Abstract
Background
Cancer cachexia is a complex syndrome associated with multiple metabolic abnormalities. Insulin resistance is present in many cancer patients and may be one mechanism through which muscle wasting occurs.
Methods and results
The present review examines evidence in support of a role for insulin resistance in the development of muscle wasting during cancer cachexia and identifies areas for future research. Patients suffering from cancer cachexia tend to exhibit insulin resistance and improvements in insulin resistance have the potential to improve cachexia symptoms. In addition, evidence suggests that insulin resistance may occur prior to the onset of cachexia symptoms.
Conclusions
Further investigation of the role of insulin resistance in cancer cachexia is needed. The use of translational research in this area is strongly encouraged, and has important implications for clinical research and the treatment and prevention of cancer cachexia.
Keywords: Insulin resistance, Muscle wasting, Cancer cachexia, Animal models
Introduction
Individuals with cancer commonly experience anorexia, weight loss, and wasting of muscle and adipose tissue [1, 2]. These symptoms are also accompanied by a number of metabolic abnormalities, including alterations in carbohydrate, protein, and lipid metabolism, and insulin resistance [3, 4]. This syndrome, known as cachexia, greatly decreases the quality of life among patients, reducing survival time, and psychological and physical health [5]. In addition, cachectic cancer patients often display more negative side effects during chemotherapy [6, 7]. Some estimates suggest that up to 80% of cancer patients exhibit some degree of cachexia [6, 8, 9], making this a clinically relevant syndrome for which the cause is currently unknown.
While weight loss is obvious in the outward appearance, the specific loss of muscle mass may be most detrimental to patient health and outcomes. As skeletal muscle mass decreases, strength, energy, and quality of life also decline sharply [5, 10]. Muscle loss is associated with decreased mobility and independence, as well as increased rates of hospitalization [10]. In many cases, it is the loss of muscle mass, and not explicit body weight, that results in the final decline in functioning and death in cachectic cancer patients [5, 10].
Despite the devastating toll this syndrome has on patients, current treatment strategies are inadequate. In addition, the reversal of cachexia symptoms is difficult once they appear [10], making early intervention and prevention efforts key. Identifying those at risk for cachexia, however, is difficult. It is currently unknown why cancer cachexia occurs in some patients but not others, and across varying types of tumors. Our understanding of which factors are present prior to cachexia development, and which are only a result of cachexia, is poor. Thus, early treatment is rarely provided, contributing to a poor prognosis in many patients. Improvements in weight loss and appetite can be obtained through a number of treatments, including progesterones, glucocorticoids, and nutritional supplementation [11]. Unfortunately, gains in body weight are typically due to increased body fat and water retention, and such gains do not last [11]. While advances have been made in the treatment of muscle wasting associated with other chronic diseases, including congestive heart failure (CHF) and chronic obstructive pulmonary disease, the prevention of muscle wasting in individuals with cancer remains illusive.
Given the metabolic nature of cancer cachexia, the study of its mechanisms ought to include an evaluation of the influence of metabolic pathways. In particular, the presence of insulin resistance in many cancer patients and animal models of cancer cachexia warrants significant attention. For the purposes of the present investigation, insulin resistance is defined as a significant decrease in insulin sensitivity. Insulin sensitivity is commonly measured via a glucose tolerance test, in which a standard dose of glucose is administered and the ability of endogenous insulin to regulate blood glucose levels is monitored. Similarly, insulin tolerance testing has been utilized for this purpose, with the glucose response to a standard insulin dose measured. While insulin acts on many systems beyond those regulating of glucose homeostasis, such testing is often relied upon as a measure of peripheral insulin sensitivity and insulin resistance in health and disease.
Insulin and the control of skeletal muscle mass
Insulin is the main hormone responsible for the control of muscle proteolysis [12]. An increase in the availability of glucose in the blood, such as when a meal is consumed, triggers a release of insulin from pancreatic β cells. This increase in endogenous insulin concentration decreases circulating blood glucose levels and suppresses proteolysis [12]. Similarly, the infusion of physiologically relevant doses of insulin can produce a decrease in skeletal muscle protein degradation, as measured by the appearance of amino acids in local circulation, without any effect on blood glucose levels [13] or protein synthesis [14]. An infusion of a higher dose of insulin decreases blood glucose levels, with no further effect on protein degradation. Thus, insulin has the potential to regulate skeletal muscle mass within a limited physiological range of concentrations, primarily through alterations in protein degradation. When insulin sensitivity is compromised, skeletal muscle mass is adversely affected. Both human and animal studies have demonstrated that insulin resistance is present in other catabolic diseases, such as diabetes mellitus, AIDS, and CHF, in which significant muscle wasting is observed [15–22].
