Abstract
Endocrine pituitary cells express numerous voltage-gated Na+, Ca2+, K+, and Cl− channels and several ligand-gated channels, and they fire action potentials spontaneously. Depending on the cell type, this electrical activity can generate localized or global Ca2+ signals, the latter reaching the threshold for stimulus-secretion coupling. These cells also express numerous G-protein-coupled receptors, which can stimulate or silence electrical activity and Ca2+ influx through voltage-gated Ca2+ channels and hormone release. Receptors positively coupled to the adenylyl cyclase signaling pathway stimulate electrical activity with cAMP, which activates hyperpolarization-activated cyclic nucleotide-regulated channels directly, or by cAMP-dependent kinase-mediated phosphorylation of K+, Na+, Ca2+, and/or non-selective cation-conducting channels. Receptors that are negatively coupled to adenylyl cyclase signaling pathways inhibit spontaneous electrical activity and accompanied Ca2+ transients predominantly through the activation of inwardly rectifying K+ channels and the inhibition of voltage-gated Ca2+ channels. The Ca2+-mobilizing receptors activate inositol trisphosphate-gated Ca2+ channels in the endoplasmic reticulum, leading to Ca2+ release in an oscillatory or non-oscillatory manner, depending on the cell type. This Ca2+ release causes a cell type-specific modulation of electrical activity and intracellular Ca2+ handling.
Keywords: action potential, calcium oscillations, cAMP, G protein-coupled receptors, IP3 receptor channels, protein kinase A, voltage-gated calcium channels
1. Introduction
The pituitary gland is composed of two embryonically, anatomically, and functionally distinct entities: the neurohypophysis and the adenohypophysis. The neurohypophysis includes the posterior pituitary lobe, whereas the adenohypophysis includes the intermediate and anterior pituitary lobes. The intermediate lobe is populated more than 95% by melanotrophs. These cells are electrically excitable cells, and spontaneous electrical activity is sufficient to trigger the release of pro-opiomelanocortin-derived peptides. Melanotrophs express GPCRs (G-protein-coupled receptors) activated by DA (dopamine), GABA (γ-aminobutyric acid), prostaglandin E2, and serotonin (5-hydroxytryptamine; 5-HT) [1]. Corticotrophs are the other pro-opiomelanocortin-producing cell type derived from the intermediate pituitary. These cells are scattered throughout the anterior lobe in adult animals and make up 10-15% of anterior pituitary cells. The main regulation of these cells is mediated by CRH (corticotropin-releasing hormone), which is secreted by paraventricular neurons that project to the median eminence and release CRH into the hypophysial portal system. In addition to CRH and the CRH family of peptides (urocortin 1-3), AVP (arginine vasopressin) acts in synergy with CRH to potentiate hormone release [2].
Lactotrophs are a non-homogenous group of endocrine cells that account for 10-25% of cells in the anterior lobe. Spontaneous electrical activity in these cells is also sufficient to trigger prolactin secretion. The predominant hypothalamic influence is inhibitory rather than stimulatory and is mediated by dopamine D2 receptors. These cells also express endothelin-activated ETA receptors, which transiently stimulate hormone release and then sustained inhibition. In contrast, TRH (thyrotropin-releasing hormone), angiotensin II, oxytocin, ATP, acetylcholine, and 5-HT stimulate prolactin release [3]. The sister cells, somatotrophs, are the most common cells in the anterior pituitary. They represent up to 50% of all cells and are localized predominantly to the lateral portions of the anterior lobe. The function of these cells is controlled by two hypothalamic neuropeptides, GHRH (growth hormone-releasing hormone), which stimulates growth hormone release, and SST (somatostatin), which inhibits growth hormone release. GHRH is secreted by neurons in the arcuate nucleus of the hypothalamus, whereas SST is secreted by neurons in the periventricular nucleus of the hypothalamus. These cells also express receptors for ghrelin, PACAP (pituitary adenylate cyclase-activating peptide), and endothelins (ETs) [4].
Thyrotrophs and gonadotrophs represent the third group of endocrine pituitary cells that express the 92 amino acid α-glucoprotein hormone α-subunit, which is needed for the formation of follicle-stimulating hormone, luteinizing hormone, and thyroid-stimulating hormone (as well as chorionic gonadotropin) heterodimers with hormone-specific beta subunits. Thyrotrophs represent less than 10% of cells in the gland and are regionally localized within the anteriomedial and lateral portions of the gland. Hypothalamic control of these cells is mediated by TRH acting as an agonist for TRH receptors. The thyrotroph function is also controlled by numerous autocrine and paracrine factors [5]. Gonadotrophs constitute about 10-15% of the anterior pituitary cell population and are localized throughout the anterior lobe, frequently adjoining lactotrophs. The decapeptide GnRH (gonadotropin-releasing hormone) is the main agonist for these cells and is secreted in a pulsatile manner by neurons that are dispersed within the mediobasal hypothalamus and preoptic areas. In addition to GnRH receptors, gonadotrophs also express functional receptors for PACAP, AVP, and substance P, which contribute to Ca2+ signaling and gonadotropin synthesis and secretion [6].
Here, we summarize our findings on the expression, signaling functions and GPCR-mediated regulation of the plasma membrane and ER (endoplasmic reticulum) ion channels in endocrine pituitary cells. For a more extensive review of this topic, see the following reviews [7-9] and the accompanying articles in this issue by Chan et al., Chang et al., Tse et al., and Zorec et al. For a review of the three-dimensional organization of the pituitary gland and cell-to-cell communication, see the article by Mollard et al. in this issue. For a review of cyclic nucleotide signaling in pituitary cells, see Antoni et al.
2. Spontaneous Electrical Activity and Calcium Signaling
The membrane potential of isolated pituitary cells in vitro is not stable; rather, it oscillates between resting potentials of −60 to −50 mV, reflecting a balance between the activities of depolarizing and hyperpolarizing channels. When membrane potential oscillations reach a threshold level, cells generate APs (action potentials). Pituitary cells fire APs independently of external stimuli, a phenomenon termed spontaneous electrical activity. Each AP is composed of a slow depolarizing phase, a rapid depolarizing phase or spiking depolarization, and a rapid or delayed repolarizing phase. Initially, it was believed that only lactotrophs and GH cells are excitable [9]. It later became obvious that other secretory pituitary cell types also fire APs spontaneously and/or in response to hypothalamic neurohormones: melanotrophs [10], corticotrophs [11, 12], somatotrophs [13], gonadotrophs [14], thyrotrophs [15]. Firing of APs causes transient elevation in [Ca2+]i (intracellular Ca2+ concentration) as it well documented in gonadotrophs, lactotrophs, somatotrophs [16] and immortalized pituitary cells [12, 17]. However, not all cells fire APs and the frequency of firing vary from cell to cell. Furthermore, other investigators found that spontaneous APs or Ca2+ transients were rarely detected in corticotrophs [18] and male gonadotrophs [19], which could indicate that cultural and/or recording conditions also influence firing.
2.1. Patterns of Electrical Activity
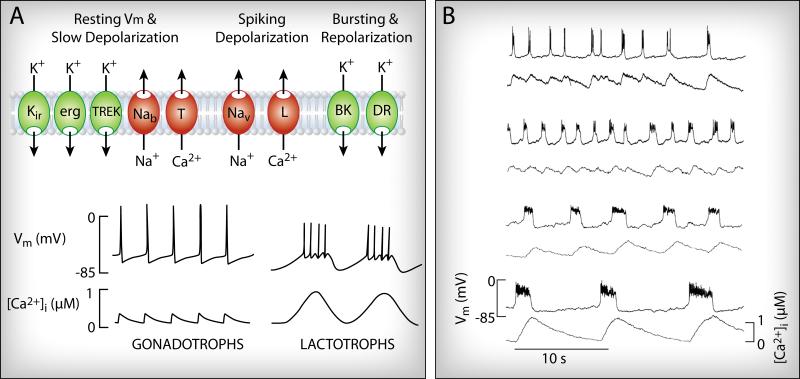
Two types of APs can be observed in pituitary cells (Fig. 1). In rat gonadotrophs, the APs are tall and narrow, with amplitudes of more than 60 mV (from initiation to peak), half-widths of less than 50 ms, and spiking frequencies that are typically ~0.7 Hz [20]. Ovine gonadotrophs also fire single APs spontaneously [21], as do rat thyrotrophs [15]. The pattern of activity in lactotrophs and somatotrophs can be similar to that in gonadotrophs, with large and narrow spikes [22, 23]. More often, however, a bursting pattern is produced, consisting of periodic depolarized potentials with superimposed small-amplitude spikes [13, 20, 22]. The bursts have a much longer duration (several seconds) than gonadotroph APs, and the burst frequency is significantly lower (~0.3 Hz). The membrane potential rarely goes above −10 mV during a plateau burst, and the spikes are quite small [20]. Corticotrophs also exhibit both spontaneous large-amplitude spiking and plateau bursting [11, 24], as do melanotrophs [10] and GH cell lines [17, 25].
Fig. 1.
