17.8% | 16.5% | 4.4% |
| Midwest | 31.0% | 31.8% | 29.3% | 32.3% | 28.3% | 27.8% | 30.9% | 23.5% |
| South | 44.4% | 43.3% | 41.7% | 41.6% | 39.5% | 44.4% | 41.2% | 66.1% |
| West | 13.9% | 13.9% | 12.3% | 7.8% | 15.6% | 10.0% | 11.4% | 6.0% |
| Setting | ||||||||
| Urban | 58.0% | 57.6% | 67.1% | 71.9% | 68.4% | 70.1% | 64.6% | 52.4% |
| Rural | 42.0% | 42.4% | 32.9% | 28.1% | 31.6% | 29.9% | 35.4% | 47.6% |
| Demographics | ||||||||
| Male | 31.1% | 28.7% | 24.9% | 27.4% | 24.2% | 26.0% | 24.6% | 28.0% |
| Age, mean | 83.3 | 83.7 | 83.3 | 81.9 | 83.4 | 82.9 | 83.6 | 82.6 |
| White race | 79.1% | 80.0% | 82.2% | 79.4% | 82.7% | 82.4% | 82.0% | 80.2% |
| Psychiatric morbidity | ||||||||
| Dementia | 70.3% | 71.4% | 75.0% | 75.1% | 74.0% | 75.5% | 75.6% | 74.4% |
| Depression | 48.8% | 48.8% | 55.5% | 63.5% | 55.6% | 58.5% | 53.2% | 59.2% |
| Anxiety | 2.9% | 2.9% | 3.3% | 2.3% | 3.5% | 3.5% | 3.0% | 4.2% |
| Delirium | 53.4% | 54.9% | 53.5% | 50.3% | 52.6% | 54.2% | 54.0% | 54.1% |
| Psychotic disorder | 10.7% | 11.1% | 11.9% | 13.7% | 11.3% | 12.5% | 11.8% | 15.8% |
| Cognitive function | ||||||||
| intact to moderate impairment | 14.3% | 14.0% | 14.7% | 17.3% | 15.3% | 14.5% | 14.2% | 16.5% |
| moderate to severe impairment | 54.6% | 55.0% | 56.8% | 58.4% | 56.6% | 56.7% | 56.7% | 58.2% |
| severe to very severe impairment | 31.0% | 31.0% | 28.5% | 24.4% | 28.1% | 28.8% | 29.0% | 25.3% |
| Delusions | 3.3% | 3.4% | 3.9% | 4.9% | 3.8% | 3.8% | 3.9% | 4.1% |
| Verbally or physically abusive behavior | 13.5% | 14.0% | 14.5% | 14.8% | 14.6% | 13.8% | 14.7% | 14.9% |
| Non-aggressive behavioral problems | 24.7% | 25.8% | 26.3% | 24.7% | 26.2% | 26.0% | 26.6% | 27.0% |
| Cardiovascular morbidity | ||||||||
| Myocardial infarction | 6.8% | 7.1% | 5.5% | 4.4% | 5.3% | 5.3% | 5.9% | 4.6% |
| Arrhythmias | 26.9% | 27.3% | 23.3% | 19.9% | 23.1% | 23.3% | 23.8% | 22.1% |
| Ischemic heart disease | 4.9% | 5.0% | 4.4% | 3.9% | 4.6% | 4.1% | 4.5% | 5.2% |
| Hypertension | 66.4% | 66.0% | 65.1% | 68.1% | 63.3% | 66.5% | 65.3% | 70.6% |
| Congestive heart failure | 37.6% | 38.2% | 31.5% | 29.8% | 30.7% | 31.5% | 32.3% | 33.9% |
| Cerebrovascular disease | 30.4% | 29.6% | 28.4% | 28.4% | 28.2% | 29.0% | 28.2% | 29.2% |
| Other comorbidities | ||||||||
| Diabetes | 25.4% | 24.8% | 22.9% | 28.5% | 20.3% | 24.5% | 23.6% | 24.7% |
| Parkinson's disease | 4.9% | 4.7% | 5.8% | 5.9% | 5.0% | 9.7% | 4.3% | 6.2% |
| Functional impairment | ||||||||
| independent, supervision or limited | 36.6% | 36.5% | 39.9% | 41.4% | 40.1% | 38.7% | 40.1% | 42.4% |
| dependence or extensive | 57.3% | 57.8% | 55.7% | 55.1% | 55.5% | 56.5% | 55.5% | 52.4% |
| total dependence | 6.1% | 5.7% | 4.5% | 3.5% | 4.4% | 4.7% | 4.5% | 5.2% |
| General indicators of comorbidity | ||||||||
| Charlson index†, mean | 3.31 | 3.29 | 3.07 | 3.17 | 2.99 | 3.11 | 3.10 | 3.10 |