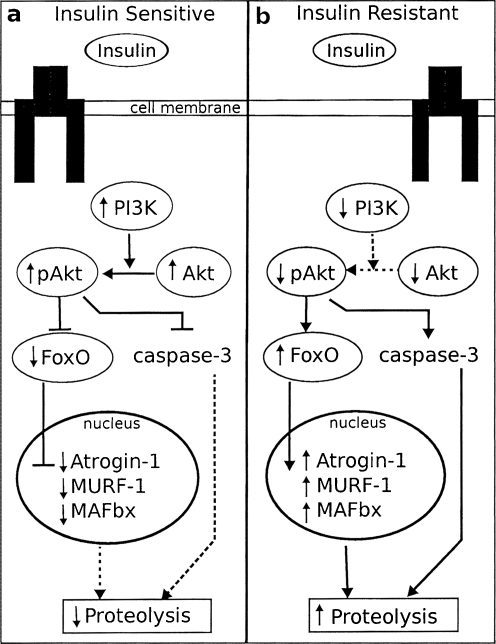
The mechanism through which muscle wasting occurs in cancer cachexia is the same as that which occurs in other catabolic diseases. Though a number of pathways contributing to muscle protein degradation may be affected in cancer cachexia, evidence suggests that the ATP-dependent ubiquitin–proteasome pathway (UPP) is particularly important, with the assistance of caspase-3 [23]. Importantly, the insulin signaling pathway activates a number of signaling molecules that overlap with the UPP [24], as illustrated in Fig. 1. Binding of insulin to its receptor activates phosphatidylinositol 3-kinase (PI3K) and Akt. The activation of Akt has been linked with suppression of FOXO and caspase-3 activity, as well as decreasing mRNA expression of atrogin-1 and MuRF-1, two important E3 enzymes in the UPP. However, when the activity of PI3K is decreased, as it is in cancer cachexia and other states of insulin resistance, the inhibition of both FOXO and caspase-3 is released and the expression of components of the UPP is increased [24]. It is through this pathway that insulin is able to control muscle protein degradation and one mechanism through which insulin resistance can result in increased protein degradation and the wasting of skeletal muscle.
Fig. 1.
Overlap between insulin signaling and ubiquitin–proteasome pathways in insulin sensitive and insulin resistant states. In insulin sensitive states (a), the binding of insulin to its receptor results in an increase in PI3K activity, which increases the phosphorylation of Akt. pAkt exerts inhibitory control over FoxO, which decreases transcription of Atrogin-1, MURF-1, and MAFbx, and caspase-3. This cascade results in decreased proteolytic activity. In contrast, in insulin-resistant states (b), PI3K activity is decreased, leading to decreased phosphorylation of Akt. Lower levels of pAkt release the inhibition of FoxO and caspase-3, resulting in increased proteolytic activity
The role of insulin resistance in cancer cachexia
Historically, insulin resistance has been classified as a consequence of muscle wasting during cancer cachexia. However, given insulin’s role in the maintenance of skeletal muscle, insulin resistance should be considered as a mechanism that contributes to the progression of muscle wasting during cancer cachexia.
Insulin resistance is present during cancer cachexia: Human subjects
The earliest known metabolic abnormality associated with cancer was glucose intolerance [25]. In 1956, Glickman and Rawson observed that approximately 37% of over 600 cancer patients exhibited “diabetic” glucose tolerance curves, with a blunted insulin response to a standard dose of glucose. This was the first large-scale study to document specific metabolic effects of cancer in the human population [26]. Since this time, additional studies have observed the presence of insulin resistance in patients with various types of tumors, as demonstrated by impaired glucose tolerance [27–33] and decreased insulin sensitivity [28, 29, 32].
