Abstract
Background
Skeletal muscle mass (SMM) can be extracted from whole-body scans obtained by X-ray-based dual-photon absorptiometry (DXA). There is a need to establish expected age-dependent values for children and adolescents.
Methods
Appendicular lean tissue mass (ALM) was extracted from whole-body DXA scans in 140 healthy children and adolescents (68 females and 72 males). Whole-body SMM was calculated from ALM using equations developed by Kim et al. (Am J Clin Nutr 84:1014–1020, 2006). Age-dependent patterns of increase in SMM were derived by fitting SMM values to equations that consisted of the sum of two logistic expressions, one accounting for SMM changes during growth and the other for SMM changes during puberty. Normal ranges were defined so that 95% of the SMM values were included. The reproducibility of SMM measurements was obtained from whole-body DXA scans repeated on three occasions in each of a separate group of 32 normal children with repositioning between scans.
Results
Normal ranges are presented as equations describing the age-dependent pattern of increase in SMM as well as population standard deviations that increased steadily with age. For 15 children below age 10, SMM reproducibility (95% CI) was 149 g (119–199 g) while for 17 children and adolescents over age 10, reproducibility was 170 g (138–223 g).
Conclusion
DXA-based measurements of SMM in children and adolescents are reproducible and can be expressed in terms of age-dependent Z scores.
Keywords: X-ray-based dual-photon absorptiometry, Whole-body scan, Skeletal muscle mass, Normal range, Reproducibility
Introduction
Heymsfield et al. [1] first showed in adults that ALM, the lean tissue mass in the arms and legs, when measured using radioisotope-based dual-photon absorptiometry (DPA) was strongly related to both total body potassium, estimated by whole-body counting of 40K, and to total body nitrogen measured by neutron activation. It followed that DPA could form the basis of a technique for the measurement of SMM, the component of muscle tissue that is used to effect locomotion and to maintain posture, especially since it was known that ALM constitutes between 73% and 75% of SMM. The technique was validated [2] when whole-body SMM was determined using MRI in a large group of healthy adults and compared with ALM measured by dual-energy X-ray absorptiometry (DXA). The derived relationship was whole-body SMM = (1.19 × ALM) − 1.01, and ALM explained 96% of the variance in whole-body SMM. This relationship was refined by accounting for the intermuscular adipose tissue that had been included previously so that the predictive relationship in adults was adjusted to become SMM = (1.19 × ALM) − 1.65 [3]. Again, 96% of the variance in whole-body SMM was explained by the variance in ALM.
The technique was extended to children by comparing MRI measures of SMM with DXA measures of appendicular lean tissue mass [4]. It was first shown that for adolescents at Tanner stage 5 and beyond, the adult predictive equation applied. However, for younger children, the adult equation was inadequate. Using regression techniques, a prediction model was developed in 65 children who were less than Tanner stage 5. The predictive equation for such children was SMM = (1.115 × ALM) − 1.135 [4]. This equation was tested in an independent sample of 18 children in whom it was found that ALM was still the strongest predictor of SMM accounting for 98% of the variance. Addition of weight and height as predictor variables added a small but significant improvement in SMM prediction.
The purpose of our study was to apply the above predictive equations in a population of normal Canadian children and adolescents in order to establish ranges for expected values of SMM. We also evaluated the reproducibility of SMM estimations from repeated measures of ALM in a separate group of children.
Materials and methods
Subjects and DXA measurement
Whole-body DXA scans were obtained using a Hologic densitometer (4500A or Discovery A) for 140 children and adolescents (68 females and 72 males) between the ages of 3.1 and 18.8 years. These ostensibly normal local children and adolescents were free of any known disease that might affect bone or body composition. Originally, these healthy volunteers had been recruited to establish normal reference ranges for whole-body areal bone mineral density (BMD) and body composition in Canadian children and adolescents [5]. A representative scan is shown in Fig. 1. Each scan was reanalyzed by a single investigator to ensure that regional markers were positioned systematically. All measurements had been approved by the Research Ethics Board of Hamilton Health Sciences and McMaster University, and all volunteers provided informed consent before any DXA scans were obtained.
Fig. 1.
