Abstract
Introduction of genetic material into cells is an essential prerequisite for current research in molecular cell biology. Although transfection with commercially available reagents results in excellent gene expression, their high costs are obstacles to experimentation with a large number or large scales of transfection. The cationic polymer linear-polyethylenimine (MW 25,000) (PEI), one of the most cost-effective vehicles, facilitates DNA compaction by polyplex formation, which leads to efficient delivery of DNA into cells by endocytosis. However, the use of PEI is still limited because of substantial cytotoxicity and intolerable deterioration in transfection efficiency by its low stability. Here, we show that acidification of PEI is important for its transfection activity. Dissolving PEI powder in 0.2N HCl confers a long shelf-life for PEI storage at 4 and −80 °C, and the polyplex formation of plasmid DNA with PEI is optimized in lactate-buffered saline at pH 4.0. Furthermore, changing the culture medium at 8–12 h posttransfection can minimize the cytotoxicity of PEI without sacrificing the high transfection efficiency comparable to that of commercial reagents. The cost per test using acidified PEI is drastically reduced to approximately 1:10,000, compared with commercial reagents. Thus, we conclude that acidification of PEI satisfactorily accomplishes cost-effective, high-efficiency transfection.
Keywords: Acidification, High efficiency transfection, Long shelf-life, Low cytotoxicity, Polyethylenimine (PEI)
Introduction
The delivery of nucleic acids into cells is indispensable for basic research in molecular and cell biology as well as medical applications such as gene therapy. Viral vectors are efficient carriers resulting in high levels of transfection, but their use is limited by the induction of immune responses and virus-associated pathogenicity. Non-viral gene delivery systems have the potential to create pharmaceuticals from nucleic acids (Davis 2002). The products should be capable of being produced in large quantities with high reproducibility and acceptable cost, and stable to storage. The two major approaches to non-viral gene delivery involve the combination of nucleic acids with cationic polymers and/or cationic lipids. Since many of the commercially available transfection reagents are quite expensive at the present time, their high costs are obstacles to experimentation with a large number and/or large scales of transfection.
Polyethylenimine (PEI) is an inexpensive, non-viral and non-liposomal reagent. PEI has a high cationic charge density potential and efficiently condenses DNA to form stable complexes termed polyplexes, which promote gene transfer into cells (Boussif et al. 1995). In addition, PEI has a high pH-buffering capacity, leading to protection of incorporated DNA from lysosomal degradation and its efficient release from lysosomes into the cytoplasm. PEI is therefore used to transfect mammalian cells with DNA in vitro and in vivo (Boussif et al. 1995; Abdallah et al. 1996; Goula et al. 1998). Extensive studies have been performed on the size, structure and chemical modification of PEI for efficient transfection (Zanta et al. 1997; Goula et al. 1998; Fischer et al. 1999; Ogris et al. 1999; Wightman et al. 2001; Durocher et al. 2002; Brissault et al. 2003; Thomas et al. 2005), and addition of supplements to a PEI/DNA polyplex or compacted DNA was also examined to improve transfection efficiency (Ogris et al. 1998; Tseng et al. 2007). However, while using PEI for transfection, we often experience considerable deterioration in transfection efficiency and notice the induction of unignorable degrees of cytotoxicity.
Although many of the previous studies focused on the size, structure and chemical modification of PEI, improvement of PEI solution has received little attention. In this study, to overcome the drawbacks of PEI, we examined the conditions of PEI solution and polyplex formation and the duration of transfection. Our results show that acidification of PEI at pH 1.0 and polyplex formation at pH 4.0 ensure high transfection efficiency without any deterioration. Furthermore, removal of PEI after 8–12 h of transfection achieves satisfactory transfection efficiency and minimal cytotoxicity. Our improvement establishes a simple, trustworthy and cost-effective transfection method.
