Abstract
Background
Cancer cachexia is characterized by loss of both adipose and skeletal muscle tissue and by an increased production of proinflammatory cytokines. Ursodeoxycholic acid (UDCA), a bile acid used for centuries in the treatment of liver disease, is known to confer anti-inflammatory and anti-apoptotic effects as well as beneficial effects on mitochondrial integrity and cell signaling. We hypothesized that UDCA ameliorates the wasting process in the Yoshida hepatoma tumor model. In addition, we sought to establish if UDCA exerts beneficial effects on survival in this model.
Methods and results
Forty-seven male rats were inoculated intraperitoneally with 108 Yoshida hepatoma AH-130 cells and treated with placebo or one of two different doses of UDCA, 25 or 100 mg/kg daily. Body weight, body composition, and activity indicators were measured over the course of study up to day 16. UDCA treatment had no effect on tumor growth, loss of body weight, and loss of fat mass. Compared with placebo, low-dose UDCA improved tissue loss in the lung (p = 0.022) and tended to reduce tissue loss in brown adipocytes (p = 0.06), gastrocnemius muscle (p = 0.06), extensor digitorum longus muscle (p = 0.09), and soleus muscle (p = 0.07). Compared with placebo, high-dose UDCA tended to reduce the loss of lean body mass (p = 0.06), lung tissue (p = 0.1), white adipose tissue (p = 0.11), and gastrocnemius muscle (p = 0.11). The activity and food intake were not altered in tumor-bearing rats by either dose of UDCA. Both doses tended to decrease the mortality rate in tumor-bearing rats, (hazard ratio (HR), 0.42; 95% confidence interval (CI), 0.17–1.04; p = 0.061 for low-dose UDCA; HR, 0.44; 95% CI, 0.18–1.05; p = 0.065 for high-dose UDCA).
Conclusion
UDCA treatment in the Yoshida hepatoma model showed a trend towards attenuation of tissue loss in animals with progressive weight loss in cancer cachexia. Tumor growth and activity indicators were not altered. Both doses of UDCA tended to reduce the mortality rates in tumor-bearing animals. Larger studies with longer follow-up are required to verify these findings.
Keywords: Cancer cachexia, Ursodeoxycholic acid, Wasting, Yoshida hepatoma animal model
Introduction
Alterations in body composition are a frequent clinical finding in patients with malignant cancer. Muscle loss with ensuing decrease in strength may develop even before weight loss becomes evident [1]. Cachexia as a terminal stage of body wasting is defined as a “complex metabolic syndrome associated with underlying illness and characterized by” weight loss as a consequence of muscle loss with or without loss of fat mass [2]. The prevalence of cachexia among patients with malignant cancer ranges between 20% and 80% during advanced disease stages, dependent on the primary tumor site, co-morbidites, and the presence or absence of metastases [3]. Furthermore, cachexia is associated with anorexia, systemic inflammation, and a reduced quality of life as a consequence of restricted mobility and a higher morbidity [2, 4, 5]. Muscle wasting occurs by activation of proteolytic systems, mainly the ubiquitin proteasome pathway, and apoptosis [4].
Ursodeoxycholic acid (UDCA) is a tertiary bile acid that has been used for centuries in the clinical treatment of gallstones, primary biliary cirrhosis, and primary sclerosing cholangitis [6–8]. It is known that UDCA suppresses secondary bile acids (cholic acid)-induced colonic carcinogenesis [9, 10]. UDCA exerts anti-inflammatory effects by inhibiting the NF-κB pathway and therefore reducing the expression of tumor necrosis factor alpha (TNF-α) [7, 11, 12]. In addition, UDCA has anti-apoptotic action mediated by preventing damage of the mitochondrial membrane, release of ROS and consequently inhibiting the induction of transcription factors responsible for proliferation and apoptosis [6, 12–15]. Here, we hypothesized that the effects of UDCA may translate into beneficial effects in an experimental cachexia model using the Yoshida hepatoma model.