Whole-body DXA scan in a healthy 13-year-old male showing positions of regional markers
Calculation of skeletal muscle mass
Skeletal muscle mass was measured using the predictive equations developed by Kim et al. [4]. In brief, from the whole-body scan, the lean tissue mass assigned to the arms and legs was summed. SMM was calculated for each child using one of the following relationships. For those at Tanner stage 5 and beyond, the equation was
| 1 |
For children yet to achieve Tanner stage 5, the equation was
| 2 |
For our whole-body DXA scans in volunteer normal children and adolescents, the Tanner stage was unknown. Based on the results of assessments of sexual maturation in US children [6], it was assumed that all children aged 16 and beyond had achieved Tanner stage 5 and that the first equation above was appropriate. The second equation was applied to all children aged 15 or below.
Normal ranges of skeletal muscle mass
The derived values of SMM were plotted as a function of age and gender. Equations analogous to those previously developed to describe the age dependence of whole-body bone mineral content, lean body mass, and fat mass in children and adolescents [5] were fitted to the SMM values. These equations had the form
| 3 |
where A–F are constants derived for each gender. The first term represents the steady increase in SMM associated with growth while the second term describes the rapid change in SMM associated with puberty. The constants A and D represent the respective contributions to the SMM at age 20 arising from growth and puberty. At younger ages, the growth contribution is modified by the exponential term that includes the age of the subject. The pubertal contribution is modified by the exponential term that includes postpubertal age (Age-F). Normal ranges were defined using the expression
| 4 |
where G and H are constants which were adjusted so that 95% of the SMM values were included within the normal range. The value 1.99 corresponds to the 95% probability of a two-tailed t distribution for 70 df.
Reproducibility of skeletal muscle mass
A separate group of 32 normal children and adolescents (18 females and 14 males) aged between 3.7 and 17.7 years underwent three consecutive whole-body DXA scans with repositioning between scans. These scans, which were reanalyzed by the same investigator to assess reproducibility of DXA-based estimates of SMM, had been used previously for the assessment of reproducibility of whole-body BMD and body composition in children and adolescents [7]. Ninety-five percent confidence intervals for reproducibility were calculated assuming a chi-square distribution for the individual variances found for each child [8].
Results
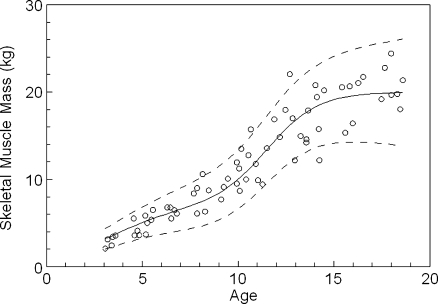
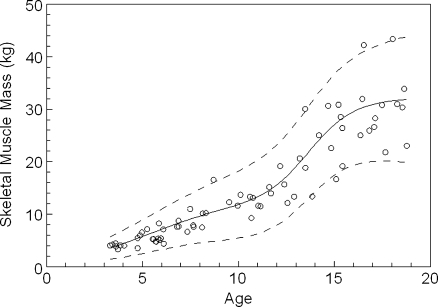
Figure 2 shows the calculated expected-for-age values of SMM for the 68 normal female children and adolescents. The values assigned to the parameters of the equation describing the pattern of increase in SMM with age in females are given in Table 1. The constants A and D reflect the increases in SMM that can be attributed to growth and to puberty respectively. In girls, approximately 65% of SMM was acquired during the pubertal growth spurt which occurred at around age 11.5 years (constant F). Figure 3 presents the corresponding results for the 72 normal male children and adolescents. The values assigned to the parameters of the equation describing the pattern of increase in SMM with age in males are given in Table 1. In boys, approximately 61% of SMM was acquired during the pubertal growth spurt which occurred at around age 13.7 years.
Fig. 2.
Derived skeletal muscle mass values for 68 normal female children and adolescents
Table 1.
Parameter values for the equations describing the age dependence of SMM for females and males
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Female | 7.0 | 6.5 | 0.55 | 13.0 | 0.75 | 11.5 |
| Male | 12.4 | 11.0 | 0.45 | 19.7 | 0.85 | 13.7 |
Fig. 3.