Materials and methods
Reagents and plasmids
Linear PEI (MW 25,000) was purchased from Polysciences, Inc. (cat. no. 23966-2, lot no. 566896, 579117, 602049, and 605705). PEI powder was dissolved in 0.2N HCl at 5 mg/mL, and aliquots were stored at 4 or −80 °C. The resulting solution shows approximately pH 1.0. Storage of PEI powder is not recommended even at −80 °C because the transfection activity of PEI decreased during storage. HEPES-buffered saline (HBS) (20 mM HEPES–KOH, pH 7.4, and 150 mM NaCl) and lactate-buffered saline (LBS) (20 mM sodium lactate, pH 3.5, 4.0 or 4.5, and 150 mM NaCl) were prepared. The TransIT-LT1 transfection reagent was purchased from Mirus Bio LLC (cat. no. MIR 2306). The pcDNA4/TO vector (Invitrogen) encoding enhanced green fluorescent protein (EGFP) (pcDNA4-TO-EGFP) was constructed previously (Nakayama et al. 2006). The pCAG vector (Miyazaki et al. 1989) encoding CD25 was constructed (pCAG/TetR-IRES-CD25), and details will be described elsewhere.
Cell lines
Human epithelial HeLa and HeLa S3 cells (Japanese Collection of Research Bioresources, Osaka) were cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 5% fetal bovine serum (FBS) and with 5% bovine serum (BS), respectively. Monkey fibroblastic COS-1 and human colon carcinoma HCT116 cells were maintained in IMDM supplemented with 5% FBS, as described (Higashiyama et al. 2009), or with 1% FBS plus 5% bovine serum (BS). Human megakaryocytic Dami cells were maintained in suspension culture in IMDM supplemented with 10% horse serum, as described previously (Greenberg et al. 1988; Hirao et al. 1998; Sato et al. 2009), and attached to culture dishes during 2–3 days of culture in IMDM supplemented with 2.5% FBS and 2.5% horse serum.
Effects of DNA compaction pH and PEI/DNA ratios on transfection efficiency
HeLa cells were seeded into 35-mm culture dishes (1 × 105 cells per dish) and grown in 2 mL of culture medium for 1–2 days. The culture medium was replaced with 2 mL of fresh serum-containing medium before transfection. To form PEI/DNA polyplexes, 1 μg of pcDNA4-TO-EGFP was mixed with PEI in 100 μL of LBS or HBS at the indicated PEI/DNA ratios, and the mixture was allowed to stand at room temperature for 20 min. In initial experiments 1 μg of pcDNA4-TO-EGFP and 5 μg (1 μL) of PEI were directly mixed in 100 μL of LBS, but in later experiments 1 μg of pcDNA4-TO-EGFP and 5 μg (1 μL) of PEI were separately diluted in 50 μL each of LBS and then mixed. The resulting PEI/DNA polyplexes were diluted with 500 μL of prewarmed serum-free IMDM and added to each culture in a dropwise manner. At 24 h posttransfection, cells were detached in phosphate-buffered saline (PBS) supplemented with 0.05% EDTA by gentle pipetting. After centrifugation, cells were resuspended in PBS supplemented with 3% FBS and 1 μg/mL propidium iodide (PI), and EGFP fluorescence for protein expression and PI staining for dead cells were analyzed by flow cytometry using a MoFlo cell sorter (Beckman Coulter). Transfected cells grown in culture dishes were examined at 24 h posttransfection under a Zeiss LSM 5 Pa deconvolution microscope (Carl Zeiss).
Mixing experiments with new and old PEI
New PEI powder (lot no. 602049) was dissolved in 0.2N HCl at 5 mg/mL, and aliquots were stored at 4 °C. Likewise, old PEI powder (lot no. 566896), stored at 4 °C for 2 years after the bottle was opened and recapped, was dissolved in 0.2N HCl and stored at 4 °C. HeLa S3 cells were seeded into 35-mm culture dishes (4 × 104 cells per dish) and grown in 2 mL of culture medium for 1 day. The culture medium was replaced with 2 mL of fresh serum-containing medium before transfection. Five microgram (1 μL) of new or old PEI was diluted in 50 μL of LBS (pH 4.0) (new or old), and 5 μg (1 μL) of new PEI was mixed with 5 μg (1 μL) of old PEI in 50 μL of LBS (pH 4.0) (mix). To form PEI/DNA polyplexes, 1 μg of pcDNA4-TO-EGFP was dissolved in 50 μL of LBS (pH 4.0) and then mixed with 50 μL of PEI solution (new, old, or mix). The resulting PEI/DNA polyplexes were diluted with 500 μL of prewarmed serum-free IMDM and added to 2 mL each of culture in a dropwise manner. Transfected cells were examined at 24 h posttransfection under a Zeiss LSM 5 Pa deconvolution microscope.