Methods
Study design
Fifty-seven 7 weeks old male Wistar Han rats weighing approximately 200 g were used. Animals were housed in groups of three, at a constant temperature of 22°C and exposed to a 12-h light cycle. Animals had free access to food and water. On day 1, 47 rats were inoculated intraperitoneally with 108 exponentially growing Yoshida hepatoma AH-130 cells [16]. Blood samples and organ collection were scheduled for day 16 but had to be performed earlier in 35 animals because ethical standards (apathy, reduced body temperature, and disturbed blood flow) ruled out killing. All organs were weighted after killing the animals. In addition, we assessed body weight and body composition before tumor implantation and on the day of killing. Quality-of-life indicators (spontaneous activity and food and water intake) were measured on days 0 and 10/11. Tumor cells were counted on the last study day.
Treatment with UDCA
Rats were randomized to control (n = 10), placebo (n = 28), 25 mg kg−1 day−1 UDCA (n = 9), and 100 mg kg−1 day−1 UDCA (n = 10). UDCA was purchased as Ursofalk suspension from Dr. Falk Pharma GmbH, Freiburg, Germany. Treatment with UDCA or placebo started 1 day after tumor inoculation and was then given daily by gavage until the end of the study.
Body composition
Body composition (lean mass, muscle mass, water content, and fluids) was analyzed using an EchoMRI-700 (Echo Medical Systems, Houston, Texas, USA). The rat was put in a tube so that it could not move during the measurement which takes 90 s. The analysis of the body structures are based on nuclear magnetic resonance which measures the resonance of magnetic active nuclei in the tissue.
Quality-of-life indicators
Spontaneous activity and food intake are indicators for quality of life [17]. Animals were housed individually with 100 g of food, and their movement was measured by an infrared scanner over 24 h with the Supermex Locomotor System (Muromachi Kikai Co., Ltd., Tokyo, Japan).
Statistics
Data were analyzed with GraphPad PRISM 5.0 (GraphPad Software, Inc, La Jolla, CA, USA). Results are shown as mean ± SEM. Data were analyzed for normal distribution using the Kolmogorov–Smirnov test. If there was a normal distribution, the data were analyzed with analysis of variance followed by Student’s t test while data without normal distribution were analyzed by Kruskal–Wallis and Mann–Whitney U test. Survival was tested by Cox proportional hazard analysis and hazard ratio (HR) and 95% confidence interval (CI) are shown. A p value of <0.05 was considered significant.
Results
We studied 57 animals; 35 of them had to be killed before day 16 because they reached ethical endpoints (day 11, n = 9; day 12, n = 10; day 13, n = 8; day 14, n = 5; and day 15, n = 3). UDCA treatment did not have an effect on the growth of the Yoshida hepatoma tumor. At the end of the study or earlier in cases that animals had to be killed, total tumor cell numbers were 2.04 ± 0.26 × 109 cells in placebo-treated animals, 1.95 ± 0.49 × 109 cells in animals that received 25 mg kg−1 day−1 of UDCA and 2.08 ± 0.46 × 109 cells in animals that received 100 mg kg−1 day−1 of UDCA (p > 0.5 both doses UDCA vs. placebo). There were no observations of other malignancies during necropsy.
Body weight and body composition
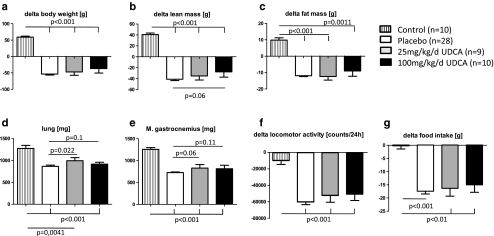
Control animals gained body weight (+59.30 ± 2.80 g), lean body mass (+40.70 ± 2.61 g), and fat mass (+9.83 ± 1.41 g). Average loss in body weight was highest in the placebo group (−53.36 ± 3.06 g), which means 35.4% and was slightly but not statistically significantly reduced in both groups with UDCA treatment (−47.22 ± 10.02 g for 25 mg kg−1 day−1 UDCA, 28.6%; −36.6 ± 13.79 g for 100 mg kg−1 day−1 UDCA, 21.6%; Fig. 1a). The loss of lean mass was slightly reduced by giving high-dose UDCA (−27.53 ± 9.73 g, p = 0.06 vs. placebo; Fig. 1b), and the loss of fat mass was not statistically significantly altered by treating tumor-bearing animals with UDCA (p > 0.5 both doses vs. placebo; Fig. 1c).
