Abstract
Damage to peripheral nerve branches triggers activation of microglia in CNS areas containing motor neuron soma and primary afferent terminals of the damaged fibers. Furthermore, microglial activation occurs in areas containing the soma and terminals of spared nerve branches of a damaged nerve. Because the abdominal viscera are innervated by spinal afferents as well as vagal afferents and efferents, we speculated that spinal nerves might respond like spared nerve branches following damage to vagal fibers. Therefore, we tested the hypothesis that damage to the abdominal vagus would result in microglial activation in vagal structures—the nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus nerve (DMV), and nodose ganglia (NG)—as well as spinal cord (SC) segments that innervate the abdominal viscera.
To test this hypothesis, rats underwent subdiaphragmatic vagotomy or sham surgery and were treated with saline or the microglial inhibitor, minocycline. Microglial activation was determined by quantifying changes in the intensity of fluorescent staining with a primary antibody against ionizing calcium adapter binding molecule 1 (Iba1).
We found that subdiaphragmatic vagotomy significantly activated microglia in the NTS, DMV, and NG two weeks post-vagotomy. Microglial activation remained significantly increased in the NG and DMV for at least 42 days. Surprisingly, vagotomy significantly decreased microglial activation in the SC. Minocycline treatment attenuated microglial activation in all studied areas. Our results indicate that microglial activation in vagal structures following abdominal vagal damage is accompanied by suppression of microglial activation in associated areas of the spinal cord.
Keywords: vagus, injury, degeneration, inflammation, microglia, Iba-1, hindbrain, sensory, rat
Introduction
Microglia are the subpopulation of macrophage-like glial cells that act as the immune defense in both the central (CNS) and peripheral nervous system. Microglia are integral to the inflammatory response that occurs following nerve damage [2;23;25;36;42]. When activated, microglia increase proliferation and undergo morphological changes from radial (resting) to amoeboid profiles, with thicker processes (activated). Furthermore, they produce and release a number of bioactive agents that modulate neuronal excitability, signaling, and recovery following injury. These agents include cytokines, chemokines, nitric oxide, prostaglandins, and growth factors [23;32;33]. Through release of these agents, activated microglia contribute to the development and maintenance of neuropathic pain [1;24;27;34;44;49]. Previous reports indicate that spinal nerve damage results in activation of microglia in ipsilateral segments of the spinal cord (SC) dorsal horn containing the central terminals of the damaged nerve. In addition, following a spared nerve injury where at least one of multiple nerve branches of a single nerve are left uninjured or “spared,” microglial activation also occurs within segments of the SC associated with uninjured nerve branches [2].
In contrast to somatic areas innervated solely by spinal nerves, abdominal viscera receive both vagal and spinal innervation [3;8–11;22;29;39;43]. The majority of spinal afferent innervation of the abdominal viscera originates from cell bodies located in lower thoracic dorsal root ganglia (DRG) and terminates within the corresponding neuromeres of the SC [13;22;48]. Vagal afferents originate from cell bodies located in the nodose ganglia (NG) and terminate centrally in the nucleus of the solitary tract (NTS), while vagal efferent neurons are located in the dorsal motor nucleus of the vagus (DMV) [12;38].
Treatments of gastrointestinal disorders, including bariatric surgery, result in either full or partial section of the vagus nerve, but little is known about microglial changes within the CNS following vagotomy [4;5;17;26;42;45]. Therefore, we tested the hypothesis that subdiaphragmatic vagotomy would result in long-lasting microglial activation in the NTS, DMV, area postrema (AP), and NG. Additionally, we hypothesized that spinal afferents would act as a spared nerve following subdiaphragmatic vagotomy, activating microglia within the SC. To test our hypothesis, we examined expression of the microglial marker, ionized calcium binding adaptor molecule 1 (Iba1), within the NTS, DMV, AP, NG, and SC. Minocycline, a member of the tetracycline family, was used to systemically inhibit microglial activation.