Spontaneous electrical activity and Ca2+ signaling in pituitary cells. (A) The resting membrane potential (Vm) and slow depolarization in endocrine pituitary cells are determined by several channels, including classic inward rectifier K+ (Kir), ether-a-go-go-related gene (ERG) and TWIK-related (TREK-1) K+-conducting channels, still unidentified Na+-conducting background channels (Nab), and T-type Ca2+ channels. Once the threshold for action potential firing is reached, L-type Ca2+ channels alone or together with voltage-gated Na+ (Nav) channels generate spiking depolarization. Depending on the cell type, spiking depolarization is followed by a rapid and sharp repolarization (bottom left) or plateau bursting type of electrical activity (bottom right). Interactions between delayed rectifier (DR) and Ca2+-activated BK-type K+ channels appear to play a major role in determining the pattern of electrical activity. (B) Influence of variable durations of bursting activity on the Ca2+ signaling pattern in pituitary cells: experimental records.
2.2. Channels Contributing to Resting Membrane Potential
Members of the Kir (inwardly rectifying K+) family of channels contribute to the regulation of resting membrane potentials in excitable cells. There are 15 members of this family of channels, and they can be divided into three groups based on the type of regulation. The majority of channel subtypes are “classical” Kir channels that are controlled by intracellular messengers (Kir1, 2, 4, 5, and 7). Kir3 channels are regulated by G-proteins, and Kir6 channels are regulated by intracellular ATP. The transcripts for the majority of these channels have been identified in GH3 cells [26]. Kir-like channels also contribute to the control of resting membrane potential in lactotrophs, somatotrophs, corticotrophs and GH3 cells [24, 27-29], but the nature of these channels has not been identified. This could be mediated by “classical” Kir 1 and 2 channels, but also by ERG (ether-a-go-go-related gene) channels, which mRNA transcripts are also expressed in GH mammosomatotrophs and native rat lactotrophs [30]. Blockade of ERG channels by E-4031 causes depolarization of the membrane potential of about 5 mV, facilitating the release of prolactin [31]. ERG currents are also expressed in MMQ lactotrophs, and their blockade facilitates AP firing and prolactin secretion [32]. Functional ERG current was also identified in mouse gonadotrophs [33]. Human prolactin-secreting tumors also express ERG and they are functionally coupled to hormone secretion [34]. Recently, contribution of TREK-1 (TWIK-related) channels in control of resting membrane potential in corticotrophs was also identified [35].
The resting membrane potential of −50 to −60 mV in pituitary cells suggests that, in addition to resting K+ conductance, there is also a depolarizing conductance due to other ions (Fig. 1). When extracellular Na+ is substituted with large organic cations, the resting membrane potential rapidly reaches approximately −85 mV. This value is close to the equilibrium potential for K+, suggesting that a Na+-conducting channel has constitutive activity. This prominent hyperpolarization of the plasma membrane in the absence of bath Na+ abolishes spontaneous firing of APs in lactotrophs, somatotrophs, gonadotrophs, and GH3 and GC cell lines. The addition of TTX (tetrodotoxin) in micromolar concentrations to inhibit all voltage-gated Na+ (Nav) channels does not mimic the effect of the removal of bath Na+ on the resting membrane potential. These and other data indicate that the constitutive activity of TTX-insensitive Na+-conducting channels, termed background Na+ channels, contributes to the control of resting membrane potential and may account for the pacemaking depolarization [23, 25, 36]. The nature of these channels is unclear.
2.3. Channels Involved in Spike Depolarization
All pituitary cells express TTX-sensitive Nav channels, which in neurons are critical for the development of the depolarizing phase of APs [9]. In a fraction of ovine gonadotrophs [21] and bovine lactotrophs [37], Nav channels are also responsible for AP generation. Furthermore, TTX-sensitive Nav channels may contribute to the firing of APs and the accompanied VGCI in frog and rat melanotrophs [38]. In the majority of rat anterior pituitary cells in vitro, these channels do not contribute to the spike depolarization because they are inactivated at the resting membrane potential. However, in hyperpolarized cells, which in vivo occurs under tonic influence by a GPCR coupled to the Gi/o signaling pathway, Nav channels could play an important role in the transition from the quiescent to the firing mode [9].
In excitable cells, Cav channels also contribute to spike depolarization. Electrophysiologically, Cav channels are high (L, N, P/Q, and R type) or low (T-type) voltage-activated channels, which are distinguished by their single-channel conductance, pharmacology, and metabolic regulation [9]. The functional expression of both high and low voltage-activated Cav currents is well documented in cultured gonadotrophs [39] and in genetically labeled mouse gonadotrophs [40]. These currents are also present in somatotrophs and lactotrophs [39], GH cells [25] and other normal and immortalized pituitary cell types [9]. In vitro, the removal of extracellular Ca2+ and the addition of Cav channel blockers abolishes electrical activity in the majority of endocrine pituitary cells without affecting the resting membrane potential, indicating that these channels are critical for spiking depolarization but not for resting membrane potential [13, 16]. In contrast, T-type Cav channels contribute to slow depolarization in pituitary cells [41].
2.4. Mechanisms for Bursting and Repolarization
The simplest explanation why some cell fire tall AP spiking, while others fire plateau-bursting type of APs could be that there is a cell-specific expression of channels, leading to different patterns of spiking. One type of channels that is larger in somatotrophs and lactotrophs than in gonadotrophs is the BK type of Ca2+-controlled K+ (KCa) channels. These channels activate rapidly upon membrane depolarization, most likely due to colocalization of the BK channels with Cav channels [13, 20, 39]. The BK current acts in conjunction with the delayed rectifying K+ current to repolarize the cell membrane during the downstroke of an AP. There is some evidence that BK channels are also a key element in the production of bursting, and that their greater expression in somatotrophs/lactotrophs is responsible for the different activity patterns of these cells vs. gonadotrophs [20]. The proximity of BK channels to Cav channels could be the major factor that determines differential (plateau-bursting vs. repolarization) role of BK channels among endocrine pituitary cells [42].
2.5. Voltage-Gated Ca2+ Influx
The high-voltage-activated Cav channels in pituitary cells not only give rise to APs in the same way as Nav channels, but they also provide an effective pathway for Ca2+ influx during transient depolarization (termed voltage-gated Ca2+ influx or VGCI). In cells not exhibiting firing of APs, a plateau depolarization also results in Ca2+ entry via Cav channels, but in a non-oscillatory manner [18]. In other excitable cells, Ca2+ influx is coupled to Ca2+ release from intracellular stores through the calcium-induced calcium release process, which involves ryanodine receptor channels expressed in the ER membrane. It is unlikely, however, that Ca2+-induced Ca2+ release contributes to the generation of Ca2+ signaling in mammalian lactotrophs and somatotrophs [43, 44]. In frog melanotrophs, the rise in Ca2+ occurs in a stepwise manner [45], and the generation of Ca2+ transients is abolished in cells that have the ER Ca2+ pump blocked [46]. It appears that in these cells, spontaneous VGCI is coupled to Ca2+-induced Ca2+-release, presumably through IP3Rs (inositol trisphosphate receptor channels) [47].
Numerous studies have indicated that patterns of spontaneous electrical activity in different cell types largely impact intracellular Ca2+ dynamics and overall Ca2+ levels. Simultaneous measurements of membrane potential and [Ca2+]i have shown that the bulk Ca2+ levels are low in spontaneously spiking gonadotrophs (20 nM to 70 nM) but much higher (300 nM to 1.2 μM) and clearly oscillatory in spontaneously bursting lactotrophs and somatotrophs [13, 16], corticotrophs [12] and immortalized pituitary cells [17, 25, 27]. Rhythmic bursts of Ca2+ transients have also been observed in acute anterior pituitary slices [48].
The difference in the patterns of Ca2+ transients between cells firing single APs and those exhibiting plateau bursting is reflected in the spatial distribution of Ca2+ within the cell (Fig. 1B). Both types of APs depolarize the membrane sufficiently to activate the various types of Cav channels that are expressed in pituitary cells [39, 49]. However, Cav channels are open only briefly during the short time that the pituitary cells are firing a single AP, and the elevated Ca2+ concentration is localized to nanodomains that form at the inner mouths of open channels. With longer durations and smaller amplitudes of bursts, channels stay open longer and significant Ca2+ entry occurs, resulting in individual Ca2+ nanodomains overlapping and producing a global signal that can be easily resolved with fluorescent Ca2+ dyes [44]. Thus, the Ca2+ influx summed over time is much greater during bursting than during large-amplitude spiking [42].