Fig. 1.
Change of body weight (a), lean body mass (b), and fat mass (c): control animals gained weight and placebo animals lost significantly body weight, lean body mass, and fat mass (p < 0.001 vs. control); 100 mg kg−1 day−1 UDCA reduced loss of body weight and fat mass but not significantly. Furthermore, the high dose of UDCA prevents slightly the loss of lean mass (p = 0.06 vs. placebo). Weight of lung (d) and GC (e): 25 mg kg−1 day−1 UDCA improved significantly the weight of the lung (p = 0.022 vs. placebo). The same dose improved the weight of the GC, but not significantly (p = 0.06 vs. placebo). Quality-of-life indicators: both doses of UDCA did not have an effect on spontaneous activity (f) and food intake (g)
Organ weights
Tumor-bearing rats lost significantly weight in nearly all analyzed organs compared with control animals. Treatment with both doses of UDCA did not attenuate the weight loss in the heart, liver, spleen, kidney, fat tissue, and tibialis muscle (Table 1). Treatment of cachectic animals with 25 mg kg−1 day−1 UDCA improved the weight of the lung significantly (p = 0.022 vs. placebo; Fig. 1d), and there was a trend towards an increase in the weight of brown adipose tissue (BAT), gastrocnemius muscle (GC; Fig. 1e), extensor digitorum longus muscle (EDL), and soleus muscle (p = 0.06 for BAT and GC, p = 0.09 for EDL, and p = 0.07 for soleus muscle; low-dose UDCA vs. placebo; Table 1). High-dose UDCA attenuated somewhat but not significantly the weight loss of the lung, white adipose tissue (WAT) and GC (p = 0.1 for lung, p = 0.11 for WAT and GC vs. placebo; Table 1 and Fig. 1e).
Table 1.
Organ and tissue weights at day 16 (end of study) or day of death
| Organ weight (mg) | Control (n = 10) | Placebo (n = 28) | 25 mg kg−1 day−1 UDCA (n = 9) | 100 mg kg−1 day−1 UDCA (n = 10) |
|---|---|---|---|---|
| Heart | 781 ± 16.5 | 520 ± 17.2* | 509 ± 21* | 529 ± 33* |
| Lung | 1,273 ± 69 | 864 ± 27* | 992 ± 71**, *** | 916 ± 43*, **** |
| Liver | 10,600 ± 393 | 6,325 ± 265* | 6,798 ± 345* | 6,265 ± 684* |
| Spleen | 641 ± 30 | 189 ± 30* | 206 ± 34* | 222 ± 57* |
| Kidney left | 1,126 ± 31 | 708 ± 19.3* | 690 ± 32* | 717 ± 45* |
| Kidney right | 1,162 ± 39 | 735 ± 22* | 724 ± 33* | 729 ± 42* |
| WAT | 1,309 ± 151 | 87 ± 24* | 301 ± 155* | 438 ± 241***, **** |
| BAT | 220 ± 12.1 | 83 ± 4.77* | 103 ± 8.6*, **** | 96 ± 14.0* |
| M. gastrocnemius | 1,255 ± 42 | 725 ± 16.0* | 828 ± 81*, **** | 814 ± 81*, **** |
| M. tibialis | 457 ± 12.4 | 268 ± 7.10* | 296 ± 23* | 300 ± 29* |
| M. EDL | 107 ± 3.66 | 64 ± 1.51* | 71 ± 5.4*, **** | 68 ± 6.3* |
| M. soleus | 98 ± 2.97 | 71 ± 1.60* | 79 ± 5.2***, **** | 69 ± 6.4* |
*p < 0.001 vs. control; **p < 0.05 vs. placebo; ***p < 0.01 vs. control; ****p < 0.12 vs. placebo
Quality of life
Indicators for quality of life are spontaneous activity and food intake. The activity was decreased in tumor-bearing animals, and there were no effects with either dose of UDCA (Fig. 1f). Food intake was reduced in the placebo group and UDCA treatment of the tumor-bearing animals did not increase food intake (Fig. 1g).