Material and methods
Animals
Male Sprague–Dawley rats (4-months-old at surgery; Simonsen Laboratories, Gilroy, CA, USA) were housed in individual hanging cages in a temperature-controlled vivarium with ad libitum access to food (Harlan Teklad F6 Rodent Diet W, Madison, WI, USA) and water. Rats were maintained on a 12-h light/dark schedule. All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee and conform to National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Subdiaphragmatic vagotomy
Beginning three days prior to surgery and continuing until sacrifice, vagotomized and sham animals received daily injections of the microglia inhibitor, minocycline (20 mg/kg i.p.; Sigma) or control injections of sterile 0.9% NaCl. Subdiaphragmatic vagotomies were performed as previously described [21]. Briefly, rats were anesthetized with a mixture of ketamine, acepromazine, and xylazine (50, 2, and 25 mg/kg, respectively), and the dorsal and the ventral vagal trunks were isolated via midline laparotomy. A 5 mm section was removed from both the dorsal and ventral nerve trunks above the point of bifurcation into the celiac and gastric or hepatic and accessory celiac branches, respectively. Sham-operated control animals had vagus nerves exposed but not cut. Completeness of vagotomies was confirmed by absence of retrogradely labeled neurons in the hindbrain and NG following intraperitoneal injection of Fast Blue (4%, EMS-CHEMIE GmbH, Germany), according to criteria described previously [38].
Tissue processing
After recovery times of 14 (n=4/group) or 42 days (n=4/group), animals were transcardially perfused with 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde; hindbrains, NG, and lower thoracic SC were then extracted. Hindbrains and SC were sectioned at 30 μm and floated in sets of three vials containing glycerol until staining. Hindbrain sections were collected between the rostral border of the AP and the calamos scriptoreus (Bregma −14.08 to −13.68) [33]. SC sections were collected beginning at the insertion of the ninth thoracic dorsal roots and continuing rostraly until a total of 36 sections were obtained. NG were sectioned at 20 μm and directly mounted onto sets of three slides. For each studied region, tissue from all animals was processed simultaneously to prevent differences in staining due to differing conditions. Prior to staining, sections were incubated for 2h in a blocking solution of 10% normal horse serum in Tris-phosphate buffered saline (TPBS, pH 7.4). Sections were subsequently incubated overnight in a primary antibody against Iba1 (rabbit polyclonal, 1:1000; cat# 019-19741, Dako) followed by an Alexa-488 secondary antibody (donkey anti-rabbit, 1:400; cat# A21206, Invitrogen). Negative controls for immunofluorescence staining were performed by omission of primary antibodies. Sections were mounted in ProLong (Molecular Probes) to reduce photo bleaching.
Intensity analysis
Sections were examined under a Nikon 80-I fluorescent microscope. The mean intensity of Iba1 immunoreactivity was analyzed in using Nikon Elements AR software. For each studied region, a representative section from each animal was used to calculate an average exposure time and background fluorescence level as determined by the pixel intensity of stained tissue regions that were negative for Iba1. Subsequently, 20×-stitched images were created using this fixed/standardized exposure time followed by removal of background fluorescence. In hindbrains, regions of interest (ROIs) were created to isolate the NTS, DMV, and AP from one another [37]. In SC, ROIs were created to isolate dorsal horns from surrounding tissue. In NG, ROIs were created to isolate cellular portions from passing fibers. In all ROIs, the mean pixel intensity was determined for each group (sham/saline; sham/minocycline; vagotomy/saline; vagotomy/minocycline). The sham/saline value was then set to 1.00, and the corresponding groups were normalized accordingly. The resulting data are expressed as mean fold change ± SEM and were analyzed using a one-way ANOVA followed by a Tukey Student's t-test for significance.
Results
Subdiaphragmatic vagotomy activates microglia in vagal structures
Fourteen days after sham surgery, hindbrain nuclei and NG contained Iba1+ microglia with resting morphology. In the hindbrain, this resting morphology was indicated by cells with small perikarya and radially branching processes (Fig. 1A & C). In the NG, resting Iba1+ microglia had small, oval perikarya with fibrous processes (Fig. 2A & C). In all vagal structures from sham-operated animals, Iba1+ microglia were evenly distributed without any clustering.