Derived skeletal muscle mass values for 72 normal male children and adolescents
The results for SMM reproducibility were considered separately for children above and below the age of 10 years. There were 15 younger children (nine females and six males). Their mean age (±standard deviation), height, and weight were 8.1 ± 1.6 years, 129.5 ± 10.7 cm, and 27.4 ± 6.1 kg, respectively. The precision for the younger group was 149 g with a 95% confidence interval of 119–199 g. The older group consisted of 17 children (nine females and eight males). Their mean age, height, and weight were 12.6 ± 2.0 years, 154.7 ± 15.3 cm, and 45.0 ± 12.5 kg, respectively. The precision for the older group was 170 g with a 95% confidence interval of 138–223 g. If all children were considered as a single group, the reproducibility was 161 g with a 95% confidence interval of 137–194 g.
Figure 2 also shows the normal range for SMM in girls. For one female, SMM was approximately 2 kg above the upper limit of the derived normal range while for another, SMM was about 2 kg below the lower limit. In total, three of 68 results (4.4%) were excluded from the selected normal range. As shown in Fig. 3, a similar outcome was obtained for males with four of 72 results (5.6%) excluded from the normal range. The values assigned to the parameters that define the upper and lower limits of the normal ranges are given in Table 2. The positive values of the constant H indicates a steadily expanding normal range as age increases with a more rapid expansion of the normal range in males.
Table 2.
Parameter values for the equations describing the age dependence of the normal range of values for SMM in female and male children and adolescents
| G | H | |
|---|---|---|
| Female | 0.10 | 0.16 |
| Male | 0.005 | 0.325 |
Discussion
A number of methods for the measurement of SMM in children have been evaluated with the objective of providing accurate, precise results in the clinical setting. Poortmans et al. [9] evaluated the merits of estimating SMM based on readily accessible variables in non-obese subjects. They measured SMM using the whole-body DXA technique in 39 children and 20 adults and examined regression relationships between the measured value and a predicted value based upon a group of variables that included height, age, and sex as well as skinfold thickness and limb circumference at each of the mid-arm, mid-thigh, and mid-calf sites; a coefficient of correlation (r 2 value) of 0.966 was observed. When the DXA-based SMM assessments were correlated with 24 h urine creatinine excretions in the same group of subjects, the r 2 value fell to 0.73. The predictive equations were not tested in an independent group of subjects.
Wang et al. [10] explored the correlation between MRI determined SMM and total body potassium determined from whole-body counting of the naturally occurring radioisotope 40K in 116 healthy children aged 5 to 17 years. SMM in children was shown to be a smaller fraction of total body potassium than in adults; in adults, the fraction is constant and independent of age [11]. Factors in addition to total body potassium that slightly improved the prediction of SMM in children were weight, height, and race.
The technique for the derivation of total body SMM from the mass of lean tissue in the arms and legs as measured by DPA was developed in adults [1] and was then extended to children [4]. The use of DXA scanning to measure ALM and hence to estimate SMM was validated by comparison with direct measures of SMM using whole-body MRI. DXA-based estimates of SMM are likely to be more acceptable than estimates of SMM based on whole-body MRI scans because of: (1) the difficulty of access to MRI scanners in comparison to DXA scanners; (2) the expense associated with MRI scanning; and (3) the need for image processing to segment MRI images.
The DXA-based technique for estimation of SMM has a reproducibility of 161 g. Expressed as a coefficient of variation, the reproducibility is 1.4%. However, it should be noted that expression of reproducibility in terms of a coefficient of variation is inappropriate, especially in children. When assessed for children below age 10, the reproducibility was 149 g; for children over age 10, the reproducibility was 170 g. When expressed as a CV, reproducibility in the younger children appeared to be worse at 1.8% compared to a value of 1.0% in the older children. However, despite an overlap of 95% confidence intervals for the standard deviations in the two groups of children, numerically, precision was better in the younger children. For a child aged 8, the age-expected value of SMM is about 8–10 kg. With a precision of 149 g, the uncertainty in the difference between two successive measurements will be (1492 + 1492)1/2 or 211 g. To be 95% confident that a change had occurred between the two measurements, the difference would have to exceed (1.97 × 211) or 413 g.