Indirect evidence also suggests that alterations in glucose tolerance are associated with the symptoms of cachexia in cancer patients. Increased glucose production has been observed in lung cancer patients, who also present with increased rates of whole body protein turnover [30]. At least a portion of this increased rate of protein turnover was occurring within skeletal muscle, as these patients experienced increased excretion of 3-methyhistidine, a marker of skeletal muscle protein degradation [30]. Additional epidemiological evidence suggests that glucose intolerance is associated with an increased risk of cancer mortality [34]. For every 50 mg/dL increase in blood glucose levels, individuals surveyed as part of the NHANES III dataset increased their risk of cancer mortality by 22%.
When cachectic cancer patients, who present with anorexia, weight loss, and muscle wasting, were compared to non-cachectic patients and healthy controls by Jasani and colleagues [28], an interesting pattern emerged. Non-cachectic cancer patients exhibited a lower rate of glucose uptake than healthy controls. However, cachectic cancer patients exhibited significantly decreased rate of glucose uptake compared to both healthy controls and the well-nourished cancer patients [28]. While the non-cachectic cancer patients had poor glucose uptake, it was greater than that of the cachectic cancer patients, suggesting that an increasing severity of glucose intolerance could be involved in the development of cachexia.
Additional evidence suggests that impairments in oral and intravenous glucose tolerance may be present in cancer patients prior to the development of significant cachexia symptoms [31]. In sarcoma patients without a significant degree of weight loss (weight loss <5% of stable body weight), intravenous glucose tolerance tests revealed significantly impaired glucose tolerance, as compared to healthy controls. Interestingly, among the cancer patients in this study, those that maintained a lower body weight showed significantly reduced glucose tolerance as compared to patients with a higher body weight [31], suggesting the possibility that a lower maintenance weight may be a result of decreased insulin sensitivity in these patients. The authors conclude that glucose intolerance and accompanying insulin resistance may play a role in the development of cachexia symptoms, and is not merely a result of cachexia itself. However, it is unknown whether either group of cancer patients later developed cachexia symptoms, making the authors’ conclusions somewhat premature.
Taken together, these data support the involvement of insulin resistance in the development of muscle wasting in patients with cancer cachexia. Additional experimental evidence from animal models offers further support for this hypothesis.
Insulin resistance is present during cancer cachexia: Animal models
Insulin resistance is a common feature of multiple animal models of cancer cachexia, including the Walker 256 carcinoma and colon-26 adenocarcinoma [35–40]. In pancreatic islets of Langerhans isolated from rats bearing the Walker 256 carcinoma, insulin secretion is decreased in response to glucose stimulation, as compared to control islets, indicating impaired insulin sensitivity [36]. In addition, despite decreased blood glucose levels in colon-26 adenocarcinoma-bearing mice, insulin sensitivity is decreased as compared to non-tumor-bearing controls, as indicated by in vivo insulin tolerance testing [39]. Importantly, structural changes in pancreatic islets have not been observed, indicating that this decrease in insulin sensitivity is not caused by structural abnormality, but instead by deficits in insulin signaling [37].
Only one study directly examines the connection between insulin resistance and the onset of cancer cachexia [39]. By using an insulin tolerance test, Asp and colleagues demonstrated that tumor-bearing mice exhibited a significantly impaired blood glucose response to a standard dose of insulin, as compared to non-tumor-bearing controls, prior to the onset of significant weight loss. The authors assert that their results support a causal role for insulin resistance in the development of cancer cachexia [39]. However, the researchers failed to measure body composition near the time when reduced insulin sensitivity was observed. Therefore, it is unknown whether insulin resistance was actually present prior to the onset of cachexia or if some muscle wasting was present prior to the onset of insulin resistance. Despite this limitation, these results support the hypothesis that insulin resistance may contribute to the development of cancer cachexia in animal models.
Treatment with insulin and insulin sensitizers improves cachexia symptoms
Given the involvement of insulin in the regulation of skeletal muscle mass and the presence of insulin resistance during cancer cachexia, treatment strategies that utilize the insulin signaling system have begun to be explored. Evidence suggests that these treatments have a positive impact on patient outcomes and cachexia symptoms.
Lundholm and colleagues have observed improvements in cachectic cancer patients following the administration of insulin [41]. Importantly, these patients had reduced insulin sensitivity but were not fully unresponsive to endogenous insulin. When a small dose of exogenous insulin (0.11 U/kg body weight) was administered daily to cachectic cancer patients, increased body weight and caloric intake were observed. These changes lead to increased survival time and increased quality of life in insulin-treated patients, without having a stimulating effect on tumor growth [41]. These data demonstrate that insulin treatment may have beneficial effects in cachectic cancer patients. However, it is important to recognize that the sample population demonstrated some degree of insulin sensitivity. If the subjects exhibited a greater degree of insulin resistance, insulin treatment may not have produced the same effect.