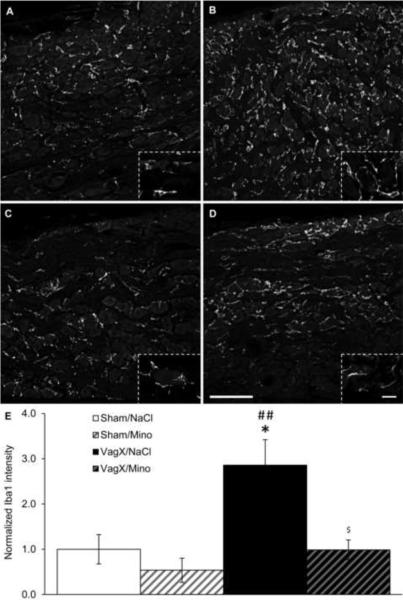
Figure 1. Vagotomy increases microglial activation in the NTS and DMV.
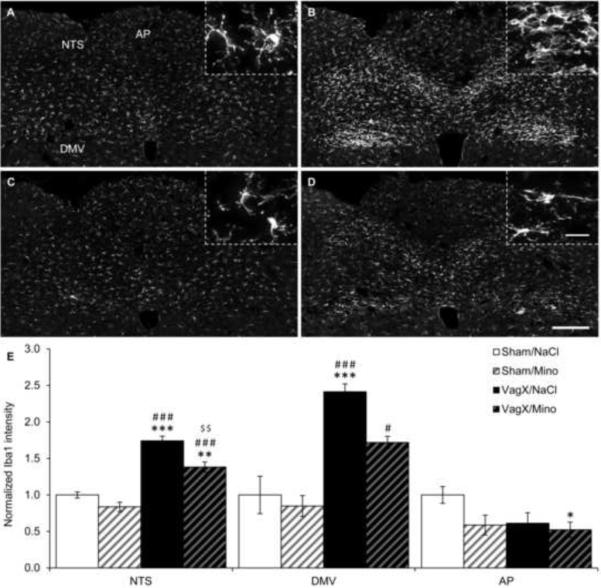
(A–D) Representative 20× images of Iba1 immunoreactivity in the hindbrain near Bregma −13.80 mm with (A) sham/saline, (B) vagotomy/saline, (C) sham/minocycline, and (D) vagotomy/minocycline. Inserts are 100× images from the same section demonstrating microglial morphology with each of the treatment combinations. (E) Graphical representation of Iba1 intensity 14 days after surgery normalized to sham/saline. NTS: nucleus of the solitary tract; DMV: dorsal motor nucleus of the vagus; AP: area postrema; scale bar = 200 μm in 20× images, 20 μm in 100× images; * p < 0.05, ** p < 0.01, *** p < 0.001 vs Sham/NaCl; # p < 0.05, ### p < 0.001 vs Sham/Mino; $$ p < 0.01 vs VagX/NaCl.
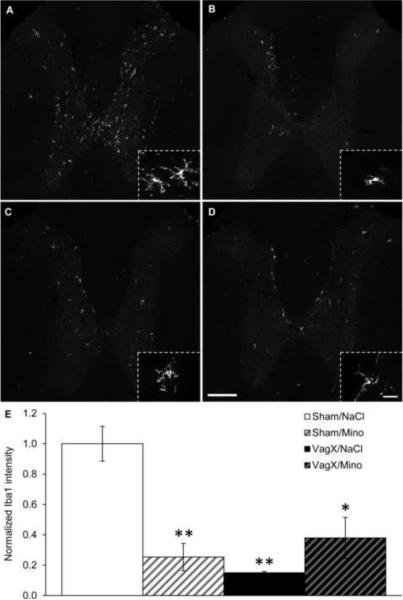
Figure 2. Vagotomy increases microglial activation in the NG.
(A–D) Representative 20× images of Iba1 immunoreactivity in the nodose ganglia with (A) sham/saline, (B) vagotomy/saline, (C) sham/minocycline, and (D) vagotomy/minocycline. Inserts are 100× images demonstrating microglial morphology with each of the treatment combinations. (E) Graphical representation of Iba1 intensity 14 days after surgery normalized to sham/saline. Scale bar = 100 μm in 20× images, 20 μm in 100× images; * p < 0.05 vs Sham/NaCl; ## p < 0.05 vs Sham/Mino; $ p < 0.05 vs VagX/NaCl.