Survival
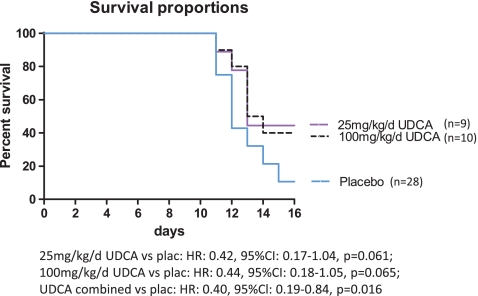
On day 16 following tumor inoculation, the survival rates in the placebo, high-, and low-dose UDCA groups were 11%, 40%, and 44%, respectively (Fig. 2). Median survival in the placebo group was 12 days, 13 days for low-dose UDCA, and 13.5 days for high-dose UDCA. Treatment with 25 and 100 mg kg−1 day−1 UDCA showed a trend towards reducing mortality of the tumor-bearing animals (HR, 0.42; 95% CI, 0.17–1.04; p = 0.061 for low-dose UDCA; HR, 0.44; 95% CI, 0.18–1.05; p = 0.065 for high-dose UDCA). Combining both UDCA-treated animal groups for the survival analysis increased the statistical power and significantly decreased mortality of tumor-bearing animals (HR, 0.40; 95% CI, 0.19–0.84; p = 0.016 for UDCA combined vs. placebo).
Fig. 2.
Kaplan–Meier survival curves, both doses UDCA slightly reduce mortality compared with placebo
Discussion
In our study, UDCA treatment of tumor-bearing animals had no effect on the growth of the tumor, loss of body weight, and loss of fat mass. Low-dose UDCA attenuated significantly the weight loss of the lung and slightly the weight loss of BAT and skeletal muscles compared with placebo. High-dose UDCA increased the lean body mass, the weight of the lung, WAT, and gastrocnemius muscle (GC). Both doses of UDCA had no effect on spontaneous activity and food intake and tended to decrease mortality rates in tumor-bearing animals.
It has been described that UDCA treatment can prevent chemically induced colonic tumorigenesis in experimental animal models and human colorectal cancer by inhibiting proliferation of tumor cells [9, 10, 12, 18, 19]. In the present study utilizing the Yoshida hepatoma animal model, we could not confirm this, because the treatment with UDCA had no effect on tumor growth. The reason therefore that UDCA was not so beneficial in our tumor model could be the type of the tumor (chemically induced tumor vs. injected tumor cells) and the duration of the treatment. Kohno et al. showed a prevention of colon carcinogenesis in mice by giving UDCA (long-term) for 17 weeks after chemical tumor induction [11]. Furthermore, Narisawa et al. treated rats with colon cancer with UDCA for 27 weeks and showed a suppression of cancer progression [9]. In our case, long-term studies of rats inoculated with Yoshida hepatoma cells are not feasible because the tumor cells are too aggressive and hence UDCA treatment of 15 days may be too short to shown any beneficial effect. Moreover, we did not see an anti-cancer effect because it could be that we did not assess the anticarcinogenic concentration of UDCA in this tumor model and hence, more dose–response studies are needed.