Additional evidence from animal models of cachexia supports these findings. However, due to differences in the models utilized, results are mixed with regards to the effectiveness of insulin treatment in cancer cachexia and may be related to the degree of insulin sensitivity exhibited. Animals implanted with Walker 256 tumors show improvement in cachectic symptoms with daily insulin administration, including increased body weight and decreased tumor mass [42–44]. Similar results have been observed in other cancer cachexia models [45, 46]. In contrast, treatment with exogenous insulin failed to have any effect on cachexia-related parameters in mice inoculated with colon-26 adenocarcinoma [47]. It is possible that insulin resistance is more complete in these animals than in other models. This is supported by the fact that the administration of insulin also had no effect on blood glucose levels in the colon-26 tumor-bearing animals [47].
While insulin treatment fails to improve cancer cachexia symptoms in colon-26 adenocarcinoma, Asp and colleagues demonstrated the potential of an insulin-sensitizing agent to prevent the development of muscle wasting in this model of cancer cachexia. Following daily treatment with the thiazolidinedione (TZD) rosiglitazone (0.3 μL per gram of body weight, I.P), tumor-bearing mice did not experience weight loss or a decrease in muscle mass as compared to non-tumor-bearing controls. In addition, the TZD-treated animals demonstrated normal insulin sensitivity, with a significantly greater insulin-stimulated glucose response as compared to PBS-treated tumor-bearing mice [39].
The utility of these treatments within the larger human population is still under investigation. However, evidence suggests that the insulin signaling pathway may be utilized with some success in the treatment of cancer cachexia.
Potential treatment strategies
While the administration of insulin may ameliorate cachexia symptoms in some patients and animal models, the effect is not universal. In addition, many of these experiments have not examined the effect of insulin on skeletal muscle mass. However, treatments that more directly affect insulin signaling hold promise as useful treatment strategies. While still in the early stages of testing in individuals with and animal models of cancer cachexia, these agents, including metformin, thiazolidinediones, and β2-adrenoceptor agonists, demonstrate a preliminary ability to increase muscle mass in catabolic states through the activation of components of the insulin signaling pathway. These treatment strategies are highlighted in Table 1.
Table 1.
Potential treatment options in cancer cachexia
| Treatment | Mechanism of action | Effective in treating MW associated with | References |
|---|---|---|---|
| Metformin | Decreased hyperinsulinemia, increased insulin sensitivity in muscle, increased PI3K and AMPK activity | DMII, PCOS, CHF | 49, 53 |
| TZDs | PPAR-γ agonist, increased GLUT4, increased PI3K and AMPK activity | DMII, CHF, CC-Aa | 39, 51, 54, 56 |
| β2-adrenoceptor agonists | Increased GLUT4, increased PI3K activity, inhibition of caspase-3 | DMII, CC-Aa | 9, 11, 57, 62 |
Treatments, which utilize mechanisms related to the insulin signaling pathway, were found to be effective in treating muscle wasting associated with chronic disease
TZDs Thiazolidinedione drugs, MW Muscle wasting, DMII Type II diabetes mellitus, PCOS Polycystic ovary syndrome, CHF Congestive heart failure, CC-A Cancer cachexia in animal models
aNot tested in all animal models
Metformin is a biguanide drug that was originally developed for the treatment of insulin resistance associated with type II diabetes mellitus. Metformin has been shown to increase muscle cell insulin sensitivity [48] and to reverse muscle wasting in a number of catabolic states, including type II diabetes [49], polycystic ovary syndrome (PCOS) [50], and CHF [51]. The effect of metformin on muscle wasting appears to be regulated, in part, through its ability to increase activity of AMP-activated protein kinase (AMPK) in muscle cells [49]. An increase in AMPK activity leads to increased glucose transporter 4 (GLUT4) activity to increase skeletal muscle glucose uptake [52]. Additionally, metformin increases PI3K activity in patients with type II diabetes and PCOS [53]. The use of metformin in cancer cachexia has yet to be investigated fully, and deserves attention, given its effectiveness in other catabolic conditions.