3. Stimulation of VGCI by GPCRs
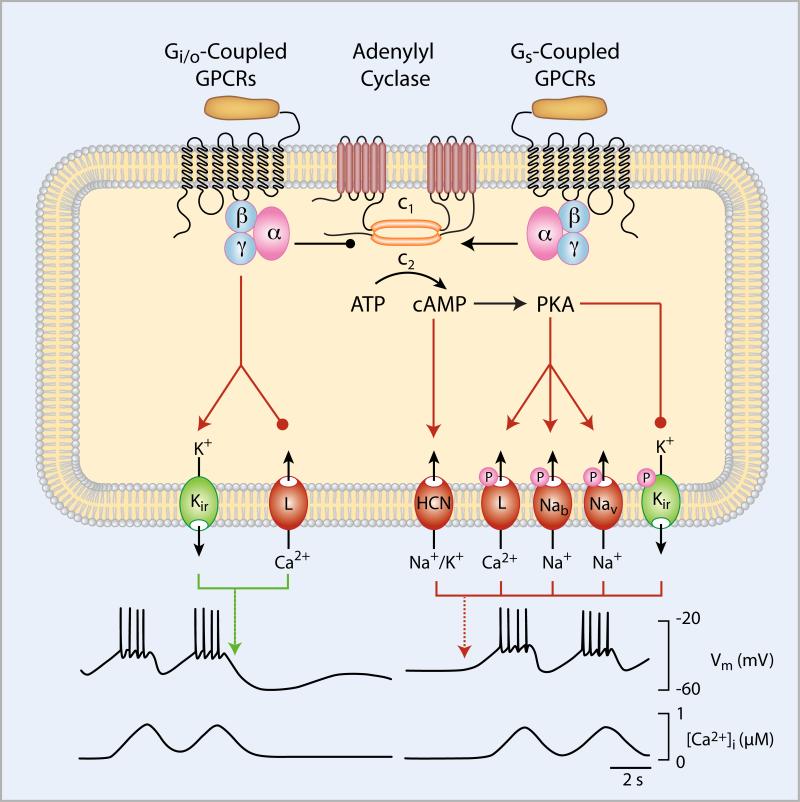
cAMP is a ubiquitous intracellular messenger that is generated by the adenylyl cyclase family of enzymes, which regulates numerous cellular responses, including electrical activity and VGCI. Adenylyl cyclase produces cAMP in the absence of hormonal stimulation; this basal activity is markedly enhanced upon the binding of Gs. In corticotrophs, the Gs-signaling pathway is triggered by CRH and in somatotrophs by GHRH. The Gs-coupled VIP/PACAP receptors operate in somatotrophs, melanotrophs, lactotrophs, and folliculostellate cells. Some eicosanoids may also signal through this pathway in pituitary cells [9]. In general, the activation of these Gs-linked GPCRs causes plasma membrane depolarization, leading to increased electrical activity and facilitated VGCI. The type of Ca2+ response that is typically obtained through this pathway is a plateau elevation of [Ca2+]i or an increase in the frequency and/or amplitude of Ca2+ transients. cAMP can modulate channel activities directly by activating HCN (hyperpolarization-activated and cyclic nucleotide-regulated) channels or indirectly through PKA-mediated phosphorylation of several plasma membrane channels (Fig. 2).
Fig. 2.
Role of adenylyl cyclase-coupled GPCRs on plasma membrane channel activity in pituitary cells. The Gi/o-coupled receptors, including dopamine D2 and somatostatin receptors, inhibit spontaneous electrical activity and Ca2+ signaling predominantly through the activation of Kir3 channels and inhibition of L-type Ca2+ channels; both are mediated by βγ dimers of heterotrimeric G proteins. The Gs-coupled receptors, including CRH and GHRH receptors, stimulate electrical activity and Ca2+ signaling in quiescent cells and increase the frequency of firing in spontaneously active cells by the cAMP-dependent stimulation of hyperpolarization-activated (HCN) channels or by protein kinase A (PKA)-mediated phosphorylation of several plasma membrane channels; this process presumably occurs in a cell type-specific manner.
3.1. Direct Effects of cAMP on VGCI
Structurally, HCN channels belong to the superfamily of Kv channels. HCN channels, however, are functionally distinct from the six other transmembrane domain K+ channels; their activation does not dampen excitation but increases the firing of APs. This paradoxical role for channels that structurally belong to the K+-channel family comes from their permeability properties; HCN channels are non-selective cation channels that are weak K+-selective channels [9]. RT-PCR and electrophysiological experiments have confirmed the presence of HCN in AtT-20 cells [50], GH3 cells [51, 52], melanotrophs [53], somatotrophs [52], and lactotrophs [54]. More recent findings indicate that these channels are also expressed in gonadotrophs and thyrotrophs [15]. The biophysical and pharmacological properties of this current are similar to those that have been described of the HCN channels in neuronal and cardiac cells. These properties include the sensitivity to both ZD7288 and Cs+ [50-52]. It appears that the basal cAMP production in the majority of pituitary cells in vitro is high enough to partially or fully activate the these channels, explaining the relatively weak effect of the activation of adenylyl cyclase on HCN gating [51]. A decrease in the AP frequency in spontaneously firing cells with inhibited HCN has also been frequently observed, indicating that these channels contribute to slow depolarization. Blocking these channels, however, does not abolish spontaneous electrical activity, indicating that other channels also contribute to slow depolarization [15]. Because basal cAMP production is down-regulated in vivo by several hypothalamic and intrapituitary factors, it is reasonable to suggest that an increase in adenylyl cyclase activity under physiological conditions should lead to the activation of HCN channels and firing of APs (Fig. 2).
3.2. Role of cAMP-Dependent Kinase in VGCI
PKAs (cAMP-dependent kinases) are present in all eukaryotic cells and function as the major mediators of the cAMP response. In the absence of cAMP, each PKA is an inactive, asymmetric tetramer containing two regulatory and two catalytic subunits that bind to each other with high affinity. The binding of cAMP to the regulatory subunit alters its affinity for the catalytic subunits by four orders of magnitude, leading to its dissociation into a dimer of regulatory subunits and two active monomeric catalytic subunits. The catalytic subunit-mediated phosphorylation of several plasma membrane channels increases the excitability of pituitary cells and thereby increases VGCI (Fig. 2).
PKA and Hyperpolarizing Currents
As discussed above, “classical” Kir channels could contribute to the regulation of the resting membrane potential. In general, PKA-dependent phosphorylation of these channels silences them, leading to cell depolarization and enhanced excitability. It is possible that GHRH decreases the intrinsic activity of a Kir channel in somatotrophs [13]. In cortocotrophs, CRH also changes the resting membrane potential and the rate of the pacemaking depolarization, causing an increase in the firing rate of spontaneously active cells and causing quiescent cells to become active [11, 12, 55]. It appears that the slow membrane depolarization is mostly caused by a reduction in the background K+ conductance mediated by a member of the Kir channel family [24]. The inhibition of Kir and the associated depolarization and increase in spike frequency last up to 15 minutes after the end of stimulation, suggesting that the phosphorylation of Kir channels could account for this memory. However, inhibition of Kir channels does not account for all effects of CRH on electrical activity [24]. Consistent with this, in these cells the presence of background hyperpolarizing current was also observed at positive potentials [18] and this current appears to be generated by TREK-1 channels and inhibited in a PKA-dependent manner [35].
PKA and Depolarizing Currents
The main effect of Gs-coupled receptors on the electrical activity in somatotrophs appears to be the facilitation of inward depolarizing currents; the phosphorylation of several channels accounts for this effect. GHRH and the synthesized growth-hormone-releasing peptide GHRP-2 increase L- and T-type Ca2+ conductance in ovine somatotrophs and human adenoma GH cells [56-59]. In contrast, the effect of GHRH on VGCI in rat somatotrophs depends on bath Na+, presumably because it enters into cells through the TTX-insensitive Nav5 and/or Nav8-9 channels [60]. A TTX-insensitive Na+ current that is upregulated by PKA phosphorylation is also present in rat somatotrophs [61]. Chen’s group also reported a stimulatory effect of GHRH on TTX-resistant Nav channels in somatotrophs from GH-green fluorescent protein transgenic mice, but they suggested that protein kinase C rather than PKA mediates the action of GHRH [62]. Electrophysiological experiments have also revealed that PACAP stimulation of melanotrophs causes an inward non-selective cation current that depolarizes cells and stimulates VGCI [63]. Recently, we provided evidence to support the hypothesis that TRPC channels could account for inward non-selective cation current and that their phosphorylation by PKA facilitates VGCI in rat somatotrophs and other pituitary subtypes [64].
4. Inhibition of VGCI by GPCRs
GPCRs linked to the Gi/o/z-signaling pathways also operate in endocrine pituitary cells [9]. Their activation leads to the inhibition of electrical activity and hormone secretion. SST and DA are two major hypothalamic factors that inhibit pituitary hormone secretion via Gi/o/z-coupled receptors. Pituitary cells also express several other GPCRs linked to this signaling pathway, including receptors activated by adenosine, ET-1, GABA, melatonin, neuropeptide Y and 5-HT. The inhibition of AC activity by Gi/o/zα is one of the mechanisms by which spontaneous electrical activity and VGCI are inhibited. The βγ dimer of these G-proteins also prominently affects electrical activity and Ca2+ signaling in a cAMP/PKA-independent manner by altering the gating of Kir and Cav channels (Fig. 2).
