Abstract
Body size, particularly large, is a matter of concern among the lay public. Whether this is justified depends upon the state of health and should be judged individually. For patients with established chronic disease, there is sufficient evidence to support the benefits of large body size, i.e., the obesity paradox. This uniform finding is shared over a variety of cardiovascular, pulmonary, and renal diseases and is counterintuitive to the current concepts on ideal body weight. The scientific community has to increase the awareness about differences for optimal body size in health and disease. Simultaneously, clinicians have to be aware about body weight dynamics implications and should interpret the changes in the context of an underlying disease in order to implement the best available management.
Perception of body size throughout history
Life expectancy and survival throughout mankind’s history was and remains dependent upon ability to cope with harmful stimuli. The human body itself developed very sophisticated defense mechanisms, which, however, are primarily based on rather primitive responses like inflammation, neurohormonal, and sympathetic nervous system activation. Vital for all processes is energy, which is derived from fat, proteins, and carbohydrates. Energetic efficacy of life organisms, although highest known in nature, is about 30% while the rest is lost primarily through heat production. Chronic food shortage and malnutrition have been the scourge of humankind throughout history. This has led to development of safety measures to accumulate energy when available to bridge over times of need. As a matter of fact, an evolutionarily conserved gene family important for fat depots, which store twice the amount of energy as carbohydrates or proteins, have recently been identified [1]. Until about a century or two ago, this gene family served its purposes for the majority of the world’s population. Nowadays, when abundant food is available all over the year, activation may be prolonged to lead to significant increase of body weight. It is therefore not surprising that attitudes of humankind towards body size changed throughout centuries. Until recently, large body size reflected wealth and wellbeing, and it was reserved for very few in the community or population. Nowadays, the other extreme is preferred and people are willing to deliberately reshape their body primarily due to aesthetic impulses, and less often due to health concerns. Interesting in this context are differences between religions in their perception and presentation of goddesses: while thin stature was associated with lack and suffering, large body size with abundance and joy. Similar observations stem from arts, literature, and medical opinion of the times, when corpulence meant something good and desirable.
Obesity and Quetelet index
Obesity in lay public is usually associated with large body size. For clinical practice and research purposes, reliable definitions are needed but are not always available. The Quetelet index is the ratio between body weight in kg and square of body height in meters. It was first described by the Belgian polymath Adolphe Quetelet during the course of developing “social physics”, which would likely correspond to current understanding of epidemiology [2]. The current name, body mass index (BMI), dates to 1972, when it was used as an estimation of body fat [3]. Thereafter, it was adopted for use in daily practice and also by the World Health Organization. According to its current definition, people with BMI <18.5 kg/m2 are considered underweight while those with BMI over 30 and 40 kg/m2 are obese and morbidly obese, respectively. These ranges are based on healthy populations and are valid only as statistical categories, a fact that is largely being ignored. International variations exist and some adjustments are needed for specific populations [4].
Body size in health and disease: bad gone good or obesity paradox
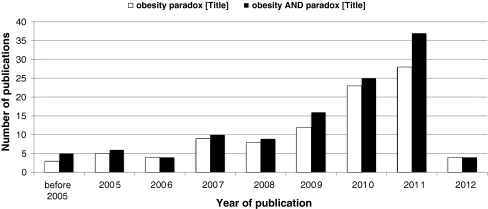
Only with sufficient and accessible food, together with modern way of life and little or no physical activity, obesity increased in prevalence to reach epidemic proportions [5]. Obesity is today considered as a chronic disease and is listed in the International Classification of Disease (ICD-9: E66.9). It is linked with increased mortality, primarily through development of cardiovascular disease [6]. Consequently, people are much more worried by weight gain than weight loss, and dieting is frequently used to obtain a “healthy weight”. Epidemiological studies, however, have demonstrated that aging attenuates association between high BMI and increased risk of death [7]. In contrast to common public beliefs and guidelines for primary preventive medicine, high BMI confers protective effects