Fourteen days after subdiaphragmatic vagotomy, microglia in the NTS, DMV, and NG were activated. Specifically, mean Iba1 intensities in the NTS and DMV of vagotomy/saline animals were increased by 1.74 ± .06 and 2.41 ± .11-fold over sham/saline controls (p < .001; Fig. 1B & E). Microglia in these animals exhibited larger perikarya with fewer and shorter processes, as is characteristic of activation (Fig. 1B). Minocycline treatment attenuated the vagotomy-induced increases in mean Iba1 intensity in both the NTS and DMV, reducing the fold changes over sham/saline to 1.38 ± .07 (p < .01) and 1.72 ± .08 (p = .078), respectively. However, activation in the NTS and DMV was still increased compared to sham-operated controls (p < .01 and p = .057, respectively). Microglia in vagotomy/minocycline animals presented with intermediate morphologies (Fig. 1D). Minocycline injections alone did not significantly reduce mean Iba1 intensity within the NTS or DMV (Fig. 1C & E).
Fourteen days following vagotomy, mean Iba1 intensity in the NG was increased by 2.86 ± .56-fold over sham/saline (p < .05; Fig. 2B & E). Active Iba1+ microglia in the NG were represented by comma-shaped profiles with shorter and thicker processes (Fig. 2B). Minocycline treatment abolished the vagotomy-induced increase in Iba1 intensity (p < .05; Fig. 2D & E). Minocycline injections alone did not significantly reduce mean Iba1 intensity within the NG (Fig. 2C & E).
Subdiaphragmatic vagotomy decreases microglial activation in the SC
As in the hindbrain, resting microglia in the dorsal horns of the SC were evenly distributed with radially branching processes (Fig. 3A). Fourteen days after vagotomy, there was a 0.15 ± .01-fold decrease in mean Iba1 intensity compared to sham/saline (p < .01; Fig. 3B & E). Despite the reduction in Iba1 intensity, the remaining microglia exhibited active morphology with fewer and shorter processes (Fig. 3B). Minocycline injections alone also decreased mean Iba1 intensity in the SC by 0.25 ± .09-fold (p < .01; Fig. 3C & E). The effects of vagotomy and minocycline injections on mean Iba1 intensity were not additive, as shown by a 0.38 ± .14-fold decrease in vagotomy/minocycline animals compared to sham/saline (p < .05; Fig. 3D & E).
Figure 3. Vagotomy decreases microglial activation in the spinal cord.
(A–D) Representative 20× images of Iba1 immunoreactivity in the spinal cord at T9 with (A) sham/saline, (B) vagotomy/saline, (C) sham/minocycline, and (D) vagotomy/minocycline. Inserts are 100× images from the same section demonstrating microglial morphology with each of the treatment combinations. (E) Graphical representation of Iba1 intensity 14 days after surgery normalized to sham/saline. Scale bar = 200 μm in 20× images, 20 μm in 100× images; * p < 0.05, ** p < 0.01 vs Sham/NaCl.
Extended activation of microglia following subdiaphragmatic vagotomy
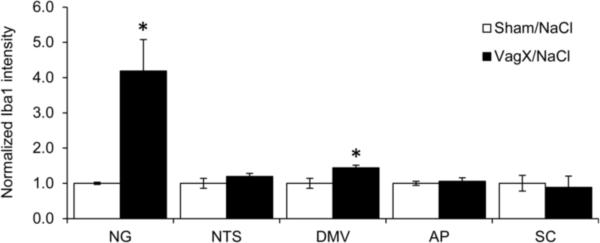
Forty-two days after subdiaphragmatic vagotomy, mean Iba1 intensity remained dramatically elevated in the NG with a fold change of 4.19 ± .89 over sham/saline (p < .05; Fig. 4). A smaller yet significant increase in Iba1 intensity was seen in the DMV 42 days after vagotomy with a fold change of 1.44 ± .07 over sham/saline (p < .05; Fig. 4). Other areas, including the NTS and SC, had returned to baseline by this time point.
Figure 4. Microglial activation persists in the NG.
Graphical representation of Iba1 intensity 42 days after surgery normalized to sham/saline. * p < 0.05 vs Sham/NaCl.
Discussion
Our results indicate that microglia in the hindbrain and nodose ganglia are activated for up to six weeks following subdiaphragmatic vagotomy. Fourteen days after vagotomy, microglial activation is shown by significantly increased Iba1 immunoreactivity and altered morphology in both the hindbrain and NG. Our observations confirm a prior report indicating that unilateral nodose ganglion removal activated microglia and increased other inflammatory markers in the ipsilateral NTS and DMV at 3 and 14 days after ganglionectomy [41;42]. Our study extends these observations by demonstrating that increased microglial activation occurs in response to more distal vagal damage. In addition, we found that increased microglial activation was detectable in the NG and DVM up to 42 days after injury. To our knowledge, this represents the longest reported microglial activation following peripheral nerve damage.