Sequential transfection of HCT116 cells, a cell line sensitive to PEI cytotoxicity
HCT116 cells were sensitive to cytotoxicity of transfection reagents. To reduce PEI cytotoxicity and enhance the transfection efficiency, we developed a sequential transfection method. In brief, the DNA/PEI polyplexes prepared with 7.5 μg of PEI and 6 μg of pCAG/TetR-IRES-CD25 were added to 2 mL of each culture medium per 35-mm dish in a dropwise manner, and the culture medium was replaced with 2 mL of fresh serum-containing medium after 8 h of incubation. For the subsequent transfection, the DNA/PEI polyplexes prepared freshly were added to the cell culture, as described in the first transfection, and the medium was replaced again with 2 mL of fresh serum-containing medium after another 8 h of incubation. Cells were further cultured for 16 h and stained with anti-CD25 antibody (7G7B6), and cell-surface expression of CD25 was analyzed by flow cytometry using a MoFlo cell sorter, as described previously (Nakayama and Yamaguchi 2005; Takahashi et al. 2009).
Generation of cell clones that stably express EGFP
HeLa S3 cells were seeded in a 60-mm dish (3 × 104 per dish) and grown in 5 mL of culture medium for 1 day. To form PEI/DNA polyplexes, 97.5 μg of PEI and 15 μg of pcDNA4-TO-EGFP were separately diluted in 50 μL each of LBS (pH 3.5), and the dilutions were mixed and used for transfection, as described above. Cells grown in culture dishes for 24 h after transfection were equally dispensed into four dishes, and stably transfected cells were selected in 250 μg/mL Zeocin (Invitrogen) from 48 h posttransfection.
Results and discussion
Effects of acidic pH on PEI storage and polyplex formation
Despite a large number of papers describing successful transfection with neutralized PEI solution (Boussif et al. 1995; Ogris et al. 1998; Fischer et al. 1999; Pham et al. 2003; Ehrhardt et al. 2006), reagent users often suffer from an unpredictable decrease in the transfection activity of PEI. We then suspected neutralized PEI solution to be unstable. When PEI (lot no. 566896) was dissolved in HBS (pH 7.4), satisfactory transfection efficiencies were observed immediately after purchase, but the transfection activity severely decreased during only one-month storage at 4 or −80 °C. Moreover, dissolving PEI in ethanol drastically decreased its transfection activity within 2 weeks during storage at 4 °C. In sharp contrast, dissolving PEI (lot no. 602049) in 0.2N HCl where the resulting solution showed at approximately pH 1.0 preserved the full transfection activity for at least 1 year during storage at 4 or −80 °C. Preservation of the transfection activity of PEI by acidification was confirmed with different lots of PEI (lot no. 579117, 602049, 605705), suggesting that variation of purity from lot to lot, if any, is negligible. In addition, the potential of PEI powder for transfection was lost to a great extent during storage at 4 or −80 °C once the bottle was opened. Because PEI powder turned yellow during storage, the fresh air inside may start to oxidize PEI, giving rise to deterioration of the transfection activity. These results suggest that protonation of the lone pair of electrons on the nitrogen of PEI at pH 1.0 is important for PEI’s stability probably due to protecting the amines against air oxidation.
Next, we compared the transfection efficiency between acidic and neutral compaction media using 5 mg/mL PEI (lot no. 602049) in 0.2N HCl. FACS analysis showed that the number of cells expressing EGFP upon use of LBS (pH 3.5) as compaction medium was much higher than that upon use of HBS (pH 7.4; Fig. 1a). Similar results were obtained from fluorescence microscopic observations (data not shown). Since it was reported that every sixth amino group is protonated at physiological pH where only a fraction of PEI is complexed with DNA, a half of amino groups are protonated at pH 5.0, and 80% of amino groups are protonated at pH 3.0 (Suh et al. 1994; Clamme et al. 2003; Menzel et al. 2003), the increased positive charge on PEI would promote the DNA/PEI polyplex formation.