Similarly, TZDs, which were originally developed for the treatment of patients with type II diabetes and have proven effective in reversing insulin resistance in this population [54], may offer a treatment path. While these insulin sensitizers stimulate peroxisome-proliferator-activated receptor γ, a nuclear transcription factor [54], they also appear to have effects on insulin receptor signaling [55]. In subjects with type II diabetes, treatment with the TZD rosiglitazone decreased hepatic glucose output and enhanced pancreatic β-cell function. In addition, rosiglitazone-treated subjects exhibit increased insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) and increased PI3K activity [56]. Similar results have been observed in patients with CHF [51]. The ability of this type of drug to increase insulin sensitivity through alterations in a pathway that overlaps with those involved in muscle proteolysis is intriguing. In addition, Asp and colleagues observed that chronic administration of rosiglitazone prevented muscle wasting associated with cancer cachexia in mice implanted with the colon-26 adenocarcinoma [39]. Due to their insulin-sensitizing effects and their ability to act directly on skeletal muscle tissue [56], the use of TZDs in the treatment of muscle wasting during cancer cachexia should be investigated further.
In addition, agonists of β2-adrenoceptors, such as clenbuterol, albutamol, and calmeterol, have shown promise in increasing skeletal muscle mass in animal models of type II diabetes and cancer cachexia [9, 11, 57–59]. Although this class of drugs has no effect on body weight or caloric intake [58], they demonstrate the remarkable ability to repartition nutrients, such that muscle mass increases preferentially over fat mass [59]. To reverse muscle wasting, these drugs appear to decrease protein degradation by inhibiting caspase-3, decreasing proteasomal activity, and reducing expression of various UPP-related genes [60]. In addition, clenbuterol improves insulin sensitivity through increases in GLUT4 and PI3K activity [59, 61]. Taken together, these data demonstrate the ability of a β2-adrenoceptor agonist to reduce muscle wasting in cancer cachexia, through a mechanism involving the UPP, as well as increase insulin sensitivity in insulin-resistant states.
Overall, metformin, TZDs, and β2-adrenoceptor agonists have been observed to improve muscle insulin resistance in catabolic conditions by utilizing elements of the insulin signaling pathway known to assist in the regulation of muscle mass. Given the effectiveness of insulin-sensitizing treatments in other catabolic conditions, the use of such treatments in patients with cancer cachexia warrants significant attention. In particular, their utility to reverse or prevent muscle wasting in cancer cachexia may prove particularly important in patient outcomes.
Conclusions and future directions
Available evidence suggests that insulin resistance may play a role in the development of muscle wasting during cancer cachexia. However, further research is necessary before this knowledge can be effectively applied to the treatment and prevention of cachexia in individuals with cancer. A number of recommended future research directions are highlighted in Table 2.
Table 2.
Future directions for research
| Insulin resistance in animal models of cancer cachexia | |
| Progression of insulin resistance during development of muscle wasting | |
| Comparison of insulin resistance across animal models of cachexia | |
| Effect of pre-existing insulin resistance on progression of cancer cachexia | |
| Cause(s) of insulin resistance in animal models of cancer cachexia | |
| Insulin resistance in human subjects with cancer cachexia | |
| Epidemiological studies of cachexia incidence in insulin resistant individuals with cancer | |
| Prospective measurement of insulin resistance and muscle status in individuals with cancer | |
| Cause(s) of insulin resistance in individuals with cancer | |
| Potential treatment options | |
| Analysis of the effects of insulin sensitizers on skeletal muscle mass in animal models and human subjects | |
| Effect of β2-adrenoceptor agonists on insulin sensitivity in animals models | |
| Effect of TZDs on existing muscle wasting in animal models |
Suggestions for future research regarding the relationship between insulin resistance and cancer cachexia
TZDs Thiazolidinedione drugs
This area of research has important potential implications for the clinical community. Currently, no evidence-based treatments are available for the treatment of cancer cachexia [11, 57]. Translational research is desperately needed in this area to determine the mechanisms through which cancer cachexia develops, as well as how these mechanisms may be exploited to prevent and treat cachexia symptoms in human cancer patients. Insulin resistance is easily screened for in a variety of healthcare settings and recent advances have allowed for better treatment of this condition in many individuals [62]. If insulin resistance could be utilized as an early signal of the development of cancer cachexia, the treatment of this metabolic abnormality could have the potential to prevent the development of cachexia symptoms and have a profound impact on morbidity and mortality in cancer patients.