for patients with established chronic diseases. Although this seemingly paradoxical message penetrates the public opinion only slowly, evidence is starting to accumulate. About a decade ago, the first publication using “obesity paradox” in the article title was published [8]. A PubMed search of February 6th, 2012 identified 96 publications that have used the exact phrase in their title, and 138 used both words in any combination (Fig. 1). Most studies used BMI to evaluate body size in relation to all-cause mortality. The message derived from small- to large-scale studies is uniform and suggests that an optimal BMI with lowest risk of death is in the overweight or even obese category. Table 1 summarizes some of the most important publications about the obesity paradox in chronic cardiovascular, pulmonary, and kidney disease as well as in intensive care patients [9–19]. In a recent study, the presence of an obesity paradox has been observed in patients with diabetes mellitus plus cardiovascular comorbidity [19]. The latter study in particular provides evidence in contrast to common thinking and to current guideline recommendations. Notably, these recommendations are commonly based on mere translation from primary prevention data and may not be applicable in patient populations with established chronic diseases. We believe that an intellectual exercise recently performed for heart failure [20] is valid for many chronic diseases. Rather than merely repeating it over a plethora of conditions, we should bring the current knowledge and experience to a next level. While the relevant clinical question of weight management in overweight and obese chronic disease patients remains unanswered, we certainly should tailor our management individually. Generally, non-edematous and particularly non-voluntary weight loss should be recorded and managed aggressively. If overlooked or ignored, a vicious circle of body wasting and eventually cachexia may ensue [21]. Even obese patients are vulnerable for such scenario, which is yet another obesity paradox to be explored in the currently ongoing Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) [22]. In real life, nothing is coincidental and you always reach a pay-off stage. Obesity-induced symptoms or syndromes like sleep-disordered breathing in heart failure [23, 24] obviously drive the trade-off between weight loss and obesity paradox and are yet another example of balancing in clinical practice. And this is where the long forgotten and neglected art of medicine should be revived [25]; to help us balance between thin and obese, between health and disease and the other way around.
Fig. 1.
PubMed search for publications with “obesity paradox” individual words or a phrase in title
Table 1.
Large-scale studies about obesity paradox in chronic disease
| Condition and study/country | N (% men) | Age | Main finding |
|---|---|---|---|
| Heart failure | |||
| Acute | 108,927 (49%) | 72 ± 14 years | Inhospital mortality decreased from 5.0% to 2.2% per BMI quartiles The mortality OR for obese, overweight, and underweight vs healthy weight was 0.74 (95% CI 0.68–0.81), 0.83 (95% CI 0.76–0.90), and 1.34 (95% CI 1.15–1.58), respectively |
| (ADHERE registry) [9] | |||
| Chronic | 7,767 (75%) | 64 ± 11 years | Higher BMI associated with lower mortality risk: adjusted HR for all-cause death for obese or overweight vs healthy weight patients was 0.81 (95% CI 0.72–0.92) and 0.88 (95% CI 0.80–0.96), respectively |
| (USA) [10] | |||
| Coronary artery disease | |||
| Coronary artery disease and hypertension | 22,576 (NA) | 66 ± 10 years | With normal weight subjects as reference, overweight and obese patients had better (HR 0.52–0.66, p < 0.001) and thin patients had worse survival (HR 1.85, p < 0.001) |
| (INVEST) [11] | |||
| Percutaneous coronary intervention | 4,880 (70%) | Median age 62 years | Patients with BMI 27.5–30 kg/m2 had lowest adjusted HR for death (0.59, 95% CI 0.39–0.90) |
| (Germany) [12] | |||
| Coronary artery bypass grafting | 22,599 (72%) | 63 ± 10 years | Lowest RR for 30-day mortality in patients with BMI of 33 kg/m2, patients with BMI < 22 kg/m2 had significantly higher RR for death |
| (Germany) [13] | |||
| Stroke | 2,785 (62%) | Mean age 70 years | Obese and overweight patients had significantly higher early (1 week and 1 month) and long-term (10 years) survival when compared to patients with normal BMI (p < 0.001 for all) |
| (Greece) [14] | |||
| Intensive care unit | 9,935 (64%) | 63 ± 15 years | Overweight (HR 0.86, 95% CI 0.74–0.99) and obese (HR 0.83, 95% CI 0.69–0.99) patients had lower 60-day inhospital mortality. |
| (Germany) [15] | |||
| Chronic obstructive pulmonary disease | |||
| Acute exacerbation | 968 (72%) | 70 ± 9 years | In an adjusted model, BMI per 1 kg/m2 unit increase was associated with 5% less chance of death (hazard ratio 0.95, 95% confidence interval 0.93–0.97) |