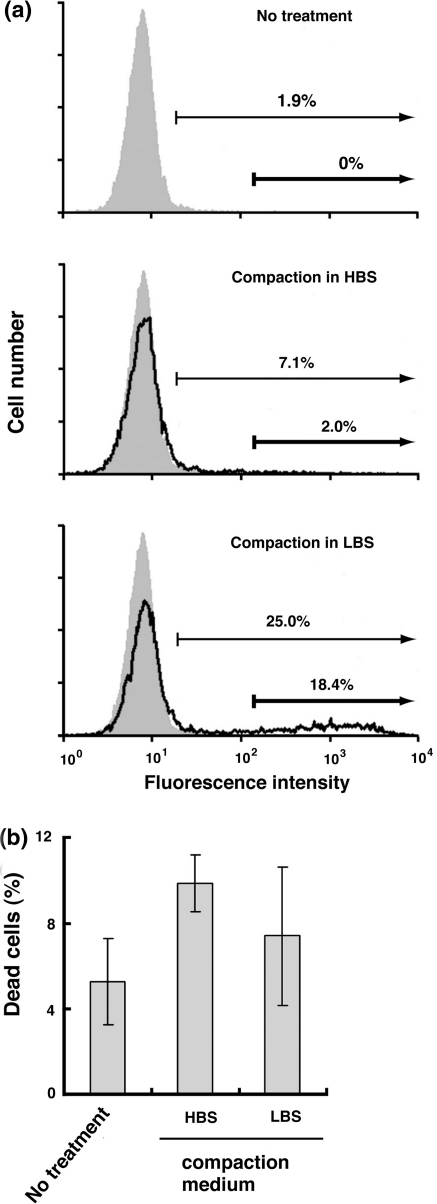
Fig. 1.
Increased transfection efficiency by acidic DNA compaction. PEI and pcDNA4-TO-EGFP were mixed in HBS (pH 7.4) or LBS (pH 3.5) [PEI/DNA ratio = 5 (μg/μg)], and the resulting PEI/DNA polyplex was transiently transfected into HeLa cells. At 24 h posttransfection, EGFP expression was analyzed by flow cytometry. a Representative histograms of control (shaded areas) and transfected cells (solid line) are shown. Two gates indicate EGFP expression at high levels (thick arrows) and at low to high levels (thin arrows). Transfection efficiencies were quantitated by counting the number of cells expressing EGFP at high levels and at low to high levels. b HeLa cells transiently transfected with the polyplex formed in HBS (pH 7.4) or LBS (pH 3.5) were stained with PI and analyzed for PI-stained dead cells by flow cytometry. Data represent means ± SD (n = 3)
In addition, LBS yielded a smaller number of dead cells stained with PI than HBS (Fig. 1b), suggesting that use of LBS alleviates the cytotoxicity of PEI. As a significant part of cytotoxicity results from free PEI molecules that do not form complexes with DNA (Godbey et al. 2001; Moghimi et al. 2005), enhancement of polyplex formation is likely to contribute to a decrease in the cytotoxicity. These results indicate that replacing neutral HBS with acidic LBS improves transfection efficiency and alleviates cytotoxicity.
Optimization of PEI/DNA ratios and compaction pH
The transfection efficiency of PEI is dependent on a ratio of PEI to DNA (Boussif et al. 1995). We thus examined various PEI/DNA ratios ranging from 1 to 10 in LBS (pH 3.5) for transfection efficiency using 5 mg/mL PEI (lot no. 602049) in 0.2N HCl. The number of cells expressing EGFP was greatly increased at PEI/DNA ratios ranging from 5.0 to 8.0 (Fig. 2a, b). The PEI/DNA ratio that we optimized at pH 3.5 corresponded to the ratio of nitrogen in PEI to phosphate in DNA (NP ratio) of 40–55 (Fig. 2a), whereas an optimal NP ratio was 6.0 under neutral compaction medium (Ferrari et al. 1997; Goula et al. 1998; Wightman et al. 2001). Given that excess free PEI molecules, which would contribute to efficient transfection, inhibited transfection efficiency owing to high cytotoxicity (Fig. 2a; Clamme et al. 2003; Ira et al. 2003), the number of dead cells stained with PI was increased over a PEI/DNA ratio of 10 (Fig. 2a). A low PEI/DNA ratio (~3.0) was preferable to large size vectors (>10 kbp) (data not shown).