This prolonged activation of microglia may have a significant effect on vagal structures through cytokine release. The cytokine IL-1β has been shown to activate vagal afferents in the NG [16]. Blocking vagal afferent signaling with subdiaphragmatic vagotomy prevents the development of fever due to circulating IL-1β [14]. Furthermore, cytokines that may be released from microglia, such as IL-1β and TNF-α, have been connected with decreased food intake, decreased gastric motility, and increased lipid metabolism, all combining to produce anorexia [6;20;21;40;46;47]. Should microglial activation seen following vagotomy be accompanied by similar physiological effects, this may be a factor in the reduction of food intake and body weight following surgeries that involve intentional or accidental vagal damage [4;5;45].
We found that minocycline treatment attenuated vagotomy-induced microglial activation in the NTS, DMV and NG. Minocycline is a tetracycline antibiotic that inhibits activation and proliferation of microglia and subsequent cytokine production [15]. Minocycline was also reported to attenuate, but not abolish, microglial activation following unilateral nodose ganglionectomy [42]. Minocycline treatment has been shown to prevent microglia-induced hyperalgesia and to be neuroprotective following damage [19;51]. Therefore, preventive minocycline treatment may reduce the side effects of bariatric surgeries that are possibly a result of microglial activation in vagal structures, including severe nausea and light-headedness [5].
Damage to a single nerve branch leads to microglial activation in terminal fields of spared branches [2]. We postulated that the abdominal spinal innervation might behave as a “spared branch” following vagotomy. However, SC sections collected 14 days after vagotomy had significantly decreased Iba1 intensities. This unexpected reduction of microglial activity may be explained by vagal inhibition of spinal sensory signaling. It has been previously demonstrated that vagal activity influences a pathway of descending inhibition from the hindbrain—presumably the NTS—to the spinal afferent terminals in the dorsal horns of the SC [30;31]. The subdiaphragmatic vagotomy performed in our study would disrupt the same descending pathway. This disruption may reduce microglial activation in the SC through a lack of descending input or the release of inhibition. Alternatively, the vagotomy may be causing the migration of microglia from the spinal cord toward the damaged nodose ganglia or hindbrain [7;28; 35].
In all studied regions, there was at least a trend towards decreased Iba1 intensity with minocycline treatment alone. The decrease in Iba1 intensity with minocycline was significant in the SC. Minocycline treatment on the order of days is not sufficient to reduce basal microglial activation [19]. However, chronic treatment on the order of weeks or relatively large doses will reduce activation below basal levels [18;42]. In our study, the apparent increase in minocycline effectiveness within the SC is likely due to lower basal density of microglia in comparison to the other studied regions rather than increased basal activity. This reduction may be the result of the anti-proliferative effect of minocycline or a reduction of Iba1 expression within still present microglia.
Conclusions
In conclusion, we found that subdiaphragmatic vagotomy has a profound and long-lasting effect on microglial activation in vagal structures. In the NTS and AP, this activation persists for at least 14 days. In the NG and DMV, activation lasts for at least 42 days. Contrary to our hypothesis, however, spinal neurons innervating the abdominal viscera do not behave as a typical spared nerve. Further examination of these phenomena may lead to a better understanding of the mechanism by which vagal manipulations decrease food intake and body weight.
Highlights
Subdiaphragmatic vagotomy activates microglia in the NTS, DMV, and nodose ganglion.
In the nodose ganglion and DMV, microglial activation lasts for at least 42 days.
Minocycline treatment attenuates vagotomy-induced microglial activation.
Vagotomy suppresses microglia in spinal cord segments innervating the GI tract.