| Number of different prescription drugs received, mean | 15.6 | 15.6 | 14.4 | 14.8 | 14.2 | 14.8 | 14.3 | 15.1 |
| Number of outpatient visits, mean | 1.1 | 1.1 | 0.9 | 0.8 | 0.9 | 0.9 | 0.9 | 1.1 |
| Number of hospital days, mean | 20.3 | 20.6 | 18.8 | 15.5 | 18.7 | 18.7 | 19.1 | 21.8 |
| History of prescriptions | ||||||||
| Antidepressants | 60.3% | 60.6% | 67.9% | 72.9% | 68.5% | 71.1% | 65.4% | 67.4% |
| Hypnotic agents | 50.2% | 51.1% | 45.8% | 43.1% | 45.6% | 48.1% | 44.8% | 46.1% |
| Other psychoactive agents‡ | 11.4% | 11.1% | 13.1% | 15.1% | 13.6% | 14.1% | 11.9% | 14.5% |
| Dementia medication | 25.8% | 26.2% | 33.7% | 41.9% | 30.9% | 37.3% | 33.2% | 41.6% |
>50mg chlorpromazine equivalents – based on the subset of patients receiving tablets or caplets
Individual comorbidities defined based on at least one hospitalization or at least one outpatient visit with the respective ICD codes.
Includes barbiturate, non-benzodiazepine anxiolytic, stimulant/ADHD medication, lithium, valproate, carbamazepine, lamotrigine.
Among patients initiating conventional APMs, haloperidol (87%) was by far the most widely used agent. Among patients initiating atypical APMs, risperidone (40%), olanzapine (33%), and quetiapine (23%) were used most frequently. Ziprasidone and aripiprazole, which were introduced in 2001 and 2002 respectively, each represented <3% of atypical APM use in our study population. Overall, patients initiating different atypical APMs resembled one another in terms of demographic and clinical characteristics. Patients treated with risperidone had slightly fewer recorded depression diagnoses, and use of antidepressants and other psychotropic medications. Patients treated with quetiapine had more diagnoses of Parkinsonism than the other groups (Table 1).
Class-Level Analyses
The overall mean follow-up since cohort entry was 45 days for patients initiating conventional APMs, and 96 days for patients initiating atypical APMs: 13% of patients initiating conventional APMs died, compared with 10% of patients initiating atypical APMs; half of the patients were censored due to a treatment switch or discontinuation, and 15% due to a hospitalization for reasons unrelated to the outcomes. For all event types considered, patients initiating conventional APMs had higher unadjusted rates than patients initiating atypical APMs (Table 2). Results of the PS-adjusted Cox regression analyses indicate that patients initiating conventional APM were at increased risk of serious bacterial infection (HR=1.37; 95%CI, 1.17-1.62), and at a decreased risk of cerebrovascular events (0.81; 95%CI, 0.65-1.01). Findings further suggest that patients initiating conventional APMs may be at an increased risk of MI (1.23; 95%CI, 0.82-1.82), hip fracture (1.27; 95%CI, 0.94-1.72), and pneumonia (1.28; 95% CI, 0.87-1.88), but these associations were imprecisely estimated. Results for the conventional PS and the hdPS analyses were consistent (Table 3).
Table 2.