4.1. G protein-regulated K+ channels
The Kir3 channels that are regulated by G-proteins are present in endocrine pituitary cells and have a well-established role in regulating electrical activity [9]. In somatotrophs, SST inhibits spontaneous and GHRH-stimulated electrical activity, VGCI, and hormone secretion. This inhibition reflects hyperpolarization of the plasma membrane [13, 65]. The effects of SST on Kir3 channels and electrical activity are blocked by pertussis toxin, an inhibitor of the Gi/o signaling pathways [66]. In lactotrophs and melanotrophs, DA also hyperpolarizes the membrane and suppresses APs and bursts, explaining the decrease in VGCI [67, 68]. The effects of DA on VGCI are sensitive to pertussis toxin, but they persist in cells with elevated cAMP [67, 69]. Furthermore, the activation of voltage-independent K+ channels by DA can be observed in excised outside-out patch [70], demonstrating that no second messenger is required. This result suggests that the coupling between the G-protein and Kir channels is mediated by the βγ dimer [69]. Similarly, the activation of GABAB receptors in pituitary cells induces late hyperpolarization, the facilitation of Kir3 channels, and the inhibition of AC activity [71]. In somatotrophs and lactotrophs, ETs activate the Ca2+-mobilization pathway, but the stimulatory effect of ET is followed by an inhibition of VGCI and hormone secretion below basal levels [72]. This inhibition reflects increase a Cs+-sensitive Kir current in both cell types [28, 73].
4.2. G-Protein-Regulated Cav Channels
There is also evidence that SST inhibits Cav channels in somatotrophs [74] and AtT-20 cells [75]. Whereas GHRH-stimulated and PKA-mediated phosphorylation facilitates Cav currents in somatotrophs, SST inhibits these channels in a cAMP/PKA-independent manner [74]. It appears that the L-type Cav channels are negatively coupled to SST receptors [76]. DA was also reported to inhibit Cav channels in rat lactotrophs [77] and melanotrophs from neonatal rats [78, 79]. DA application was shown to inhibit Cav currents after a short (1-10 min) and a prolonged (over 24 h) application in GH4C1 cells transfected with D2 receptors. Porcine pituitary melanotrophs also express 5-HT1A and 5-HT1C receptors; their activation inhibits L-type Cav channels [80]. The inhibition of L-type and Q-type Cav channels by 5-HT also occurs in rat melanotrophs [81]. In both cell types, the inhibition of Cav currents is abolished by pertussis toxin, indicating that 5-HT receptors are coupled to the Gi/o signaling pathway. Adenosine inhibits electrical activity-driven Ca2+ transients in GH cell lines [82] and in frog melanotrophs [83], presumably reflecting an inhibition of Cav channels. Neuropeptide Y also inhibits spontaneous electrical activity and Cav currents in these cells [84]. Similarly, the activation of GABAB receptors in pituitary cells induces attenuation of Cav currents [71].
5. Calcium-Mobilizing Receptors and Ca2+ Signaling
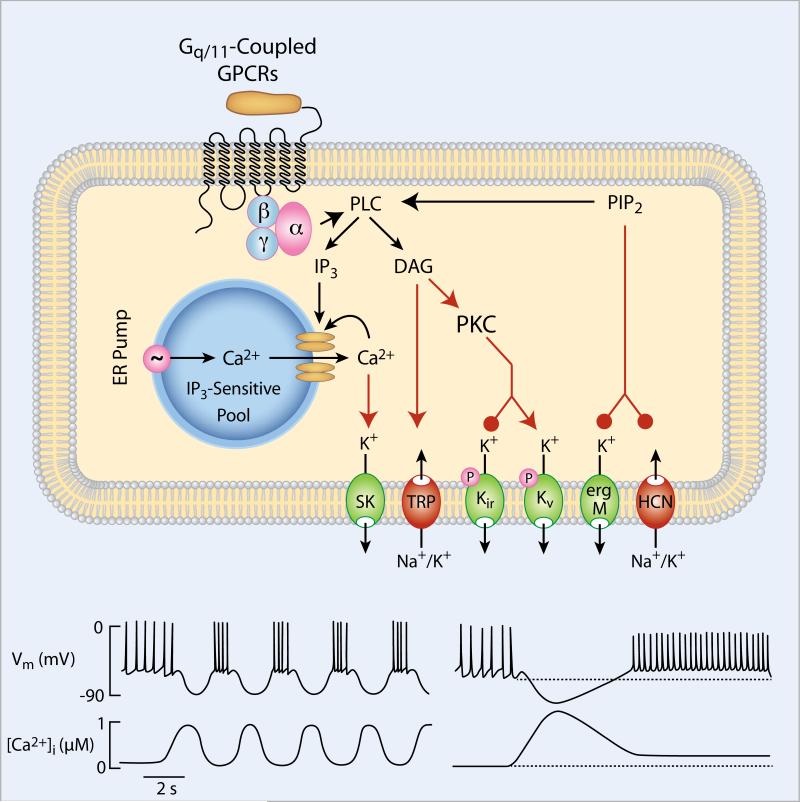
Ca2+-mobilizing GPCRs are expressed in all endocrine pituitary cells. In gonadotrophs, Ca2+ mobilization is triggered not only by GnRH, the main agonist for these cells, but also ETs, PACAP, substance P, and AVP/oxytocin. In thyrotrophs, the Gq/11 signaling pathway is activated by TRH, the main agonist for these cells, and ETs. Lactotrophs express Ca2+-mobilizing receptors activated by acetylcholine, angiotensin II, ATP, ETs, oxytocin, 5-HT, substance P, TRH and galanin. Mammalian melanotrophs express muscarinic receptors, and frog melanotrophs express Ca2+-mobilizing receptors for TRH and neuropeptide Y, in addition to muscarinic receptors. In corticotrophs, the Ca2+-mobilizing pathway is activated by AVP, norepinephrine and potentially 5-HT. Somatotrophs express Ca2+-mobilizing ghrelin and ETA receptors [9]. In general, the activated receptors trigger phosphoinositide hydrolysis and the production of IP3 and DAG. IP3 binds to IP3Rs expressed in the ER membrane, causing the release of Ca2+ (Fig. 3).
Fig. 3.
Role of Ca2+-mobilizing receptors on channel activity and Ca2+ signaling in pituitary cells. The Gq/11-coupled receptors stimulate phospholipase C (PLC), leading to the depletion of phosphatidyl inositol-bisphosphate (PIP2) and the formation of two intracellular messengers: inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to IP3 receptors in the endoplasmic reticulum (ER), causing two types of Ca2+ signaling: baseline Ca2+ oscillations (bottom left) or biphasic Ca2+ signaling (bottom right). In gonadotrophs, the generation of baseline Ca2+ oscillations reflects periodic activation of IP3 receptors mediated by IP3 and Ca2+, whereas the generation of a biphasic response reflects the tonic activation of IP3 receptors. Due to the activation of SK-type Ca2+-activated K+ channels, spontaneous electrical activity in pituitary cells is also affected, generating a bursting type of electrical activity in gonadotrophs and sustained plateau firing in non-oscillating cells. DAG may stimulate transient receptor potential (TRP) channels or may alter the gating properties of Kir and voltage-gated K+ (Kv) channels through protein kinase C (PKC)-dependent phosphorylation. Depletion of PIP2 may also influence ERG and M type K+ channels and HCN channels. TRP, Kir, ERG and/or M channels could play an important role in sustaining plasma membrane depolarization in non-oscillating cells.
5.1. Pattern of Ca2+ Release from the ER
The ER is the primary storehouse for Ca2+ in most cells, including pituitary cells. The ER’s resting Ca2+ concentration ([Ca2+]ER) is a few hundred micromolar, in contrast to the resting level of [Ca2+]i, which is ~100 nM. The high [Ca2+]ER is maintained by SERCA pumps. Ca2+ effluxes from the ER through passive leakage and through IP3Rs and/or RyRs [9]. Because of the large concentration difference, the activation of IP3Rs by a Gq/11 agonist results in a large and sudden increase in [Ca2+]i. Following this initial Ca2+ pulse, one of two behaviors can be observed in pituitary cells, depending on the cell type and, in some cases, the agonist. In lactotrophs, somatotrophs, thyrotrophs and cells from the GH cell lines, the pulse is typically followed by a slow decline to a plateau in [Ca2+]i, although some cells may only a have pulse or a plateau [72]. This non-oscillatory pattern of Ca2+ response is termed biphasic or pulse-decay-plateau. In mammalian gonadotrophs, the pulse is typically followed by large baseline [Ca2+]i oscillations [85, 86] (Fig. 3, bottom). Fish gonadotrophs also show an oscillatory Ca2+ response to the application of GnRH [87], as do corticotrophs in response to norepinephrine application [88].
5.2. Mechanism of Biphasic Ca2+ Response
The biphasic Ca2+ response requires only that the IP3Rs open and remain open during agonist application. The initial rapid increase in [Ca2+]i is followed by a slow decline, reflecting the removal of Ca2+ from the cell by plasma membrane ATPase pumps and Na+-dependent Ca2+ efflux and reuptake of Ca2+ by SERCA pump and mitochondria. The decline in [Ca2+]i is mirrored by a decline in [Ca2+]ER, although [Ca2+]ER is much larger. As [Ca2+]ER declines to a sufficiently low level, a voltage-gated Ca2+ entry pathway is activated, bringing additional Ca2+ into the cell and producing an elevated plateau or fluctuations in [Ca2+]i that are evident near the end of the agonist application [9]. In cells bathed in Ca2+-deficient medium or blocked VGCI, [Ca2+]i drops to basal levels within a few minutes, indicating that sustained Ca2+ signaling by Ca2+ mobilizing GPCRs is critically dependent on Ca2+ influx. This phenomenon is well illustrated in TRH-stimulated lactotrophs bathed in Ca2+-deficient medium and by lactotrophs and somatotrophs stimulated with ETs in the presence of Ca2+ [72, 89].