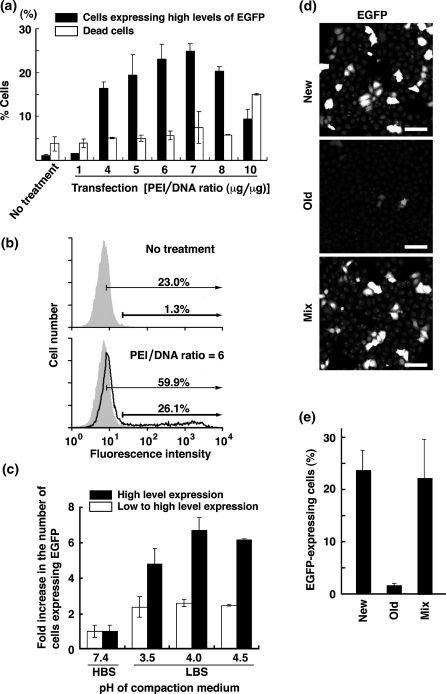
Fig. 2.
Effects of PEI/DNA ratios and pH of compaction medium on transfection efficiency. a, b PEI and pcDNA4-TO-EGFP were mixed in LBS (pH 3.5) at various PEI/DNA ratios (μg/μg), and the resulting PEI/DNA polyplex was transiently transfected into HeLa cells. a At 24 h posttransfection, cells were stained with PI for dead cells, and analyzed by flow cytometry for EGFP expression (filled bars) and cytotoxicity (open bars). Data represent means ± SD (n = 3). b PEI and pcDNA4-TO-EGFP were mixed in LBS (pH 3.5) at a ratio of 6 (μg/μg). Representative histograms of control (shaded areas) and transfected cells (solid line) are shown. Two gates indicate EGFP expression at high levels (thick arrows) and at low to high levels (thin arrows). c PEI and pcDNA4-TO-EGFP were mixed at a ratio of 5 (μg/μg) in HBS (pH 7.4) or LBS (pH 3.5, 4.0, 4.5), and the resulting PEI/DNA polyplex was transiently transfected into HeLa cells. Transfection efficiencies were quantitated by counting the number of cells expressing EGFP at high levels (filled bars) and at low to high levels (open bars). Results are expressed as values relative to the number of cells expressing EGFP using HBS as DNA compaction medium (pH 7.4). Data represent means ± SD (n = 3). Transfection efficiencies were 2.5 and 16.5% at pH 7.4 and pH 4.0, respectively in cells expressing EGFP at high levels. d, e Five microgram of new or old PEI and 1 μg of pcDNA4-TO-EGFP were mixed in LBS (pH 4.0), and 5 μg of new PEI and 5 μg of old PEI (mix) and 1 μg of pcDNA4-TO-EGFP were mixed in LBS (pH 4.0). The resulting PEI/DNA polyplex was transiently transfected into HeLa S3 cells, and transfection efficiencies were quantitated by counting the number of cells expressing EGFP. Data represent means ± SD (n = 3)
Furthermore, we compared the effect of compaction pH on transfection efficiency and found that LBS (pH 4.0) was the most efficient compaction medium (Fig. 2c). Note that 20 mM lactate buffer in LBS was diluted to a final concentration of 0.77 mM in culture medium. Owing to 20 mM HEPES buffer as an ingredient of IMDM, the addition of LBS did not affect pH of culture medium. Therefore, we recommend using LBS (pH 4.0) to achieve the highest level of PEI-mediated transfection efficiency.