Major medical events leading to hospitalization within 180 days after start of antipsychotics*
| Conventional APMs | Haloperidol | Atypical APMs | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=7,463) | (n=6,477) | (n=76,496) | (n=1,989) | (n=25,068) | (n=17,336) | (n=30,945) | (n=1,158) | |||||||||
| N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | |
| MI | 32 | 3.49 | 29 | 3.79 | 403 | 2.02 | 11 | 2.19 | 126 | 2.01 | 86 | 1.99 | 148 | 1.98 | <11(2) | |
| Stroke/TIA | 93 | 10.18 | 75 | 9.84 | 1,835 | 9.22 | 48 | 9.63 | 539 | 8.63 | 386 | 8.96 | 718 | 9.66 | 28 | 11.09 |
| Bacterial infection | 188 | 20.68 | 149 | 19.63 | 2,100 | 10.54 | 47 | 9.41 | 657 | 10.52 | 406 | 9.41 | 845 | 11.36 | 22 | 8.70 |
| Pneumonia | 33 | 3.60 | 27 | 3.53 | 384 | 1.92 | <11† | 139 | 2.22 | 76 | 1.76 | 139 | 1.86 | <11(2) | ||
| Hip fracture | 49 | 5.35 | 45 | 5.89 | 761 | 3.81 | 16 | 3.19 | 242 | 3.86 | 176 | 4.07 | 266 | 3.57 | <11(2) | |
Rate expressed per 100 patient-years
Actual event number is not provided in accordance with CMS’ cell size suppression policy
The total number of events at the class level does not necessarily equal the sum of the events for the individual drugs due to the censoring mechanisms.
Table 3.
Hazard Ratios of Study Outcomes within 180 Days
| Event Types | HR (95% CI) |
|||
|---|---|---|---|---|
| Unadjusted | Age-, sex-, and calendar year-adjusted | PS-adjusted | hdPS-adjusted | |
| APM CLASS (referent = Atypical APMs) | ||||
| Conventional APMs | ||||
| MI | 1.38 (0.93-2.04) | 1.36 (0.92-2.02) | 1.23 (0.82-1.82) | 1.23 (0.81-1.86) |
| Stroke/TIA | 0.89 (0.72-1.11) | 0.88 (0.70-1.09) | 0.81 (0.65-1.01) | 0.82 (0.65-1.02) |
| Bacterial infection | 1.63 (1.38-1.91) | 1.59 (1.35-1.87) | 1.37 (1.17-1.62) | 1.25 (1.05-1.49) |
| Pneumonia | 1.63 (1.11-2.39) | 1.56 (1.07-2.29) | 1.28 (0.87-1.88) | 1.22 (0.82-1.82) |
| Hip fracture | 1.22 (0.91-1.65) | 1.24 (0.92-1.67) | 1.27 (0.94-1.72) | 1.29 (0.95-1.76) |
| INDIVIDUAL AGENTS (referent = Risperidone) | ||||
| Aripiprazole | ||||
| MI | 0.88 (0.43-1.78) | 0.91 (0.44-1.88) | 0.95 (0.46-1.96) | 1.07 (0.51-2.23) |
| Stroke/TIA | 1.01 (0.75-1.37) | 0.98 (0.72-1.34) | 0.99 (0.72-1.35) | 0.97 (0.70-1.33) |
| Bacterial infection | 0.84 (0.62-1.14) | 0.81 (0.60-1.10) | 0.87 (0.64-1.19) | 0.88 (0.64-1.21) |
| Pneumonia | 0.46 (0.17-1.25) | 0.51 (0.19-1.40) | 0.63 (0.23-1.71) | 0.64 (0.23-1.78) |
| Hip fracture | 0.92 (0.54-1.54) | 1.01 (0.59-1.71) | 1.06 (0.62-1.79) | 1.03 (0.60-1.76) |
| Olanzapine | ||||
| MI | 1.06 (0.83-1.35) | 1.04 (0.81-1.32) | 1.11 (0.87-1.42) | 1.03 (0.81-1.32) |
| Stroke/TIA | 0.91 (0.81-1.02) | 0.90 (0.80-1.01) | 0.94 (0.84-1.05) | 0.91 (0.81-1.02) |
| Bacterial infection | 0.94 (0.85-1.05) | 0.94 (0.85-1.04) | 0.96 (0.86-1.07) | 0.99 (0.89-1.09) |
| Pneumonia | 1.18 (0.93-1.50) | 1.15 (0.91-1.47) | 1.17 (0.91-1.49) | 1.20 (0.94-1.53) |