5.3. Mechanism of Oscillatory Ca2+ Release
Unlike AP-driven Ca2+ oscillations, those induced by GnRH in gonadotrophs or norepinephrine in corticotrophs persist for 3-15 min when the Ca2+ bath is removed, as do cells that are bathed in the presence of Ca2+ but clamped at potentials that silence Ca2+ influx through Cav channels [88, 90]. Thus, oscillation is intrinsic to the Ca2+-handling properties of the cell. These two cell types differ in their oscillatory Ca2+ mobilization mechanisms. In gonadotrophs, the frequency of Ca2+ oscillations varies from 5 to 20 pulses per minute [86, 91, 92], whereas norepinephrine generates Ca2+ oscillations in corticotrophs that have a frequency of about one per minute [88]. In gonadotrophs, oscillations in IP3 are not required to generate oscillatory Ca2+ release, as demonstrated by the injection of non-metabolized IP3 analogs, and the concentration of IP3 underlies the frequency of Ca2+ spiking [92]. The [Ca2+]i influences the IP3-dependent Ca2+ release in these cells. The rapid stimulatory effect of Ca2+ on IP3-depenent Ca2+ release in gonadotrophs is nicely demonstrated by the phase resetting of GnRH-induced oscillations by a brief pulse of Ca2+ entry [93]. The inhibitory effect of high [Ca2+]i on GnRH-induced Ca2+ oscillations has also been demonstrated [94]. Thus, in contrast to cells that exhibit continuous opening of IP3Rs, resulting in biphasic Ca2+ signals, IP3Rs in gonadotrophs undergo periodic activation and inhibition mediated by cytosolic Ca2+ (Fig. 3). The mechanism responsible for IP3-dependent oscillations in corticotrophs is unknown.
5.4. Contribution of Mitochondria and SERCA Pumps to IP3-Induced Ca2+ Release
In general, Ca2+ is transported into mitochondria through Ca2+ uniporters, which are powered by the membrane potential across the inner membrane. Calcium is transported out of mitochondria primarily by Na+/Ca2+ exchangers. In corticotrophs, the rate of Ca2+ clearance after depolarization-induced Ca2+ influx is dramatically slowed by mitochondrial uncouplers or inhibitors of the mitochondrial uniporter. This inhibition enhances the exocytotic response [95]. In oscillating gonadotrophs, Ca2+ released from the ER is partly taken up again by the ER and partly pumped into other intracellular compartments or out of the cell [96]. In these cells, collapsing the mitochondrial inner membrane potential with the protonophore carbonyl cyanide m-chlorophenylhydrazone inhibits Ca2+ uptake by mitochondria and slows or inhibits GnRH-induced [Ca2+]i oscillations [97, 98]. In non-oscillating GnRH-stimulated gonadotrophs, the removal of Ca2+ from cells in a Na+-dependent manner dominates over reuptake of Ca2+ by mitochondria [99]. In contrast, the inhibition of SERCA pumps causes a transition from an oscillatory to non-oscillatory mode of Ca2+ release in GnRH-stimulated gonadotrophs, indicating that the reuptake of Ca2+ by the ER is essential for periodic activation of IP3R by [Ca2+]i [100, 101].
5.5. Dependence of IP3-Mediated Ca2+ Signaling on VGCI
The Ca2+ response to a Gq/11-activating agonist impacts the plasma membrane potential. In cells that respond with biphasic Ca2+ signals, the rapid rise in [Ca2+]i activates the apamin-sensitive SK-type KCa channels in the plasma membrane. The KCa current hyperpolarizes the membrane, terminating any spontaneous electrical activity that was present prior to agonist application [72, 102]. The rapid hyperpolarization phase is followed by a sustained depolarization phase due to the modulation of a still unidentified current, presumably the downregulation of an M/ERG channel [30, 103, 104], and/or the stimulation of a TRPC current [64]. This depolarization activates Cav channels, further depolarizing the cell and initiating single spiking or bursting, depending on the cell type [72]. Such electrical activity would then be reflected in the [Ca2+]i time course as small oscillations on top of the plateau and would contribute to the plateau (Fig. 3).
In gonadotrophs, coupling between the ER and the plasma membrane is also mediated through the apamin-sensitive SK type KCa channels [85, 86], but the integration of VGCI into IP3-dependent Ca2+ oscillations is more complex. In cells at a hyperpolarized potential, few Cav channels are open, and the Ca2+ current is small, whereas at depolarized holding potentials, many Cav channels are open, and thus, there is a larger Ca2+ current. In addition to demonstrating that GnRH-induced Ca2+ oscillations persist in the absence of membrane potential oscillations, these studies have shown that the oscillations die out if the holding potential is not sufficiently depolarized, due to gradual depletion of the ER. These experiments also showed that no patterned electrical activity is required to keep the ER-Ca2+ store replenished [90, 105].
Stimulated gonadotrophs do produce electrical bursting, however, due to bidirectional interactions between the plasma membrane and the ER [42, 106]. Ion channels in the plasma membrane bring Ca2+ into the cell during each spike, which replenishes the ER and thereby enables coupling between the membrane and the ER. The key feature is the antiphasic pattern of electrical activity and Ca2+ spikes (Fig. 3) due to the inhibitory action of each Ca2+ spike on the plasma membrane mediated by the SK current. The electrical spiking resumes once [Ca2+]i returns to a low level following the Ca2+ spike. Thus, the Ca2+ oscillator periodically interrupts the plasma membrane oscillator, producing a bursting pattern of electrical activity. Recent investigation also revealed that inhibition of ERG channel by GnRH receptors facilitates the depolarizing phase, promoting Ca2+ influx [33]. The electrical activity and secretion are out of phase; the former serves to refill the ER, which provides the periodic Ca2+ pulse that is needed to evoke secretion [107].
6. Concluding Remarks
Endocrine pituitary cells express numerous Nav, Cav, Kv, KCa, and Kir channels, as well as cation-conducting HCN and TRPC channels, and generate APs spontaneously and in response to depolarization of the cell membrane. Physiologically, electrically driven Ca2+ signals resemble neuronal cell signaling, which requires high Ca2+ in the extracellular medium and APs as a driving force for Ca2+ influx. In cells that fire single APs, Cav channels are open for a short amount of time, and the elevated Ca2+ concentration is localized to nanodomains that form at the inner mouth of open channels. Cells that exhibit bursting activity generate oscillatory global Ca2+ signals of sufficient amplitude to trigger exocytosis (Fig. 1). The activation of Gs-coupled receptors initiates the firing of APs in quiescent cells and increases the frequency of firing or duration of bursting in spontaneously active cells in a cAMP-dependent manner. Both direct and indirect (through PKA) action of cAMP accounts for the enhanced electrical activity and accompanying VGCI (Fig. 2). This signaling pathway plays a major role in somatotrophs and corticotrophs, operated by GHRH and CRH receptors, respectively. In contrast, activation of the Gi/o signaling pathway inhibits electrical activity and the accompanying VGCI by stimulating Kir channels and/or inhibiting Cav channels in a cAMP/PKA-independent manner (Fig. 2). Of the Gi/o-coupled receptors that are expressed in pituitary cells, D2 receptors play a major role in cellular Ca2+ homeostasis in melanotrophs and lactotrophs and SST receptors in somatotrophs.
All pituitary cells have an additional system to control intracellular Ca2+, which is composed of the IP3Rs in the ER membrane. Endocrine pituitary cells express at least 15 subtypes of Gq/11-coupled GPCRs and several receptor tyrosine kinases, whose activation leads to the mobilization of intracellular Ca2+ in an IP3-dependent manner (Fig. 3). In melanotrophs, somatotrophs, and lactotrophs, Ca2+ mobilization provides only a transient source of non-oscillatory [Ca2+]i elevation due to the continuous opening of IP3Rs in the presence of agonist; Ca2+ influx through Cav channels is critical for sustaining Ca2+ signaling. Gonadotrophs have the most sophisticated Ca2+ mobilization pathway; they release Ca2+ in an oscillatory manner in response to the activation of any of the Ca2+-mobilizing receptors that are expressed in these cells, with the frequency of spiking being determined by the IP3 concentration. In these cells, oscillations in [Ca2+]i are generated by the periodic activation of IP3Rs during continuous stimulation of Ca2+-mobilizing receptors due to bidirectional actions of cytosolic Ca2+ on the IP3-dependent gating of these channels. Intracellular Ca2+ is redistributed between the ER and mitochondria, providing a relatively long-lasting spike when cells are bathed in Ca2+-deficient medium. In this way, gonadotrophs resemble skeletal muscle cells, relying on Ca2+ mobilization for a prolonged period of time and with VGCI controlling the “excitability” of the ER membrane during receptor activation.