Acknowledgments
This work was supported by National Institutes of Health DK078654 (KPK) and by the National Institutes of Health, National Cancer Institute R25CA128770 (D. Teegarden) Cancer Prevention Internship Program (MAH) administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University. The authors would like to thank Dr. Terry Powley and Dr. Terry Davidson for their comments and assistance. All authors of this manuscript comply with the guidelines of ethical authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [63].
Conflict of interest
The authors declare that they have no conflict of interest
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 2.Kolter DP. Cachexia. Ann Intern Med. 2000;133:622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 3.Argiles JM, Alvarez B, Lopez-Soriano FJ. The metabolic basis of cancer cachexia. Med Res Rev. 1997;17:477–498. doi: 10.1002/(SICI)1098-1128(199709)17:5<477::AID-MED3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Wasting in cancer. J Nutr. 1999;129:243S–246S. doi: 10.1093/jn/129.1.243S. [DOI] [PubMed] [Google Scholar]
- 5.Baracos VE. Hypercatabolism and hypermetabolism in wasting states. Curr Opin Clin Nutr Metab Care. 2002;5:237–239. doi: 10.1097/00075197-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Gordon JN, Green SR, Goggin PM. Cancer cachexia. Q J Med. 2005;98:779–788. doi: 10.1093/qjmed/hci127. [DOI] [PubMed] [Google Scholar]
- 7.Coats AJS. Treatment goals. In: Hofbauer KG, Anker SD, Inui A, Nicholson JR, editors. Pharmacotherapy of cachexia. Boca Raton, FL: CRC Press; 2006. pp. 261–266. [Google Scholar]
- 8.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 9.Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- 10.Winkler MF. Body composition changes in cancer cachexia: are they reversible? Top Clin Nutr. 2004;19:85–94. [Google Scholar]
- 11.Mantovani G, Maccios A, Massa E, Madeddu C. Managing cancer-related anorexia/cachexia. Drugs. 2001;61:499–514. doi: 10.2165/00003495-200161040-00004. [DOI] [PubMed] [Google Scholar]
- 12.Cahill GF, Aoki EE, Brennan MF, Muller WA. Insulin and muscle amino acid balance. Proc Nutr Soc. 1972;31:233–238. doi: 10.1079/PNS19720042. [DOI] [PubMed] [Google Scholar]
- 13.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biolo G, Wolfe RR. Insulin action on protein metabolism. Baillieres Clin Endocrinol Metab. 1993;7:989–1005. doi: 10.1016/S0950-351X(05)80242-3. [DOI] [PubMed] [Google Scholar]
- 16.Wing SS, The UPS. In diabetes and obesity. BMC Biochem. 2003;9:56. doi: 10.1186/1471-2091-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinol. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 18.Price SR, Bailey JL, Wang X, Jurkovitz C, England BK, Ding X, Phillips LS. Muscle wasting in insulinopenic rats results from activation of the ATP-dependent ubiquitin-proteasome proteolytic pathway by a mechanism involving gene transcription. J Clin Invest. 1996;98:1703–1708. doi: 10.1172/JCI118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol Cell Physiol. 1999;276:C1132–C1138. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Klein JD, Du J, Wang XH. Cardiac muscle protein catabolism in diabetes mellitus: activation of the ubiquitin-proteasome system by insulin deficiency. Endocrinol. 2008;149:5384–5390. doi: 10.1210/en.2008-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kino T, Mirani M, Alesci S, Chrousos GP. AIDS-related lipodystrophy/insulin resistance syndrome. Horm Metab Res. 2003;35:129–136. doi: 10.1055/s-2003-39072. [DOI] [PubMed] [Google Scholar]
- 22.Coats AJS, Anker S. Insulin resistance in chronic heart failure. J Cardiovasc Pharmacol. 2000;35:S9–S14. doi: 10.1097/00005344-200000004-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 24.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 25.Rohdenburg GL, Bernard A, Krehbiel O. Sugar tolerance in cancer. JAMA. 1919;72:1528–1530. doi: 10.1001/jama.1919.02610210024007. [DOI] [Google Scholar]