| Hip fracture | 1.13 (0.94-1.35) | 1.12 (0.94-1.34) | 1.17 (0.97-1.40) | 1.08 (0.91-1.29) |
| Quetiapine | ||||
| MI | 1.02 (0.77-1.34) | 1.05 (0.80-1.39) | 1.07 (0.81-1.42) | 1.02 (0.77-1.34) |
| Stroke/TIA | 0.93 (0.82-1.05) | 0.92 (0.81-1.04) | 0.91 (0.80-1.03) | 0.89 (0.79-1.02) |
| Bacterial infection | 0.84 (0.74-0.94) | 0.82 (0.73-0.93) | 0.85 (0.75-0.96) | 0.83 (0.73-0.94) |
| Pneumonia | 0.94 (0.70-1.25) | 1.01 (0.75-1.36) | 0.98 (0.73-1.32) | 1.05 (0.78-1.42) |
| Hip fracture | 1.20 (0.99-1.46) | 1.25 (1.02-1.52) | 1.25 (1.02-1.52) | 1.17 (0.96-1.43) |
| Ziprasidone | ||||
| MI | 0.66 (0.21-2.06) | 0.68 (0.22-2.15) | 0.62 (0.20-1.96) | 1.53 (0.70-3.35) |
| Stroke/TIA | 0.99 (0.65-1.51) | 0.97 (0.63-1.48) | 0.92 (0.60-1.41) | 0.98 (0.65-1.49) |
| Bacterial infection | 0.66 (0.41-1.07) | 0.64 (0.40-1.04) | 0.59 (0.36-0.95) | 0.75 (0.48-1.17) |
| Pneumonia | 1.23 (0.50-2.99) | 1.30 (0.53-3.19) | 1.01 (0.41-2.49) | 1.45 (0.62-3.38) |
| Hip fracture | 1.24 (0.66-2.33) | 1.33 (0.70-2.51) | 1.18 (0.62-2.25) | 1.03 (0.52-2.04) |
Analyses stratified by dose confirmed the findings for serious bacterial infection (PS-adjusted HR=1.42 (95%CI, 1.10-1.84) for low-dose, and 1.47 (95%CI, 1.13-1.91) for high-dose) and indicated that the lower risk of cerebrovascular events is driven by the high-dose category (1.01 (95%CI, 0.71-1.42) for low-dose and 0.62 (95%CI, 0.42-0.93) for high-dose). Results for the other outcomes are not presented due to the small (<10) event numbers in some dose strata and resulting unstable estimates.
Individual-Agent Analyses
All associations were estimated with risperidone as the reference group. In view of the fact that 87% of residents initiating conventional APMs were treated with haloperidol, the findings for haloperidol mirror those of the class-level analyses shown above.
The mean follow-up in days since cohort entry was 94 for risperidone, 97 for olanzapine, 96 for quetiapine, 99 for aripiprazole, and 92 for ziprasidone. Results from hdPS-adjusted analyses tended to be somewhat closer to the null than those from conventional PS-adjusted analyses. Among patients initiating olanzapine, the associations for MI (hdPS-adjusted HR=1.03; 95%CI, 0.81-1.32), serious bacterial infections (0.99; 95%CI, 0.89-1.09), and hip fracture (1.08; 95%CI, 0.91-1.29) were near null. The HR for cerebrovascular events was 0.91 (95%CI, 0.81-1.02), and for pneumonia 1.20 (95%CI, 0.94-1.53) (Table 3).
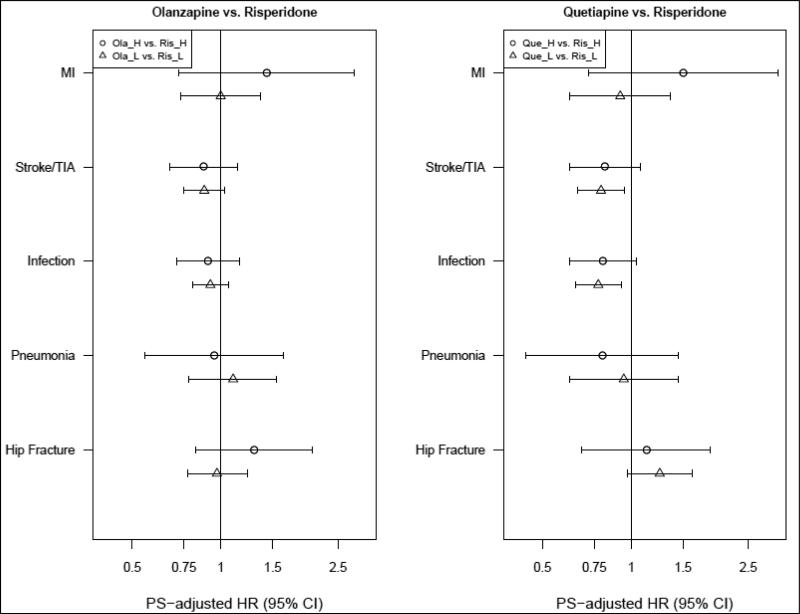
Among patients initiating quetiapine, the associations for MI (1.02; 95%CI, 0.77-1.34) and pneumonia (1.05; 95%CI, 0.78-1.42) were near null. Patients treated with quetiapine had a lower risk of bacterial infections (0.83; 95%CI, 0.73-0.94). The HR for cerebrovascular events was 0.89 (95%CI, 0.79-1.02), and for hip fracture 1.17 (95%, 0.96-1.43) (Table 3). There was no evidence of effect measure modification by dose for either olanzapine or quetiapine (Figure 2A).