Acknowledgment
SS is supported by an NIH grant from the Intramural Research Program of the NICHD, NIH. The author is thankful to Sir Michael J. Berridge for helpful discussions of calcium signaling in pituitary cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jenks BG. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann N Y Acad Sci. 2009;1163:17–30. doi: 10.1111/j.1749-6632.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- 2.Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:319–29. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 4.Porter TE. Regulation of pituitary somatotroph differentiation by hormones of peripheral endocrine glands. Domest Anim Endocrinol. 2005;29:52–62. doi: 10.1016/j.domaniend.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Pazos-Moura CC, Ortiga-Carvalho TM, de Moura E Gaspar. The autocrine/paracrine regulation of thyrotropin secretion. Thyroid. 2003;13:167–75. doi: 10.1089/105072503321319477. [DOI] [PubMed] [Google Scholar]
- 6.Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–99. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- 7.Stojilkovic SS, Catt KJ. Calcium oscillations in anterior pituitary cells. Endocr Rev. 1992;13:256–80. doi: 10.1210/edrv-13-2-256. [DOI] [PubMed] [Google Scholar]
- 8.Kwiecien R, Hammond C. Differential management of Ca2+ oscillations by anterior pituitary cells: a comparative overview. Neuroendocrinology. 1998;68:135–51. doi: 10.1159/000054360. [DOI] [PubMed] [Google Scholar]
- 9.Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Foll F, Castel H, Soriani O, Vaudry H, Cazin L. Gramicidin-perforated patch revealed depolarizing effect of GABA in cultured frog melanotrophs. J Physiol. 1998;507(Pt 1):55–69. doi: 10.1111/j.1469-7793.1998.055bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996;137:2269–77. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- 12.Guerineau N, Corcuff JB, Tabarin A, Mollard P. Spontaneous and corticotropin-releasing factor-induced cytosolic calcium transients in corticotrophs. Endocrinology. 1991;129:409–20. doi: 10.1210/endo-129-1-409. [DOI] [PubMed] [Google Scholar]
- 13.Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic SS. Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol. 2007;98:131–44. doi: 10.1152/jn.00872.2006. [DOI] [PubMed] [Google Scholar]
- 14.Stojilkovic SS, Kukuljan M, Iida T, Rojas E, Catt KJ. Integration of cytoplasmic calcium and membrane potential oscillations maintains calcium signaling in pituitary gonadotrophs. Proc Natl Acad Sci U S A. 1992;89:4081–5. doi: 10.1073/pnas.89.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kretschmannova K, Kucka M, Gonzalez-Iglesias AE, Stojilkovic SS. The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells. Mol. Endocrinol. 2011 doi: 10.1210/me.2011-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Goor F, Zivadinovic D, Martinez-Fuentes AJ, Stojilkovic SS. Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. J Biol Chem. 2001;276:33840–6. doi: 10.1074/jbc.M105386200. [DOI] [PubMed] [Google Scholar]
- 17.Schlegel W, Winiger BP, Mollard P, Vacher P, Wuarin F, Zahnd GR, Wollheim CB, Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987;329:719–21. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- 18.Lee AK, Tse A. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol. 1997;504(Pt 2):367–78. doi: 10.1111/j.1469-7793.1997.367be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tse A, Hille B. Role of voltage-gated Na+ and Ca2+ channels in gonadotropin-releasing hormone-induced membrane potential changes in identified rat gonadotropes. Endocrinology. 1993;132:1475–81. doi: 10.1210/endo.132.4.8384989. [DOI] [PubMed] [Google Scholar]
- 20.Van Goor F, Li YX, Stojilkovic SS. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J Neurosci. 2001;21:5902–15. doi: 10.1523/JNEUROSCI.21-16-05902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyward PM, Chen C, Clarke IJ. Inward membrane currents and electrophysiological responses to GnRH in ovine gonadotropes. Neuroendocrinology. 1995;61:609–21. doi: 10.1159/000126887. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Zhang J, Vincent JD, Israel JM. Sodium and calcium currents in action potentials of rat somatotrophs: their possible functions in growth hormone secretion. Life Sci. 1990;46:983–9. doi: 10.1016/0024-3205(90)90021-i. [DOI] [PubMed] [Google Scholar]
- 23.Sankaranarayanan S, Simasko SM. A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. Am J Physiol. 1996;271:C1927–34. doi: 10.1152/ajpcell.1996.271.6.C1927. [DOI] [PubMed] [Google Scholar]
- 24.Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. J Physiol. 1997;502(Pt 2):265–79. doi: 10.1111/j.1469-7793.1997.265bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwiecien R, Robert C, Cannon R, Vigues S, Arnoux A, Kordon C, Hammond C. Endogenous pacemaker activity of rat tumour somatotrophs. J Physiol. 1998;508(Pt 3):883–905. doi: 10.1111/j.1469-7793.1998.883bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wulfsen I, Hauber HP, Schiemann D, Bauer CK, Schwarz JR. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J Neuroendocrinol. 2000;12:263–72. doi: 10.1046/j.1365-2826.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- 27.Charles AC, Piros ET, Evans CJ, Hales TG. L-type Ca2+ channels and K+ channels specifically modulate the frequency and amplitude of spontaneous Ca2+ oscillations and have distinct roles in prolactin release in GH3 cells. J Biol Chem. 1999;274:7508–15. doi: 10.1074/jbc.274.11.7508. [DOI] [PubMed] [Google Scholar]
- 28.Tomic M, Van Goor F, He ML, Zivadinovic D, Stojilkovic SS. Ca(2+)-mobilizing endothelin-A receptors inhibit voltage-gated Ca(2+) influx through G(i/o) signaling pathway in pituitary lactotrophs. Mol Pharmacol. 2002;61:1329–39. doi: 10.1124/mol.61.6.1329. [DOI] [PubMed] [Google Scholar]
- 29.Xu R, Zhao Y, Chen C. Growth hormone-releasing peptide-2 reduces inward rectifying K+ currents via a PKA-cAMP-mediated signalling pathway in ovine somatotropes. J Physiol. 2002;545:421–33. doi: 10.1113/jphysiol.2002.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer R, Wulfsen I, Behrens S, Weinsberg F, Bauer CK, Schwarz JR. The erg-like potassium current in rat lactotrophs. J Physiol. 1999;518(Pt 2):401–16. doi: 10.1111/j.1469-7793.1999.0401p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer CK, Schafer R, Schiemann D, Reid G, Hanganu I, Schwarz JR. A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs. Mol Cell Endocrinol. 1999;148:37–45. doi: 10.1016/s0303-7207(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 32.Lecchi M, Redaelli E, Rosati B, Gurrola G, Florio T, Crociani O, Curia G, Cassulini RR, Masi A, Arcangeli A, Olivotto M, Schettini G, Possani LD, Wanke E. Isolation of a long-lasting eag-related gene-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release. J Neurosci. 2002;22:3414–25. doi: 10.1523/JNEUROSCI.22-09-03414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirdes W, Dinu C, Bauer CK, Boehm U, Schwarz JR. Gonadotropin-releasing hormone inhibits ether-a-go-go-related gene K+ currents in mouse gonadotropes. Endocrinology. 2010;151:1079–88. doi: 10.1210/en.2009-0718. [DOI] [PubMed] [Google Scholar]
- 34.Bauer CK, Wulfsen I, Schafer R, Glassmeier G, Wimmers S, Flitsch J, Ludecke DK, Schwarz JR. HERG K(+) currents in human prolactin-secreting adenoma cells. Pflugers Arch. 2003;445:589–600. doi: 10.1007/s00424-002-0980-0. [DOI] [PubMed] [Google Scholar]
- 35.Lee AK, Smart JL, Rubinstein M, Low MJ, Tse A. Reciprocal regulation of TREK-1 channels by arachidonic acid and CRH in mouse corticotropes. Endocrinology. 2011;152:1901–10. doi: 10.1210/en.2010-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucka M, Kretschmannova K, Murano T, Wu CP, Zemkova H, Ambudkar SV, Stojilkovic SS. Dependence of multidrug resistance protein-mediated cyclic nucleotide efflux on the background sodium conductance. Mol Pharmacol. 2010;77:270–9. doi: 10.1124/mol.109.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobbett P, Ingram CD, Mason WT. Sodium and potassium currents involved in action potential propagation in normal bovine lactotrophs. J Physiol. 1987;392:273–99. doi: 10.1113/jphysiol.1987.sp016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentijn JA, Valentijn K. Two distinct Na+ currents control cytosolic Ca2+ pulsing in Xenopus laevis pituitary melanotrophs. Cell Calcium. 1997;21:241–51. doi: 10.1016/s0143-4160(97)90048-8. [DOI] [PubMed] [Google Scholar]