- 26.Glickman AS, Rawson RW. Diabetes and altered carbohydrate metabolism in patients with cancer. Cancer. 1956;9:1127–1134. doi: 10.1002/1097-0142(195611/12)9:6<1127::AID-CNCR2820090610>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Holroyde CP, Gabuzda TG, Putnam RC, Paul P, Reichard GA. Altered glucose metabolism in metastatic cancer. Cancer Res. 1975;35:3710–3714. [PubMed] [Google Scholar]
- 28.Jasani B, Donaldson LJ, Ratcliffe JG, Sokhi GS. Mechanism of impaired glucose tolerance in patients with neoplasia. Br J Cancer. 1978;38:287–292. doi: 10.1038/bjc.1978.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundholm K, Holm G, Schersten T. Insulin resistance in patients with cancer. Cancer Res. 1978;38:4665–4670. [PubMed] [Google Scholar]
- 30.Heber D, Chiebowski RT, Ishibashi DE, Herrold JN, Black JB. Abnormalities in glucose and protein metabolism in noncachectic lung cancer patients. Cancer Res. 1982;42:4815–4819. [PubMed] [Google Scholar]
- 31.Norton JA, Maher M, Wesley R, White D, Brennan MF. Glucose intolerance in sarcoma patients. Cancer. 1984;54:3022–3027. doi: 10.1002/1097-0142(19841215)54:12<3022::AID-CNCR2820541234>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Tayek JA. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J Am Coll Nutr. 1992;11:445–456. doi: 10.1080/07315724.1992.10718249. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa T, Noguchi Y, Doi C, Makino T, Okamoto T, Matsumoto A. Insulin resistance was connected with the alterations of substrate utilization in patients with cancer. Cancer Lett. 1999;141:93–98. doi: 10.1016/S0304-3835(99)00086-5. [DOI] [PubMed] [Google Scholar]
- 34.Parekh N, Lin Y, Hayes RB, Albu JB, Lu-Yao GL. Longitudinal associations of blood markers of insulin and glucose metabolism and cancer mortality in the third National Health and Nutrition Examination Survey. Cancer Cause Control. 2010;21:631–642. doi: 10.1007/s10552-009-9492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guaitani A, Recchia M, Carli M, Rocchetti M, Bartosek I, Garattini S. Walker carcinoma 256: a model for studies on tumor-induced anorexia and cachexia. Oncol. 1982;39:173–178. doi: 10.1159/000225631. [DOI] [PubMed] [Google Scholar]
- 36.Fernandes LC, Machado UF, Nogueira CR, Carpinelli AR, Curi R. Insulin secretion in Walker 256 tumor cachexia. Am J Physiol Endocrinol Metab. 1990;258:E1033–E1036. doi: 10.1152/ajpendo.1990.258.6.E1033. [DOI] [PubMed] [Google Scholar]
- 37.el Razi Neto SER, Zorn TMT, Curi R, Carpinelli AR. Impairment of insulin secretion in pancreatic islets isolated from Walker 256 tumor-bearing rats. Am J Physiol Cell Physiol. 1996;271:C804–C809. doi: 10.1152/ajpcell.1996.271.3.C804. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, et al. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:2290–2295. [PubMed] [Google Scholar]
- 39.Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer. 2009;126:756–763. doi: 10.1002/ijc.24784. [DOI] [PubMed] [Google Scholar]
- 40.Lazarus DD, Destree AT, Mazzola LM, McCormack TA, Dick LR, Xu B, Huang JQ, Pierce JW, et al. A new model of cancer cachexia: contribution of the ubiquitin-proteasome pathway. Am J Physiol Endocrinol Metab. 1999;277:E332–E341. doi: 10.1152/ajpendo.1999.277.2.E332. [DOI] [PubMed] [Google Scholar]
- 41.Lundholm K, Korner U, Gunnebo L, Sixt-Ammilon P, Pouladiun M, Daneryd P, Bosaeus I. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res. 2007;13:2699–2706. doi: 10.1158/1078-0432.CCR-06-2720. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes LC, Carpinelli AR, Hell NS, Curi R. Improvement of cancer cachexia and decrease of Walker 256 tumor growth by insulin administration in rats. Cancer Ther Control. 1991;1:259–268. [Google Scholar]
- 43.Fernandes LC, Curi R. Reversion of Walker 256 tumor cachexia by insulin treatment: possible mechanisms involved and perspectives for future research. Endocr Relat Cancer. 1997;4:465–474. doi: 10.1677/erc.0.0040465. [DOI] [Google Scholar]