Figure 2A.
Analyses stratified by dose* *: restricted to users of tablets or caplets; H: high dose; L: low dose
Given the few patients treated with aripiprazole and ziprasidone during the study period, there was insufficient information to draw meaningful conclusions for most event types, except a near null association for cerebrovascular events and hip fracture (Table 3).
There was no evidence of effect measure modification by the presence of dementia, behavioral disturbances, or delirium for the class level or individual agent analyses (data not shown).
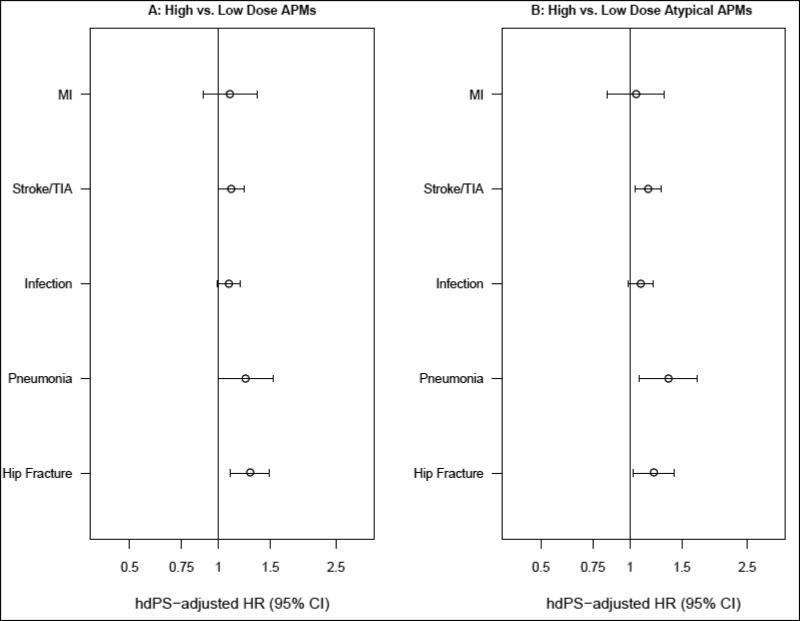
Dose-Response Analyses
When comparing the risk of major medical events in residents treated with high (>50 mg chlorpromazine equivalents) versus low (≤50 mg) dose of all APMs combined, an increased risk was observed for pneumonia (hdPS-adjusted HR=1.24; 95%CI, 1.00-1.53) and hip fracture (1.28; 95%CI, 1.10-1.49). The associations for MI (1.09; 95%CI, 0.89-1.35), cerebrovascular events (1.11; 95%CI, 1.00-1.22) and serious bacterial infections (1.09; 95%CI, 0.99-1.19) were weaker, but still pointed toward an increased risk (Figure 2B, Panel A). The association for the risk of hospitalization for any of the events considered was 1.12 (95%CI, 1.05-1.19). Results were similar when restricted to all atypical APMs (Figure 2B, Panel B). Results for users of conventional APMs are not presented owing to the small event numbers.
Figure 2B.