- 39.Van Goor F, Zivadinovic D, Stojilkovic SS. Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol. 2001;15:1222–36. doi: 10.1210/mend.15.7.0668. [DOI] [PubMed] [Google Scholar]
- 40.Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–11. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- 41.Li YX, Rinzel J, Vergara L, Stojilkovic SS. Spontaneous electrical and calcium oscillations in unstimulated pituitary gonadotrophs. Biophys J. 1995;69:785–95. doi: 10.1016/S0006-3495(95)79952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stojilkovic SS, Zemkova H, Van Goor F. Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab. 2005;16:152–9. doi: 10.1016/j.tem.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Zimber MP, Simasko SM. Recruitment of calcium from intracellular stores does not occur during the expression of large spontaneous calcium oscillations in GH(3) cells and lactotropic cells in primary culture. Neuroendocrinology. 2000;72:242–51. doi: 10.1159/000054593. [DOI] [PubMed] [Google Scholar]
- 44.Tomic M, Koshimizu T, Yuan D, Andric SA, Zivadinovic D, Stojilkovic SS. Characterization of a plasma membrane calcium oscillator in rat pituitary somatotrophs. J Biol Chem. 1999;274:35693–702. doi: 10.1074/jbc.274.50.35693. [DOI] [PubMed] [Google Scholar]
- 45.Lieste JR, Koopman WJ, Reynen VC, Scheenen WJ, Jenks BG, Roubos EW. Action currents generate stepwise intracellular Ca2+ patterns in a neuroendocrine cell. J Biol Chem. 1998;273:25686–94. doi: 10.1074/jbc.273.40.25686. [DOI] [PubMed] [Google Scholar]
- 46.Scheenen WJ, Jenks BG, van Dinter RJ, Roubos EW. Spatial and temporal aspects of Ca2+ oscillations in Xenopus laevis melanotrope cells. Cell Calcium. 1996;19:219–27. doi: 10.1016/s0143-4160(96)90023-8. [DOI] [PubMed] [Google Scholar]
- 47.Jenks BG, Roubos EW, Scheenen WJ. Ca2+ oscillations in melanotropes of Xenopus laevis: their generation, propagation, and function. Gen Comp Endocrinol. 2003;131:209–19. doi: 10.1016/s0016-6480(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 48.Bonnefont X, Fiekers J, Creff A, Mollard P. Rhythmic bursts of calcium transients in acute anterior pituitary slices. Endocrinology. 2000;141:868–75. doi: 10.1210/endo.141.3.7363. [DOI] [PubMed] [Google Scholar]
- 49.Kuryshev YA, Childs GV, Ritchie AK. Three high threshold calcium channel subtypes in rat corticotropes. Endocrinology. 1995;136:3916–24. doi: 10.1210/endo.136.9.7649100. [DOI] [PubMed] [Google Scholar]
- 50.Tian L, Shipston MJ. Characterization of hyperpolarization-activated cation currents in mouse anterior pituitary, AtT20 D16:16 corticotropes. Endocrinology. 2000;141:2930–7. doi: 10.1210/endo.141.8.7617. [DOI] [PubMed] [Google Scholar]
- 51.Kretschmannova K, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells. J Neuroendocrinol. 2006;18:484–93. doi: 10.1111/j.1365-2826.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- 52.Simasko SM, Sankaranarayanan S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am J Physiol. 1997;272:E405–14. doi: 10.1152/ajpendo.1997.272.3.E405. [DOI] [PubMed] [Google Scholar]
- 53.Mei YA, Soriani O, Castel H, Vaudry H, Cazin L. Adenosine potentiates the delayed-rectifier potassium conductance but has no effect on the hyperpolarization-activated Ih current in frog melanotrophs. Brain Res. 1998;793:271–8. doi: 10.1016/s0006-8993(98)00184-x. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Iglesias AE, Kretschmannova K, Tomic M, Stojilkovic SS. ZD7288 inhibits exocytosis in an HCN-independent manner and downstream of voltage-gated calcium influx in pituitary lactotrophs. Biochem Biophys Res Commun. 2006;346:845–50. doi: 10.1016/j.bbrc.2006.05.194. [DOI] [PubMed] [Google Scholar]
- 55.Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulation of Ca2+ entry in corticotropes is partially dependent on protein kinase A. Endocrinology. 1995;136:3925–35. doi: 10.1210/endo.136.9.7649101. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Xu R, Clarke IJ, Ruan M, Loneragan K, Roh SG. Diverse intracellular signalling systems used by growth hormone-releasing hormone in regulating voltage-gated Ca2+ or K channels in pituitary somatotropes. Immunol Cell Biol. 2000;78:356–68. doi: 10.1046/j.1440-1711.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 57.Chen C, Clarke IJ. Modulation of Ca2+ influx in the ovine somatotroph by growth hormone-releasing factor. Am J Physiol. 1995;268:E204–12. doi: 10.1152/ajpendo.1995.268.2.E204. [DOI] [PubMed] [Google Scholar]
- 58.Takei T, Takano K, Yasufuku-Takano J, Fujita T, Yamashita N. Enhancement of Ca2+ currents by GHRH and its relation to PKA and [Ca2+]i in human GH-secreting adenoma cells. Am J Physiol. 1996;271:E801–7. doi: 10.1152/ajpendo.1996.271.5.E801. [DOI] [PubMed] [Google Scholar]
- 59.Chen C, Clarke IJ. Effects of growth hormone-releasing peptide-2 (GHRP-2) on membrane Ca2+ permeability in cultured ovine somatotrophs. J Neuroendocrinol. 1995;7:179–86. doi: 10.1111/j.1365-2826.1995.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 60.Kato M, Hattori MA, Suzuki M. Inhibition by extracellular Na+ replacement of GRF-induced GH secretion from rat pituitary cells. Am J Physiol. 1988;254:E476–81. doi: 10.1152/ajpendo.1988.254.4.E476. [DOI] [PubMed] [Google Scholar]
- 61.Naumov AP, Herrington J, Hille B. Actions of growth-hormone-releasing hormone on rat pituitary cells: intracellular calcium and ionic currents. Pflugers Arch. 1994;427:414–21. doi: 10.1007/BF00374255. [DOI] [PubMed] [Google Scholar]
- 62.Yang SK, Wang K, Parkington H, Chen C. Involvement of tetrodotoxin-resistant Na+ current and protein kinase C in the action of growth hormone (GH)-releasing hormone on primary cultured somatotropes from GH-green fluorescent protein transgenic mice. Endocrinology. 2008;149:4726–35. doi: 10.1210/en.2008-0405. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka K, Shibuya I, Harayama N, Nomura M, Kabashima N, Ueta Y, Yamashita H. Pituitary adenylate cyclase-activating polypeptide potentiation of Ca2+ entry via protein kinase C and A pathways in melanotrophs of the pituitary pars intermedia of rats. Endocrinology. 1997;138:4086–95. doi: 10.1210/endo.138.10.5442. [DOI] [PubMed] [Google Scholar]
- 64.Tomic M, Kucka M, Kretschmannova K, Li S, Nesterova M, Stratakis CA, Stojilkovic SS. Role of nonselective cation channels in spontaneous and protein kinase A-stimulated calcium signaling in pituitary cells. Am J Physiol Endocrinol Metab. 2011;301:E370–9. doi: 10.1152/ajpendo.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwiecien R, Tseeb V, Kurchikov A, Kordon C, Hammond C. Growth hormone-releasing hormone triggers pacemaker activity and persistent Ca2+ oscillations in rat somatotrophs. J Physiol. 1997;499(Pt 3):613–23. doi: 10.1113/jphysiol.1997.sp021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrucci C, Cervia D, Buzzi M, Biondi C, Bagnoli P. Somatostatin-induced control of cytosolic free calcium in pituitary tumour cells. Br J Pharmacol. 2000;129:471–84. doi: 10.1038/sj.bjp.0703075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Iglesias AE, Murano T, Li S, Tomic M, Stojilkovic SS. Dopamine inhibits basal prolactin release in pituitary lactotrophs through pertussis toxin-sensitive and -insensitive signaling pathways. Endocrinology. 2008;149:1470–9. doi: 10.1210/en.2007-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valentijn JA, Louiset E, Vaudry H, Cazin L. Dopamine-induced inhibition of action potentials in cultured frog pituitary melanotrophs is mediated through activation of potassium channels and inhibition of calcium and sodium channels. Neuroscience. 1991;42:29–39. doi: 10.1016/0306-4522(91)90147-g. [DOI] [PubMed] [Google Scholar]
- 69.Gregerson KA, Flagg TP, O’Neill TJ, Anderson M, Lauring O, Horel JS, Welling PA. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142:2820–32. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- 70.Einhorn LC, Oxford GS. Guanine nucleotide binding proteins mediate D2 dopamine receptor activation of a potassium channel in rat lactotrophs. J Physiol. 1993;462:563–78. doi: 10.1113/jphysiol.1993.sp019569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–47. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 72.Lachowicz A, Van Goor F, Katzur AC, Bonhomme G, Stojilkovic SS. Uncoupling of calcium mobilization and entry pathways in endothelin-stimulated pituitary lactotrophs. J Biol Chem. 1997;272:28308–14. doi: 10.1074/jbc.272.45.28308. [DOI] [PubMed] [Google Scholar]