To examine whether old PEI affected the transfection activity of new PEI (lot no. 602049), mixing experiments were performed using old PEI powder (lot no. 566896), which were stored at 4 °C for 2 years after the bottle was opened and recapped. Both new and old PEI were dissolved in 0.2N HCl and tested for EGFP transfection, as described under Materials and methods. During storage of old PEI powder, the transfection activity was almost completely lost (Fig. 2d, e). The mixture with new and old PEI was found to show the transfection activity comparable to new PEI alone (Fig. 2d, e), and old PEI did not show cytotoxicity despite inclusion of 2-fold amounts (10 μg) of PEI in ‘mix’ samples, meaning that old PEI does not contain any cytotoxic breakdown products. These results suggest that old PEI is neither regenerated in 0.2N HCl nor affects the transfection activity of new PEI.
Minimization of PEI cytotoxicity for efficient transfection
We were able to successfully transfect various cell lines, such as HeLa, HeLa S3, A431, COS-1, MCF7, NIH3T3, HCT116, HEK293 and MDCK cells, with the PEI/DNA polyplexes formed in LBS (pH 4.0; Fig. 3; data not shown). When 25 μg of PEI and 5 μg of DNA (5-fold increase in amounts) were added to each 35-mm culture dish and the culture medium was replaced with fresh medium at 12 h posttransfection, transfection efficiencies in COS-1 and HeLa S3 cells were ~80 and ~60%, respectively (Fig. 3a). We next compared the transfection efficiencies between PEI in LBS (pH 4.0) and TransIT-LT1, a commercial transfection reagent, and found that the transfection efficiency using PEI transfection was comparable to that using TransIT-LT1 (Fig. 3b).
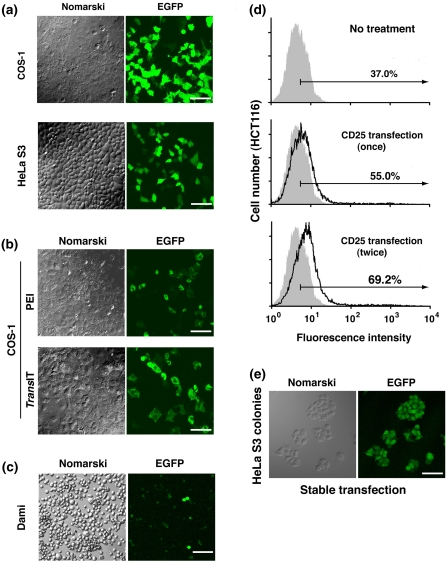
Fig. 3.
Transfection into COS-1, HeLa S3, Dami, and HCT116 cells. a–c EGFP expression was examined by fluorescence microscopy. Scale bars, 10 μm. a COS-1 and HeLa S3 cells were transiently transfected with the polyplex formed by mixing 5 μg of pcDNA4-TO-EGFP with 25 μg of PEI in LBS (pH 4.0), and after 12 h of culture the medium was replaced with fresh serum-containing medium. b COS-1 cells were transiently transfected with the polyplex formed by mixing 1 μg of pcDNA4-TO-EGFP with 5 μg of PEI in LBS (pH 4.0) or an optimal amount of TransIT-LT1. c Dami cells in suspension culture were attached to culture dishes, as described under “Materials and methods”, and transiently transfected with the polyplex formed by mixing 4 μg of pcDNA4-TO-EGFP with 26 μg of PEI in LBS (pH 4.0). d HCT116 cells were transiently transfected once or twice with the polyplex formed by mixing 6 μg of pCAG/TetR-IRES-CD25 with 7.5 μg of PEI in LBS (pH 4.0), as described under “Materials and methods”, and stained with anti-CD25 antibody. Representative histograms of control (shaded areas) and transfected cells (solid line) are shown for CD25 expression at low to high levels (thin arrows). e HeLa S3 cells were stably transfected with the polyplex formed by mixing pcDNA4-TO-EGFP with PEI in LBS, as described under “Materials and methods”. Zeocin-resistant colonies were cloned in 2 weeks and observed by fluorescence microscopy. Scale bar, 10 μm
Since many suspension culture cells are poorly transfected using chemical transfection reagents, electroporation is usually performed with special equipment and a large number of cells in suspension despite induction of massive cell death. To transfect suspension cells using PEI, we chose human megakaryocytic Dami cells, which were found to attach to culture dishes when they were grown in medium supplemented with FBS and horse serum (see “Materials and methods”). We found that Dami cells were successfully transfected with the PEI/DNA polyplexes formed in LBS (pH 4.0) at a 3–5% transfection rate comparable to that by electroporation (Fig. 3c).