Dose-response analyses* *: Restricted to users of tablets or caplets
DISCUSSION
In this population of Medicaid-eligible NH residents, we observed risks of serious bacterial infections, MI and hip fracture that were higher and risks of cerebrovascular events that were lower among patients initiating conventional compared to atypical agents. Little variation in event risk was observed among individual atypical agents, except for a somewhat lower risk of cerebrovascular events with olanzapine and quetiapine, a lower risk of serious bacterial infections, and possibly a higher risk of hip fracture with quetiapine, all compared with risperidone. A dose-response relation was observed for all event types for all agents combined.
Conventional APMs have been associated with increased risks of cardiovascular death compared with atypical APMs.35 Our findings are suggestive of an increased risk of MI leading to hospitalization for conventional APMs. Although olanzapine shares with risperidone warnings related to an increased risk of stroke,8 our findings suggest that the risk of stroke/TIA for olanzapine is lower than that for risperidone. We found a similar risk of stroke/TIA for aripiprazole and risperidone, but did not have sufficient data to examine the effect of dose. The increased risk of serious bacterial infections associated with use of conventional vs. atypical APMs in our study is consistent with an earlier report of increased risk of death and hospitalization due to infection35, but stands in contrast with the increased risk of pneumonia observed among patients using atypical compared to conventional agents in two case-control studies of community-dwelling elderly.36, 37 Current evidence about the differential risk of hip fracture conveyed by conventional and atypical APMs is still inconclusive,38-41 and the risks among patients initiated on different atypical agents have not previously been examined. It is believed that differences in receptor-affinity profile within this heterogeneous class of drugs may explain differences in side-effect profiles between agents, but the specific associations are not yet well understood.42
We used multiple approaches in the design and analysis of the study to reduce the possibility of confounding: including, an incident-user design; implementation of different methods to mitigate confounding by predefined covariates and by proxies for unobserved factors (hdPS adjustment); prospective follow-up of the population for the outcomes of interest; documentation of and accounting for changes in exposure status.
Despite these strengths, the current analyses should be interpreted in the context of several limitations. First, as with all non-randomized studies, residual confounding by indication is a factor to consider as an alternative explanation of our findings. This would occur if patients who were frail and at increased risk of undesirable health outcomes were more or less likely to be prescribed certain antipsychotic medications than others. Confounder information derived from claims data was supplemented with clinical assessment data from the MDS. Since NH residents may be at increased risk of adverse health outcomes simply by being admitted to a facility with poor quality indicators,43 we accounted for potential NH quality indicators through use of the OSCAR database which reflects findings from state inspections and complaint investigations. Nevertheless, if the presence or the severity of important clinical conditions or quality-of-care measures were incompletely captured, this could lead to residual confounding. High-dimensional proxy adjustment based on PS techniques was therefore used in an effort to further improve confounding control compared with adjustment limited to predefined covariates.26 Second, there is likely to be under-ascertainment of the outcomes (e.g., not all pneumonia cases in NH residents will require hospitalization). As long as the misclassification is non-differential and the specificity of the disease definition is high (ensured through the implementation of strict disease definitions), we expect the relative effect measures to be unbiased.44 Third, there is potential for misclassification of exposure status. This would occur if there is a lack of consumption of filled prescriptions (e.g., due to non-adherence or occasional use). However, adherence is expected to be high in a population of NH residents that is closely monitored. Nonetheless, occasional (‘as needed’) use might be an important source of misclassification since it is imperfectly captured in claims data. Fourth, patients were classified into low- and high-dose groups based on the initial prescription filled. In case of dose titration, this could have resulted in exposure misclassification in the analyses stratified by dose. We verified both the second and last prescription filled before end of follow-up, and the dose assignment remained unchanged for >90% of the patients, suggesting this is not an important source of misclassification. Fifth, the known potential for drug intolerance or treatment failure may lead to drug discontinuation and thus informative censoring. This could make treatment switches or discontinuation a predictor for adverse health outcomes that would not be observed in an as-treated analysis, therefore introducing bias towards the null. To minimize this potential bias, we allowed for a 30-day grace period at the time of treatment change. Any outcomes observed during this time period were still attributed to the initial exposure. Varying the length of the grace period did not meaningfully affect the findings. Finally, by not imposing the presence of a recorded dementia diagnosis as an inclusion criterion, it is possible that some patients in the cohort received APMs for reasons other than dementia-related behavioral problems. Given the under-recording of dementia diagnoses in elderly patients with multiple comorbidities and the exclusion of patients with a schizophrenia and/or bipolar diagnosis, this number is expected to be small however. Moreover, analyses stratified by the presence of a recorded dementia diagnosis showed no evidence of effect measure modification.