The body weight and the fat mass were unaltered in UDCA-treated tumor-bearing rats compared with placebo. Only high-dose UDCA affected the lean body mass; 25 mg kg−1 day−1 UDCA significantly increased the weight of the lung and showed a trend towards increasing the weight of BAT, GC, EDL, and soleus muscle (p < 0.12 vs. placebo) while high-dose UDCA improved the weight of the lung, WAT, and GC (p < 0.12 vs. placebo). This effect may be mediated by UDCA reported to be anti-inflammatory and anti-apoptotic. In vitro and in vivo cancer studies showed that UDCA treatment inhibits the NF-κB pathway leading to the expression of the proinflammatory cytokine TNF-α from which is known that it is associated with wasting in cancer cachexia [7, 11, 12, 20]. A possible mechanism explaining the anti-inflammatory and anti-apoptotic effect could be that UDCA blocks the cancer induced generation of reactive oxygen species and subsequently inhibiting the transcription factors AP-1, and NF-κB important for carcinogenesis through the PKC signaling pathway, established from other studies [7, 12, 21–24].
It was seen that high dose of UDCA did not exert improved effects on body wasting than low-dose UDCA, so there was no dose-dependent correlation. This may be mediated by the fact that UDCA has side-effects like diarrhea, and therefore UDCA is less absorbed.
The locomotor activity and food intake were unchanged at day 10 after tumor inoculation compared with placebo showing no effect of UDCA on improving quality of life. The mortality of tumor-bearing animals treated with both doses UDCA was reduced compared with placebo but did reach significance when analyzing the combined UDCA doses with placebo because of an increased statistical power.
The results indicate that a low (25 mg kg−1 day−1) and high (100 mg kg−1 day−1) dosage of UDCA had no effect on tumor growth but slightly on body wasting and survival in an experimental Yoshida hepatoma animal model. This could be due to the kind of tumor, the fast tumor progression, the unknown efficient UDCA concentration and because of the short treatment time of UDCA. More studies are needed to show an anti-cancer effect and a more ameliorated cachexia to identify effects of higher doses or longer treated periods.
Study limitations
The primary endpoint of this study was to investigate if UDCA treatment could reduce body wasting in an experimental cancer cachexia model. Due to the small overall effects of UDCA treatment on body weight, we did not analyze signaling mechanisms including TNF-α measurement. The study was not powered for a survival endpoint, but a combination of both treated groups resulted in improved outcome. This suggests that a study powered for survival should be performed. However, since the effects on body weight and body composition were minimal, it may not be relevant to patient’s health.
A second limitation of the study is that we have used the fast-growing and aggressive tumor AH-130 as a model for cachexia in rats, which may be the reason that UDCA had limited effects. However, further experiments could include UDCA treatment in a less aggressive and therefore long-term cancer cachexia animal model followed by measurement of proinflammatory cytokines.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [25].
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.von Haehling S. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 5.Matthys P, Billiau A. Cytokines and cachexia. Nutrition. 1997;13:763–770. doi: 10.1016/S0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R, Prichard D, Majer F, Byrne AM, Kelleher D, Long A, et al. Ursodeoxycholic acid amides as novel glucocorticoid receptor modulators. J Med Chem. 2011;54:122–130. doi: 10.1021/jm100860s. [DOI] [PubMed] [Google Scholar]
- 7.Cronin J, Williams L, McAdam E, Eltahir Z, Griffiths P, Baxter J, et al. The role of secondary bile acids in neoplastic development in the oesophagus. Biochem Soc Trans. 2010;38:337–342. doi: 10.1042/BST0380337. [DOI] [PubMed] [Google Scholar]