Acknowledgments
This work was supported by NIH grants DK052849 and NS 020561 and by WSU Start-up Funds with graduate student support from an Aven Foundation endowed ARCS fellowship. We would like to acknowledge Stephen Johnston for his efforts in creating high-quality images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Aldskogius H. Mechanisms and consequences of microglial responses to peripheral axotomy. Front Biosci. (Schol. Ed) 2011;3:857–868. doi: 10.2741/192. [DOI] [PubMed] [Google Scholar]
- [2].Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav. Immun. 2007;21:624–633. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol. Motil. 2004;16(Suppl 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- [4].Berthoud HR. The vagus nerve, food intake and obesity. Regul. Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Butera PC, Briffa CF, Whitaker EE. Devazepide fails to reverse the inhibitory effect of interleukin-1beta on food intake in female rats. Physiol Behav. 2004;82:777–783. doi: 10.1016/j.physbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- [7].Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp. Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- [8].Clerc N. Afferent innervation of the lower oesophageal sphincter of the cat. An HRP study. J. Auton. Nerv. Syst. 1983;9:623–636. doi: 10.1016/0165-1838(83)90118-2. [DOI] [PubMed] [Google Scholar]
- [9].Cottrell DF, Greenhorn JG. The vagal and spinal innervation of the gastro-duodenal junction of sheep. Q. J. Exp. Physiol. 1987;72:513–524. doi: 10.1113/expphysiol.1987.sp003093. [DOI] [PubMed] [Google Scholar]
- [10].Czaja K, Ritter RC, Burns GA. Vagal afferent neurons projecting to the stomach and small intestine exhibit multiple N-methyl-D-aspartate receptor subunit phenotypes. Brain Res. 2006;1119:86–93. doi: 10.1016/j.brainres.2006.08.042. [DOI] [PubMed] [Google Scholar]
- [11].Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography 9. J. Comp Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [12].Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am. J. Physiol. 1999;277:G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- [13].Gallaher ZR, Larios RM, Ryu V, Sprunger LK, Czaja K. Recovery of viscerosensory innervation from the dorsal root ganglia of the adult rat following capsaicin-induced injury. J Comp Neurol. 2010;518:3529–3540. doi: 10.1002/cne.22412. [DOI] [PubMed] [Google Scholar]
- [14].Gaykema RP, Goehler LE, Hansen MK, Maier SF, Watkins LR. Subdiaphragmatic vagotomy blocks interleukin-1beta-induced fever but does not reduce IL-1beta levels in the circulation. Auton. Neurosci. 2000;85:72–77. doi: 10.1016/s1566-0702(00)00222-8. [DOI] [PubMed] [Google Scholar]
- [15].Giuliani F, Hader W, Yong VW. Minocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interaction. J. Leukoc. Biol. 2005;78:135–143. doi: 10.1189/jlb.0804477. [DOI] [PubMed] [Google Scholar]
- [16].Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804:306–310. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- [17].Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140:1415–1434. doi: 10.1016/j.neuroscience.2006.03.048. [DOI] [PubMed] [Google Scholar]
- [18].Guasti L, Richardson D, Jhaveri M, Eldeeb K, Barrett D, Elphick MR, Alexander SP, Kendall D, Michael GJ, Chapman V. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol. Pain. 2009;5:35. doi: 10.1186/1744-8069-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–118. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hermann GE, Van Meter MJ, Rogers RC. CXCR4 receptors in the dorsal medulla: implications for autonomic dysfunction. Eur. J. Neurosci. 2008;27:855–864. doi: 10.1111/j.1460-9568.2008.06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hermann GE, Rogers RC. TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: implications for autonomic control. Brain Res. 2009;1273:72–82. doi: 10.1016/j.brainres.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holzer P, Michl T, Danzer M, Jocic M, Schicho R, Lippe IT. Surveillance of the gastrointestinal mucosa by sensory neurons. J Physiol Pharmacol. 2001;52:505–521. [PubMed] [Google Scholar]
- [23].Ichikawa H, Sato T, Kano M, Suzuki T, Matsuo S, Kanetaka H, Shimizu Y. Masseteric nerve injury increases expression of brain-derived neurotrophic factor in microglia within the rat mesencephalic trigeminal tract nucleus. Cell Mol. Neurobiol. 2011;31:551–559. doi: 10.1007/s10571-011-9648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- [25].Jergova S, Cizkova D. Microglial activation in different models of peripheral nerve injury of the rat. J. Mol. Histol. 2007;38:245–251. doi: 10.1007/s10735-007-9094-5. [DOI] [PubMed] [Google Scholar]