Some cell types, including HCT116 cells, are highly sensitive to PEI exposure, leading to cell aggregation and cell death. To reduce the cytotoxicity of PEI, the PEI/DNA ratio was decreased to 1.25 and the amount of DNA was increased (6 μg), as described under “Materials and methods”. FACS analysis showed that our sequential transfection procedure yielded a transfection efficiency of around 30% (Fig. 3d). The high transfection efficiency and low cytotoxicity attained by our transfection procedure using PEI prompted us to examine whether stably transfected cell clones were easily generated. According to our protocol described under “Materials and methods”, more than 200 single cell-derived colonies that stably expressed EGFP were obtained from 3 × 104 HeLa S3 cells (Fig. 3e). Furthermore, stably transfected cell clones were successfully generated from PEI-sensitive HCT116 cells. In addition, our transfection procedure using PEI enabled us to efficiently transfect various cell lines, including HeLa, COS-1 and Dami cells, with short hairpin RNA constructs for gene knockdown (Nakayama et al. 2009; data not shown).
In conclusion, we developed a transfection method using acidified PEI as a stock solution and LBS (pH 4.0) as a compaction medium. Our standard procedure for efficient transfection is summarized in Fig. 4. Acidification of PEI ensures long-term preservation of its transfection activity and achieves high transfection efficiency and minimal cytotoxicity in introduction of DNA into a wide variety of cell lines. Notably, the cost per test using acidified PEI is drastically reduced to approximately 1:10,000, compared with various commercial reagents. Thus, acidification of PEI accomplishes cost-effective, high-efficiency transfection.
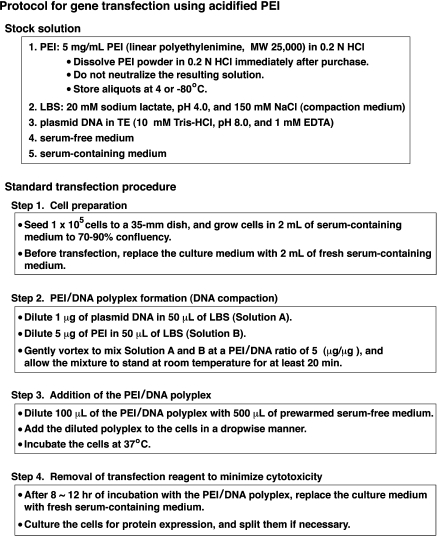
Fig. 4.
Overview of our gene transfection protocol using acidified PEI. Our standard transfection procedure is summarized together with preparation of stock solutions
Acknowledgments
The hybridoma cell line 7G7B6 was obtained from The Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University. This work was supported in part by grants-in-aid for Scientific Research, Global COE Program (Global Center for Education and Research in Immune Regulation and Treatment) and Special Funds for Education and Research (Development of SPECT probes for Pharmaceutical Innovation) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and a research grant from the Suzuken Memorial Foundation. Y.O. is a G-COE Research Assistant.