- 73.Tomic M, Zivadinovic D, Van Goor F, Yuan D, Koshimizu T, Stojilkovic SS. Expression of Ca(2+)-mobilizing endothelin(A) receptors and their role in the control of Ca(2+) influx and growth hormone secretion in pituitary somatotrophs. J Neurosci. 1999;19:7721–31. doi: 10.1523/JNEUROSCI.19-18-07721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scherubl H, Hescheler J, Riecken EO. Molecular mechanisms of somatostatin’s inhibition of hormone release: participation of voltage-gated calcium channels and G-proteins. Horm Metab Res Suppl. 1993;27:1–4. [PubMed] [Google Scholar]
- 75.Luini A, Lewis D, Guild S, Schofield G, Weight F. Somatostatin, an inhibitor of ACTH secretion, decreases cytosolic free calcium and voltage-dependent calcium current in a pituitary cell line. J Neurosci. 1986;6:3128–32. doi: 10.1523/JNEUROSCI.06-11-03128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang SK, Parkington HC, Epelbaum J, Keating DJ, Chen C. Somatostatin decreases voltage-gated Ca2+ currents in GH3 cells through activation of somatostatin receptor 2. Am J Physiol Endocrinol Metab. 2007;292:E1863–70. doi: 10.1152/ajpendo.00047.2007. [DOI] [PubMed] [Google Scholar]
- 77.Lledo PM, Legendre P, Israel JM, Vincent JD. Dopamine inhibits two characterized voltage-dependent calcium currents in identified rat lactotroph cells. Endocrinology. 1990;127:990–1001. doi: 10.1210/endo-127-3-990. [DOI] [PubMed] [Google Scholar]
- 78.Gomora JC, Avila G, Cota G. Ca2+ current expression in pituitary melanotrophs of neonatal rats and its regulation by D2 dopamine receptors. J Physiol. 1996;492(Pt 3):763–73. doi: 10.1113/jphysiol.1996.sp021344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valentijn JA, Louiset E, Vaudry H, Cazin L. Dopamine regulates the electrical activity of frog melanotrophs through a G protein-mediated mechanism. Neuroscience. 1991;44:85–95. doi: 10.1016/0306-4522(91)90252-j. [DOI] [PubMed] [Google Scholar]
- 80.Ciranna L, Mouginot D, Feltz P, Schlichter R. Serotonin inhibits Ca2+ currents in porcine melanotrophs by activating 5-HT1C and 5-HT1A receptors. J Physiol. 1993;463:17–38. doi: 10.1113/jphysiol.1993.sp019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ciranna L, Feltz P, Schlichter R. Selective inhibition of high voltage-activated L-type and Q-type Ca2+ currents by serotonin in rat melanotrophs. J Physiol. 1996;490(Pt 3):595–609. doi: 10.1113/jphysiol.1996.sp021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mollard P, Guerineau N, Chiavaroli C, Schlegel W, Cooper DM. Adenosine A1 receptor-induced inhibition of Ca2+ transients linked to action potentials in clonal pituitary cells. Eur J Pharmacol. 1991;206:271–7. doi: 10.1016/0922-4106(91)90109-u. [DOI] [PubMed] [Google Scholar]
- 83.Mei YA, Le Foll F, Vaudry H, Cazin L. Adenosine inhibits L- and N-type calcium channels in pituitary melanotrophs. Evidence for the involvement of a G protein in calcium channel gating. J Neuroendocrinol. 1996;8:85–91. doi: 10.1111/j.1365-2826.1996.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 84.Valentijn JA, Vaudry H, Kloas W, Cazin L. Melanostatin (NPY) inhibited electrical activity in frog melanotrophs through modulation of K+, Na+ and Ca2+ currents. J Physiol. 1994;475:185–95. doi: 10.1113/jphysiol.1994.sp020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kukuljan M, Stojilkovic SS, Rojas E, Catt KJ. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett. 1992;301:19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- 86.Tse A, Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992;255:462–4. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- 87.Mollard P, Kah O. Spontaneous and gonadotropin-releasing hormone-stimulated cytosolic calcium rises in individual goldfish gonadotrophs. Cell Calcium. 1996;20:415–24. doi: 10.1016/s0143-4160(96)90004-4. [DOI] [PubMed] [Google Scholar]
- 88.Tse A, Tse FW. alpha-adrenergic stimulation of cytosolic Ca2+ oscillations and exocytosis in identified rat corticotrophs. J Physiol. 1998;512(Pt 2):385–93. doi: 10.1111/j.1469-7793.1998.385be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andric SA, Zivadinovic D, Gonzalez-Iglesias AE, Lachowicz A, Tomic M, Stojilkovic SS. Endothelin-induced, long lasting, and Ca2+ influx-independent blockade of intrinsic secretion in pituitary cells by Gz subunits. J Biol Chem. 2005;280:26896–903. doi: 10.1074/jbc.M502226200. [DOI] [PubMed] [Google Scholar]
- 90.Kukuljan M, Vergara L, Stojilkovic SS. Modulation of the kinetics of inositol 1,4,5-trisphosphate-induced [Ca2+]i oscillations by calcium entry in pituitary gonadotrophs. Biophys J. 1997;72:698–707. doi: 10.1016/s0006-3495(97)78706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stojilkovic SS, Torsello A, Iida T, Rojas E, Catt KJ. Calcium signaling and secretory responses in agonist-stimulated pituitary gonadotrophs. J Steroid Biochem Mol Biol. 1992;41:453–67. doi: 10.1016/0960-0760(92)90371-o. [DOI] [PubMed] [Google Scholar]
- 92.Stojilkovic SS, Kukuljan M, Tomic M, Rojas E, Catt KJ. Mechanism of agonist-induced [Ca2+]i oscillations in pituitary gonadotrophs. J Biol Chem. 1993;268:7713–20. [PubMed] [Google Scholar]
- 93.Vergara LA, Stojilkovic SS, Rojas E. GnRH-induced cytosolic calcium oscillations in pituitary gonadotrophs: phase resetting by membrane depolarization. Biophys J. 1995;69:1606–14. doi: 10.1016/S0006-3495(95)80033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stojilkovic SS, Tomic M, Kukuljan M, Catt KJ. Control of calcium spiking frequency in pituitary gonadotrophs by a single-pool cytoplasmic oscillator. Mol Pharmacol. 1994;45:1013–21. [PubMed] [Google Scholar]
- 95.Lee AK, Tse A. Dominant role of mitochondria in calcium homeostasis of single rat pituitary corticotropes. Endocrinology. 2005;146:4985–93. doi: 10.1210/en.2005-0358. [DOI] [PubMed] [Google Scholar]
- 96.Tse FW, Tse A, Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc Natl Acad Sci U S A. 1994;91:9750–4. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaftan EJ, Xu T, Abercrombie RF, Hille B. Mitochondria shape hormonally induced cytoplasmic calcium oscillations and modulate exocytosis. J Biol Chem. 2000;275:25465–70. doi: 10.1074/jbc.M000903200. [DOI] [PubMed] [Google Scholar]
- 98.Hehl S, Golard A, Hille B. Involvement of mitochondria in intracellular calcium sequestration by rat gonadotropes. Cell Calcium. 1996;20:515–24. doi: 10.1016/s0143-4160(96)90094-9. [DOI] [PubMed] [Google Scholar]
- 99.Zemkova H, Balik A, Kretschmannova K, Mazna P, Stojilkovic SS. Recovery of Ins(1,4,5)-trisphosphate-dependent calcium signaling in neonatal gonadotrophs. Cell Calcium. 2004;36:89–97. doi: 10.1016/j.ceca.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Iida T, Stojilkovic SS, Izumi S, Catt KJ. Spontaneous and agonist-induced calcium oscillations in pituitary gonadotrophs. Mol Endocrinol. 1991;5:949–58. doi: 10.1210/mend-5-7-949. [DOI] [PubMed] [Google Scholar]
- 101.Tse A, Tse FW, Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994;477(Pt 3):511–25. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sartor P, Dufy-Barbe L, Corcuff JB, Taupignon A, Dufy B. Electrophysiological response to thyrotropin-releasing hormone of rat lactotrophs in primary culture. Am J Physiol. 1990;258:E311–9. doi: 10.1152/ajpendo.1990.258.2.E311. [DOI] [PubMed] [Google Scholar]
- 103.Sankaranarayanan S, Simasko SM. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. J Neurosci. 1996;16:1668–78. doi: 10.1523/JNEUROSCI.16-05-01668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barros F, del Camino D, Pardo LA, Palomero T, Giraldez T, de la Pena P. Demonstration of an inwardly rectifying K+ current component modulated by thyrotropin-releasing hormone and caffeine in GH3 rat anterior pituitary cells. Pflugers Arch. 1997;435:119–29. doi: 10.1007/s004240050491. [DOI] [PubMed] [Google Scholar]
- 105.Kukuljan M, Rojas E, Catt KJ, Stojilkovic SS. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994;269:4860–5. [PubMed] [Google Scholar]
- 106.Li YX, Stojilkovic SS, Keizer J, Rinzel J. Sensing and refilling calcium stores in an excitable cell. Biophys J. 1997;72:1080–91. doi: 10.1016/S0006-3495(97)78758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tse A, Tse FW, Almers W, Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993;260:82–4. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]