- 8.Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–1013. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 9.Narisawa T, Fukaura Y, Terada K, Sekiguchi H. Prevention of N-methylnitrosourea-induced colon tumorigenesis by ursodeoxycholic acid in F344 rats. Jpn J Cancer Res. 1998;89:1009–1013. doi: 10.1111/j.1349-7006.1998.tb00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earnest DL, Holubec H, Wali RK, Jolley CS, Bissonette M, Bhattacharyya AK, et al. Chemoprevention of azoxymethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res. 1994;54:5071–5074. [PubMed] [Google Scholar]
- 11.Kohno H, Suzuki R, Yasui Y, Miyamoto S, Wakabayashi K, Tanaka T. Ursodeoxycholic acid versus sulfasalazine in colitis-related colon carcinogenesis in mice. Clin Cancer Res. 2007;13:2519–2525. doi: 10.1158/1078-0432.CCR-06-2727. [DOI] [PubMed] [Google Scholar]
- 12.Shah SA, Volkov Y, Arfin Q, Abdel-Latif MM, Kelleher D. Ursodeoxycholic acid inhibits interleukin 1 beta [corrected] and deoxycholic acid-induced activation of NF-kappaB and AP-1 in human colon cancer cells. Int J Cancer. 2006;118:532–539. doi: 10.1002/ijc.21365. [DOI] [PubMed] [Google Scholar]
- 13.Tsagarakis NJ, Drygiannakis I, Batistakis AG, Kolios G, Kouroumalis EA. A concentration-dependent effect of ursodeoxycholate on apoptosis and caspases activities of HepG2 hepatocellular carcinoma cells. Eur J Pharmacol. 2010;640:1–7. doi: 10.1016/j.ejphar.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Feldman R, Martinez JD. Growth suppression by ursodeoxycholic acid involves caveolin-1 enhanced degradation of EGFR. Biochim Biophys Acta. 2009;1793:1387–1394. doi: 10.1016/j.bbamcr.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, et al. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31:111–118. doi: 10.1080/01635589809514689. [DOI] [PubMed] [Google Scholar]
- 16.Costelli P, Carbo N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argiles JM, et al. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783–2789. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauhofer A, Witte K, Celik I, Pummer S, Lemmer B, Lorenz W. Sickness behaviour, an animal equivalent to human quality of life, is improved in septic rats by G-CSF and antibiotic prophylaxis. Langenbecks Arch Surg. 2001;386:132–140. doi: 10.1007/s004230100206. [DOI] [PubMed] [Google Scholar]
- 18.Garioud A, Seksik P, Chretien Y, Corphechot C, Poupon R, Poupon RE, et al. Characteristics and clinical course of primary sclerosing cholangitis in France: a prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:842–847. doi: 10.1097/MEG.0b013e328331c2b7. [DOI] [PubMed] [Google Scholar]
- 19.Sjoqvist U, Tribukait B, Ost A, Einarsson C, Oxelmark L, Lofberg R. Ursodeoxycholic acid treatment in IBD-patients with colorectal dysplasia and/or DNA-aneuploidy: a prospective, double-blind, randomized controlled pilot study. Anticancer Res. 2004;24:3121–3127. [PubMed] [Google Scholar]
- 20.Tisdale MJ. Catabolic mediators of cancer cachexia. Curr Opin Support Palliat Care. 2008;2:256–261. doi: 10.1097/SPC.0b013e328319d7fa. [DOI] [PubMed] [Google Scholar]
- 21.Shiraki K, Ito T, Sugimoto K, Fuke H, Inoue T, Miyashita K, et al. Different effects of bile acids, ursodeoxycholic acid and deoxycholic acid, on cell growth and cell death in human colonic adenocarcinoma cells. Int J Mol Med. 2005;16:729–733. [PubMed] [Google Scholar]
- 22.Byrne AM, Foran E, Sharma R, Davies A, Mahon C, O’Sullivan J, et al. Bile acids modulate the Golgi membrane fission process via a protein kinase Ceta and protein kinase D-dependent pathway in colonic epithelial cells. Carcinogenesis. 2010;31:737–744. doi: 10.1093/carcin/bgq011. [DOI] [PubMed] [Google Scholar]
- 23.Pongracz J, Clark P, Neoptolemos JP, Lord JM. Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int J Cancer. 1995;61:35–39. doi: 10.1002/ijc.2910610107. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Jin B, Huang C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr Cancer Drug Targets. 2007;7:305–316. doi: 10.2174/156800907780809741. [DOI] [PubMed] [Google Scholar]
- 25.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. [DOI] [PMC free article] [PubMed]