- 44.Piffar PM, Fernandez R, Tchaikovski O, Hirabara SM, Folador A, Pinto GJ, Jakobi S, Gobbo-Bordon D, et al. Naproxen, clenbuterol, and insulin administration ameliorates cancer cachexia and reduce tumor growth in Walker 256 tumor-bearing rats. Cancer Lett. 2003;201:139–148. doi: 10.1016/S0304-3835(03)00472-5. [DOI] [PubMed] [Google Scholar]
- 45.Moley JF, Morrison SD, Norton JA. Insulin reversal of cancer cachexia in rats. Cancer Res. 1985;45:4925–4931. [PubMed] [Google Scholar]
- 46.Moley JF, Morrison SD, Gorschboth CM, Norton JA. Body composition changes in rats with experimental cancer cachexia: improvement with exogenous insulin. Cancer Res. 1988;48:2784–2787. [PubMed] [Google Scholar]
- 47.Lazarus DD, Kambayashi T, Lowry SF, Strassmann G. The lack of an effect by insulin or insulin-like growth factor-1 in attenuating colon-26 mediated cancer cachexia. Cancer Lett. 1996;103:71–77. doi: 10.1016/0304-3835(96)04197-3. [DOI] [PubMed] [Google Scholar]
- 48.Komer R, Vrana A. Thiazolidinediones: tools for the research of metabolic syndrome X. Physiol Res. 1998;47:215–225. [PubMed] [Google Scholar]
- 49.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Willimason JM, et al. Metformin increased AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 50.Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eu J Endocrinol. 2010;162:193–212. doi: 10.1530/EJE-09-0733. [DOI] [PubMed] [Google Scholar]
- 51.Wong AKF, al Zadjali MA, Choy AJ, Lang CC. Insulin resistance: a potential new target for therapy in patients with heart failure. Cardiovasc Ther. 2008;26:203–213. doi: 10.1111/j.1755-5922.2008.00053.x. [DOI] [PubMed] [Google Scholar]
- 52.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 53.Diamanti-Kandarakis E, Economou F, Palimeri S, Christakou C. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:192–198. doi: 10.1111/j.1749-6632.2010.05679.x. [DOI] [PubMed] [Google Scholar]
- 54.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 55.Miyazaki Y, He H, Mandarino LJ, DeFronzo RA. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes. 2003;52:1943–1950. doi: 10.2337/diabetes.52.8.1943. [DOI] [PubMed] [Google Scholar]
- 56.Kintscher U, Law RE. PPARγ-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab. 2005;288:E287–E291. doi: 10.1152/ajpendo.00440.2004. [DOI] [PubMed] [Google Scholar]
- 57.Gagnon B, Bruera E. A review of the drug treatment of cachexia associated with cancer. Drugs. 1998;55:675–688. doi: 10.2165/00003495-199855050-00005. [DOI] [PubMed] [Google Scholar]
- 58.Lambert CP, Uc EY, Evans WJ. β2-adrenergic agonists in the treatment of muscle atrophy. In: Hofbauer KG, Anker SD, Inui A, Nicholson JR, editors. Pharmacotherapy of cachexia. Boca Raton, FL: CRC Press; 2006. pp. 3311–3324. [Google Scholar]
- 59.Castle A, Yaspelkis BB, Kuo C, Ivy JL. Attenuation of insulin resistance by chronic β2-adrenergic agonist treatment: possible muscle specific contributions. Life Sci. 2001;69:599–611. doi: 10.1016/S0024-3205(01)01149-3. [DOI] [PubMed] [Google Scholar]
- 60.Busquet S, Figueras MT, Fuster G, Almendro V, Moore-Carraso R, Ametller E, Argiles JM, Lopez-Soriano FJ. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 2004;64:6725–6731. doi: 10.1158/0008-5472.CAN-04-0425. [DOI] [PubMed] [Google Scholar]
- 61.Nevzorova J, Evans BA, Bengtsson T, Summers RJ. Multiple signaling pathways involved in β2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br J Pharmacol. 2006;147:446–454. doi: 10.1038/sj.bjp.0706626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matthael S, Stumvoli M, Kellerer M, Haring H. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. 2000;21:585–618. doi: 10.1210/er.21.6.585. [DOI] [PubMed] [Google Scholar]
- 63.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachex Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]