A gender specific, age-expected value of SMM for a child between the ages of 3 and 18 years (SMMpredicted) can be calculated using Eq. 3 with the appropriate values provided in Table 1. A measured value (SMMmeasured) can then be expressed in terms of a Z-score by deriving the appropriate, age-dependent standard deviation from the data given in Table 2 and using the following equation. That is,
| 5 |
The derived Z-score can be used to interpret a measurement of SMM in terms of an expected value based on age and gender.
A limitation of our study is that Eqs. 1 and 2 were developed using whole-body scans obtained from a Lunar Densitometer [10]. The whole-body DXA scans for our normal subjects were obtained from a Hologic Densitometer. Software differences between the two manufacturers will likely mean that the values of the slope and intercept in Eqs. 1 and 2 need to be adjusted for Hologic equipment. However, this will have little, if any, effect on the Z-scores calculated from Eq. 5 since the measured SMM and the SMM predicted for age will both include the same systematic error. Since the difference between these two variables is required for the Z-score calculation, the influence of the systematic error is minimized. This conclusion needs to be validated by comparing, in a group of children and adolescents, absolute SMM measured with whole-body MRI and SMM values determined from ALM measured on an Hologic densitometer. Another criticism might be that Tanner stages for our normal population were unknown. The influence of this effect can be estimated. If, for example, a 13-year-old female had a measured ALM of 17 kg, our procedure would have estimated her SMM from Eq. 2 as 17.82 kg. Since she was, in fact, at Tanner stage 5, Eq. 1 should have been used which would have yielded a SMM of 18.58 kg. The difference between these values is somewhat greater than the smallest detectable change for the DXA technique of SMM measurement but is very much smaller than the normal ranges established for boys and girls. Finally, it must be stressed that the prediction equations presented here apply only to children and adolescents below age 20 and do not account for the expected increases in SMM beyond that age.
This work has provided a means of interpreting DXA-based measurements of SMM in children and adolescents in terms of expected-for-age values. Our results also permit the interpretation of the significance of the differences between consecutive measurements of SMM.
Acknowledgments
The authors declare that they have no conflict of interest. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [12].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, Shen W, Freda PU, Heymsfield SB. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol. 2004;97:655–660. doi: 10.1152/japplphysiol.00260.2004. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Shen W, Gallagher D, Jones A, Wang Z, Wang J, Heshka S, Heymsfield SB. Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. Am J Clin Nutr. 2006;84:1014–1020. doi: 10.1093/ajcn/84.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sala A, Webber CE, Morrison J, Beaumont LF, Barr RD. Whole-body bone mineral content, lean body mass, and fat mass measured by dual-energy X-ray absorptiometry in a population of normal Canadian children and adolescents. Can Assoc Radiol J. 2007;58:46–52. [PubMed] [Google Scholar]
- 6.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 7.Leonard CM, Roza MA, Barr RD, Webber CE. Reproducibility of DXA measurements of bone mineral density and body composition in children. Pediatr Radiol. 2008;39:148–154. doi: 10.1007/s00247-008-1067-7. [DOI] [PubMed] [Google Scholar]
- 8.Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteop Inter. 1995;5:262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 9.Poortmans JR, Boisseau N, Moraine J-J, Moreno-Reyes R, Goldman S. Estimation of total-body skeletal muscle mass in children and adolescents. Med Sci Sports Exer. 2005;37:316–322. doi: 10.1249/01.MSS.0000152804.93039.CE. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Heshka S, Pietrobelli A, Chen Z, Silva AM, Sardinha LB, Wang J, Gallager D, Heymsfield SB. A new total body potassium method to estimate total body skeletal muscle mass in children. J Nutr. 2007;137:1988–1991. doi: 10.1093/jn/137.8.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zhu S, Wang J, Pierson RN, Heymsfield SB. Whole-body skeletal muscle mass: validation of estimates by total-body potassium—cellular level model. Am J Clin Nutr. 2003;77:76–82. doi: 10.1093/ajcn/77.1.76. [DOI] [PubMed] [Google Scholar]
- 12.Von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopemia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]