- [26].Ji JF, Dheen ST, Kumar SD, He BP, Tay SS. Expressions of cytokines and chemokines in the dorsal motor nucleus of the vagus nerve after right vagotomy. Brain Res. Mol. Brain Res. 2005;142:47–57. doi: 10.1016/j.molbrainres.2005.09.017. [DOI] [PubMed] [Google Scholar]
- [27].Keller AF, Beggs S, Salter MW, De KY. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, ssing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Brain Res. Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- [29].McMahon SB, Morrison JF. Two group of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J. Physiol. 1982;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miao FJ, Janig W, Levine JD. Nociceptive neuroendocrine negative feedback control of neurogenic inflammation activated by capsaicin in the rat paw: role of the adrenal medula. J Physiol. 2000;527:601–610. doi: 10.1111/j.1469-7793.2000.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miao FJ, Janig W, Jasmin L, Levine JD. Spino-bulbo-spinal pathway mediating vagal modulation of nociceptive-neuroendocrine control of inflammation in the rat. J Physiol. 2001;532:811–822. doi: 10.1111/j.1469-7793.2001.0811e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 2001;65:322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- [33].Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J. Neurochem. 2002;80:697–705. doi: 10.1046/j.0022-3042.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- [34].Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, Yajima Y, Suzuki T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J. Neurochem. 2006;97:1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- [35].Nutile-McMenemy N, Elfenbein A, Deleo JA. Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J. Neurochem. 2007;103:2035–2046. doi: 10.1111/j.1471-4159.2007.04889.x. [DOI] [PubMed] [Google Scholar]
- [36].Patro N, Nagayach A, Patro IK. Iba1 expressing microglia in the dorsal root ganglia become activated following peripheral nerve injury in rats. Indian J. Exp. Biol. 2010;48:110–116. [PubMed] [Google Scholar]
- [37]. Paxinos G, Watson CH. The Rat Brain in Stereotaxic Coordinates. Elsevier; 2004. [DOI] [PubMed] [Google Scholar]
- [38].Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies 1. Am. J. Physiol. 1987;253:R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- [39].Renehan WE, Zhang X, Beierwaltes WH, Fogel R. Neurons in the dorsal motor nucleus of the vagus may integrate vagal and spinal information from the GI tract. Am. J. Physiol. 1995;268:G780–G790. doi: 10.1152/ajpgi.1995.268.5.G780. [DOI] [PubMed] [Google Scholar]
- [40].Reyes TM, Sawchenko PE. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J. Neurosci. 2002;22:5091–5099. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roulston CL, Lawrence AJ, Jarrott B, Widdop RE. Non-angiotensin II [(125)I] CGP42112 binding is a sensitive marker of neuronal injury in brainstem following unilateral nodose ganglionectomy: comparison with markers for activated microglia. Neuroscience. 2004;127:753–767. doi: 10.1016/j.neuroscience.2004.04.062. [DOI] [PubMed] [Google Scholar]
- [42].Roulston CL, Lawrence AJ, Widdop RE, Jarrott B. Minocycline treatment attenuates microglia activation and non-angiotensin II [125I] CGP42112 binding in brainstem following nodose ganglionectomy. Neuroscience. 2005;135:1241–1253. doi: 10.1016/j.neuroscience.2005.06.087. [DOI] [PubMed] [Google Scholar]
- [43].Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res. 2005;1039:108–115. doi: 10.1016/j.brainres.2005.01.050. [DOI] [PubMed] [Google Scholar]
- [44].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- [45].Shin AC, Berthoud HR. Food reward functions as affected by obesity and bariatric surgery. Int. J. Obes. (Lond) 2011;35(Suppl 3):S40–S44. doi: 10.1038/ijo.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sonti G, Ilyin SE, Plata-Salaman CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. 1996;270:R1394–R1402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- [47].Suto G, Kiraly A, Plourde V, Tache Y. Intravenous interleukin-1-beta-induced inhibition of gastric emptying: involvement of central corticotrophin-releasing factor and prostaglandin pathways in rats. Digestion. 1996;57:135–140. doi: 10.1159/000201326. [DOI] [PubMed] [Google Scholar]
- [48].Tanaka K, Matsugami T, Chiba T. The origin of sensory innervation of the peritoneum in the rat. Anat Embryol. 2002;205:307–313. doi: 10.1007/s00429-002-0254-9. [DOI] [PubMed] [Google Scholar]
- [49].Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- [51].Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]