The fact that no previous studies have directly evaluated the relationship between different antipsychotic agents and risk of multiple adverse health outcomes in routine care, the consideration of dose, the large study size used, the focus on the most vulnerable and most widely treated segment of the elderly population, and the access to detailed clinical and quality-of-care related variables make this investigation valuable to clinical practice. The findings provide new information concerning the comparative safety of the antipsychotic treatment options available for residents with dementia-related behavioral problems. The observed associations lend further support to the premise that conventional APMs are less safe than atypical APMs in elderly patient with dementia-related behavioral symptoms and generally should not be used in this population. Overall, little variation existed among individual atypical agents, but for some event types effect estimates ranged from about 10 to 17% on the relative scale. While these differences are relatively small, they are not inconsequential in a vulnerable population of older nursing home residents already burdened by an array of medical illnesses and complex medication regimens. While awaiting confirmation of these findings, it therefore seems prudent for clinicians to evaluate a given drug's risk profile against an individual patient's vulnerabilities when selecting a specific APM agent and dose, and to closely monitor its safety.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by AHRQ/FDA Award HS017918 and AHRQ Award HS016097 to Rutgers University. Drs. Huybrechts and Schneeweiss were partially funded by National Institute of Mental Health R01-MH078708.
Sponsor's Role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis or preparation of the paper.
Footnotes
Author's contributions
Dr. Huybrechts had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schneeweiss, Crystal, Huybrechts, Gerhard, Olfson, Avorn, Lucas
Acquisition of the data: Crystal, Schneeweiss
Analysis and interpretation of data: Huybrechts, Schneeweiss, Levin, Gerhard, Olfson, Avorn, Crystal, Lucas
Drafting of the manuscript: Huybrechts
Critical revision of the manuscript for important intellectual content: Huybrechts, Schneeweiss, Gerhard, Olfson, Avorn, Crystal, Lucas, Levin
Statistical analysis: Huybrechts, Schneeweiss, Levin
Administrative, technical, or material support: Schneeweiss, Crystal
Study supervision: Crystal, Schneeweiss
Conflict of Interest: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Beers M, Avorn J, Soumerai S, et al. Psychoactive medication use in intermediate-care facility residents. JAMA. 1988;260:3016–3020. [PubMed] [Google Scholar]
- 2.Ray WA, Federspiel CF, Schaffner W. A study of antipsychotic drug use in nursing homes: Epidemiologic evidence suggesting misuse. Am J Public Health. 1980;70:485–491. doi: 10.2105/ajph.70.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liperoti R, Mor V, Lapane KL, et al. The use of atypical antipsychotics in nursing homes. J Clin Psychiatry. 2003;64:1106–1112. doi: 10.4088/jcp.v64n0918. [DOI] [PubMed] [Google Scholar]
- 4.Briesacher BA, Limcangco MR, Simoni-Wastila L, et al. The quality of antipsychotic drug prescribing in nursing homes. Arch Intern Med. 2005;165:1280–1285. doi: 10.1001/archinte.165.11.1280. [DOI] [PubMed] [Google Scholar]
- 5.Bronskill SE, Anderson GM, Sykora K, et al. Neuroleptic drug therapy in older adults newly admitted to nursing homes: Incidence, dose, and specialist contact. J Am Geriatr Soc. 2004;52:749–755. doi: 10.1111/j.1532-5415.2004.52212.x. [DOI] [PubMed] [Google Scholar]
- 6.Rochon PA, Stukel TA, Bronskill SE, et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167:676–683. doi: 10.1001/archinte.167.7.676. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Briesacher B, Field T, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170:89–95. doi: 10.1001/archinternmed.2009.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: Update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2007;33:957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [May 3, 2010];FDA Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. [URL: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm053171.htm].