| (Slovenia) [16] | |||
| Stable disease | 2,132 (57%) | 56 ± 11 years | Adjusted RR for death in underweight vs normal weight patients was 1.64 (95% CI 1.20–2.23) in men and 1.42 (95% CI 1.07–1.89) in women |
| (Copenhagen city heart study) [17] | |||
| Chronic kidney disease | 121,762 (54%) | 63 ± 15 years | Higher BMI (optimal range 40–45) was independently associated with better survival after adjustment for available surrogates of nutritional status and inflammation |
| (DaVita dialysis facilities, USA) [18] | |||
| Diabetes plus cardiovascular disease | 5,202 (66) | 62 ± 7 years | Higher BMI (optimum range 30–35 kg/m2) was associated with lower mortality and hospitalization. Weight loss but not weight gain was predictive of increased mortality. |
| PROactive Study [19] | |||
BMI body mass index, OR odds ratio, HR hazard ratio; RR relative risk; 95% CI 95% confidence interval
Clinical implications
BMI is an easily accessible parameter that carries important information for patient risk stratification, and the so-called obesity paradox relates to beneficial effects of large body size in terms of mortality. The risks and benefits of overweight or obese BMI categories in healthy individuals and chronic disease patients, however, is diametrically different, and, thus, public and also medical healthcare providers’ perception has to be modified accordingly.
Acknowledgments
The authors of this manuscript certify that they comply with the Principles of Ethical Publishing in the Journal of Cachexia, Sarcopenia, and Muscle [26].
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kadereit B, Kumar P, Wang WJ, Miranda D, Snapp EL, Severina N, et al. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci USA. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eknoyan G. Adolphe Quetelet (1796–1874)—the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- 3.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 4.Shiwaku K, Anuurad E, Enkhmaa B, Nogi A, Kitajima K, Shimono K, et al. Overweight Japanese with body mass indexes of 23.0–24.9 have higher risks for obesity-associated disorders: a comparison of Japanese and Mongolians. Int J Obes Relat Metab Disord. 2004;28:152–158. doi: 10.1038/sj.ijo.0802486. [DOI] [PubMed] [Google Scholar]
- 5.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 6.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 8.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/S0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. ADHERE Scientific Advisory Committee and Investigators. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-DeHoff RM, Zhou Q, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Hastie CE, Padmanabhan S, Slack R, Pell ACH, Oldroyd KG, Flapan AD, et al. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:222–226. doi: 10.1093/eurheartj/ehp317. [DOI] [PubMed] [Google Scholar]
- 13.Potapov EV, Loebe M, Anker SD, Stein J, Bondy S, Nasseri BA, et al. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart J. 2003;24:1933–1941. doi: 10.1016/j.ehj.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, et al. Association between obesity and mortality after acute first-ever stroke: the obesity–stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 15.Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F, et al. The obesity paradox in surgical intensive care units. Intensive Care Med. 2011;37:1793–1799. doi: 10.1007/s00134-011-2321-2. [DOI] [PubMed] [Google Scholar]
- 16.Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2:81–86. doi: 10.1007/s13539-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2011. doi:10.1016/j.ijcard.2011.09.039. [DOI] [PubMed]
- 20.Anker SD, von Haehling S. The obesity paradox in heart failure: accepting reality and making rational decisions. Clin Pharm Ther. 2011;90:188–190. doi: 10.1038/clpt.2011.72. [DOI] [PubMed] [Google Scholar]
- 21.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Haehling S, Lainscak M, Doehner W, Ponikowski P, Rosano G, Jordan J, et al. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) J Cachexia Sarcopenia Muscle. 2010;1:187–194. doi: 10.1007/s13539-010-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V, et al. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail. 2011;13:68–75. doi: 10.1093/eurjhf/hfq183. [DOI] [PubMed] [Google Scholar]
- 24.Lainscak M, Blue L, Clark AL, Dahlström U, Dickstein K, Ekman I, et al. Self-care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:115–126. doi: 10.1093/eurjhf/hfq219. [DOI] [PubMed] [Google Scholar]
- 25.Reng CM, Konrad S, Schölmerich J. The art of medicine. Med Klin. 2003;98:672–678. doi: 10.1007/s00063-003-1326-1. [DOI] [PubMed] [Google Scholar]
- 26.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]