References
- Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissault B, Kichler A, Guis C, Leborgne C, Danos O, Cheradame H. Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconjug Chem. 2003;14:581–587. doi: 10.1021/bc0200529. [DOI] [PubMed] [Google Scholar]
- Clamme JP, Azoulay J, Mély Y. Monitoring of the formation and dissociation of polyethylenimine/DNA complexes by two photon fluorescence correlation spectroscopy. Biophys J. 2003;84:1960–1968. doi: 10.1016/S0006-3495(03)75004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME. Non-viral gene delivery systems. Curr Opin Biotechnol. 2002;13:128–131. doi: 10.1016/S0958-1669(02)00294-X. [DOI] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:e9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, Ludwig S. Polyethylenimine, a cost-effective transfection reagent. Signal Transduction. 2006;6:179–184. doi: 10.1002/sita.200500073. [DOI] [Google Scholar]
- Ferrari S, Moro E, Pettenazzo A, Behr JP, Zacchello F, Scarpa M. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Ther. 1997;4:1100–1106. doi: 10.1038/sj.gt.3300503. [DOI] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/A:1014861900478. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu K, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/S0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix BA. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998;5:712–717. doi: 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988;72:1968–1977. [PubMed] [Google Scholar]
- Higashiyama Y, Takahashi A, Fukumoto Y, Nakayama Y, Yamaguchi N. Induction of chromatin condensation by nuclear expression of a novel arginine-rich cationic protein genetically engineered from the enhanced green fluorescent protein. Cytotechnology. 2009;60:153–159. doi: 10.1007/s10616-009-9227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Huang XL, Suda T, Yamaguchi N. Overexpression of C-terminal Src kinase homologous kinase suppresses activation of Lyn tyrosine kinase required for VLA5-mediated Dami cell spreading. J Biol Chem. 1998;273:10004–10010. doi: 10.1074/jbc.273.16.10004. [DOI] [PubMed] [Google Scholar]
- Ira, Mély Y, Krishnamoorthy G. DNA vector polyethyleneimine affects cell pH and membrane potential: a time-resolved fluorescence microscopy study. J Fluoresc. 2003;13:339–347. doi: 10.1023/A:1025381812568. [DOI] [Google Scholar]
- Menzel H, Horstmann S, Behrens P, Bärnreuther P, Krueger I, Jahns M. Chemical properties of polyamines with relevance to the biomineralization of silica. Chem Commun. 2003;2003:2994–2995. doi: 10.1039/b310201g. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Takaki S, Araki K, Tashiro F, Tominaga A, Takatsu K, Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yamaguchi N. Multi-lobulation of the nucleus in prolonged S phase by nuclear expression of Chk tyrosine kinase. Exp Cell Res. 2005;304:570–581. doi: 10.1016/j.yexcr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Kawana A, Igarashi A, Yamaguchi N. Involvement of the N-terminal unique domain of Chk tyrosine kinase in Chk-induced tyrosine phosphorylation in the nucleus. Exp Cell Res. 2006;312:2252–2263. doi: 10.1016/j.yexcr.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Igarashi A, Kikuchi I, Obata Y, Fukumoto Y, Yamaguchi N. Bleomycin-induced over-replication involves sustained inhibition of mitotic entry through the ATM/ATR pathway. Exp Cell Res. 2009;315:2515–2528. doi: 10.1016/j.yexcr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferring-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- Ogris M, Brunner S, Schüller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- Pham PL, Perret S, Doan HC, Cass B, St-Laurent G, Kamen A, Durocher Y. Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioeng. 2003;84:332–342. doi: 10.1002/bit.10774. [DOI] [PubMed] [Google Scholar]
- Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, Yokoyama KK, Saito T, Yamaguchi N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–975. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- Suh J, Paik HJ, Hwang BK. Ionization of poly(ethylenimine) and poly(allylamine) at various pH’s. Bioorg Chem. 1994;22:318–327. doi: 10.1006/bioo.1994.1025. [DOI] [Google Scholar]
- Takahashi A, Obata Y, Fukumoto Y, Nakayama Y, Kasahara K, Kuga T, Higashiyama Y, Saito T, Yokoyama KK, Yamaguchi N. Nuclear localization of Src-family tyrosine kinases is required for growth factor-induced euchromatinization. Exp Cell Res. 2009;315:1117–1141. doi: 10.1016/j.yexcr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci USA. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WC, Tang CH, Fang TY, Su LY. Trehalose enhances transgene expression mediated by DNA-PEI complexes. Biotechnol Prog. 2007;23:1297–1304. doi: 10.1021/bp070224m. [DOI] [PubMed] [Google Scholar]
- Wightman L, Kircheis R, Rössler V, Carotta S, Ruzicka R, Kursa M, Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med. 2001;3:362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- Zanta MA, Boussif O, Adib A, Behr JP. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug Chem. 1997;8:839–844. doi: 10.1021/bc970098f. [DOI] [PubMed] [Google Scholar]