- 10.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 11.Vigen C, Mack W, Keefe R, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: Outcomes from CATIE-AD. Am J Psychiatry. 2011;168:831–839. doi: 10.1176/appi.ajp.2011.08121844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [May 3, 2010];FDA: Information for Healthcare Professionals - Antipsychotics. [URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm]
- 13.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 14.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Can Med Assoc J. 2007;176:627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill SS, Bronskill SE, Normand S-LT, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Brookhart M, Setoguchi S, et al. Psychotropic medication use for behavioral symptoms of dementia. Curr Neurol Neurosci Rep. 2006;6:490–495. doi: 10.1007/s11910-006-0051-6. [DOI] [PubMed] [Google Scholar]
- 17.Trifiro G, Spina E, Gambassi G. Use of antipsychotics in elderly patients with dementia: Do atypical and conventional agents have a similar safety profile? Pharmacol Res. 2009;59:1–12. doi: 10.1016/j.phrs.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA. Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Safe. doi: 10.1002/pds.1926. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins M, Burgess A, Bottomley C, et al. Chlorpromazine equivalents: A consensus of opinion for both clinical and research applications. Pscyhiatr Bull. 1997;21:224–226. [Google Scholar]
- 21.Lehman A, Steinwachs D. Translating research into practice: The Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophrenia Bull. 1998;24:1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- 22.Woods S. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 23.Romano P, Roos L, Jollis J. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 25.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: Analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 26.Schneeweiss S, Rassen J, Glynn R, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 28.Rothman KJ, Greenland S, Lash T. Precision and Statistics in Epidemiologic Studies. In: Rothman K, Greenland S, Lash T, editors. Modern Epidemiology. 3 edn Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 148–167. [Google Scholar]
- 29.Lang J, Rothman K, Cann C. That confounded p-value. Epidemiology. 1998;9:7–8. doi: 10.1097/00001648-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Poole C. Beyond the confidence interval. Am J Public Health. 1987;77:195–199. doi: 10.2105/ajph.77.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ. A show of confidence. N Engl J Med. 1978;299:1362–1363. doi: 10.1056/NEJM197812142992410. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ. Significance questing. Ann Intern Med. 1986;105:445–447. doi: 10.7326/0003-4819-105-3-445. [DOI] [PubMed] [Google Scholar]
- 33.Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25:225–230. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 34.International Committee of Medical Journal Editors Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication, 2010. [URL: www.icmje.org]
- 35.Setoguchi S, Wang P, Brookhart M, et al. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56:1644–1650. doi: 10.1111/j.1532-5415.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 36.Knol W, van Marum R, Jansen P, et al. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56:661–666. doi: 10.1111/j.1532-5415.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 37.Trifirò G, Gambassi G, Sen E, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: A nested case-control study. Ann Int Med. 2010;152:418–425. doi: 10.7326/0003-4819-152-7-201004060-00006. [DOI] [PubMed] [Google Scholar]
- 38.Hugenholtz G, Heerdink E, van Staa T, et al. Risk of hip/femur fractures in patients using antipsychotics. Bone. 2005;37:864–870. doi: 10.1016/j.bone.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Liperoti R, Onder G, Lapane K, et al. Conventional or atypical antipsychotics and the risk of femur fracture among elderly patients: Results of a case-control study. J Clin Psychiatry. 2007;68:929–934. doi: 10.4088/jcp.v68n0616. [DOI] [PubMed] [Google Scholar]
- 40.Rochon PA, Normand S- L, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168:1090–1096. doi: 10.1001/archinte.168.10.1090. [DOI] [PubMed] [Google Scholar]
- 41.Bolton JM, Metge C, Lix L, et al. Fracture Risk From Psychotropic Medications: A Population-Based Analysis. J Clin Psychopharmacol. 2008;28:384–391.310.1097/JCP.1090b1013e31817d35943. doi: 10.1097/JCP.0b013e31817d5943. [DOI] [PubMed] [Google Scholar]
- 42.Nasrallah H. Atypical antipsychotic-induced metabolic side effects: Insights from receptor-binding profiles. Mol Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- 43.Bronskill S, Rochon P, Gill S, et al. The relationship between variations in antipsychotic prescribing across nursing homes and short-term mortality: Quality of care implications. Med Care. 2009;47:1000–1008. doi: 10.1097/MLR.0b013e3181a3943f. [DOI] [PubMed] [Google Scholar]
- 44.Rothman K, Greenland S, Lash T. Validity in Epidemiologic Studies. In: Rothman KJ, Greenland S, Lash T, editors. Modern Epidemiology. 3 edn. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 128–147. [Google Scholar]
- 45.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: Estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Birman-Deych E, Waterman A, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 47.Schneeweiss S, Robicsek A, Scranton R, et al. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.