Abstract
Vascular endothelial growth factors receptor 2 (VEGFR-2) has been implicated in playing an important role in the formation of new blood vessels in tumors and other diseases. A high affinity human/mouse cross-reactive anti-VEGFR-2 monoclonal antibody (mAb) named A8H1 was established by hybridoma technology. Several immunological methods were used to characterize the A8H1, including ELISA, affinity and kinetics assay, MALDI-TOF MS, WB, IP, IF, FASC and IHC. The results suggested that A8H1 could bind with linear and conformational epitopes of the VEGFR-2 antigen. The mAb had good specific reactivity with three forms of VEGFR-2 in HUVEC, and two forms in NIH-3T3 mouse fibroblast cells, which are regarded as non-expressive for VEGFR-2. The A8H1 mAb associated with intracellular and plasma membranes in HUVEC and with the nuclei in NIH-3T3 cells. This mAb also effectively identified VEGFR-2 over-expressing cells in a number of archived human cancer tissues.
Keywords: Vascular endothelial growth factor receptor 2, Monoclonal antibody, Hybridoma technology, Linear epitope, Conformational epitope
Introduction
Vascular angiogenesis, a microvascular process of formation of new blood vessels out of pre-existing capillaries, has been shown to play a key role in many diseases, such as retinopathies, arthritis, endometriosis and malignant tumors (Benedetta Donati and Gozdzikiewicz 2008). Blockade of angiogenesis is an attractive approach for the treatment of these diseases. The idea that tumor growth can be accompanied by increased vascular proliferation was introduced over a hundred years ago (Ferrara 2002). Thus, angiogenesis not only provides a method for estimating metastatic potential of different tumors, but it also offers a possibility for testing chemotherapeutic agents and their therapeutic potential (Benedetta Donati and Gozdzikiewicz 2008).
Vascular endothelial growth factor (VEGF) and its receptors: VEGFR-1, VEGFR-2, VEGFR-3, especially VEGFR-2, play a particularly important role in angiogenesis under both physiological and pathological conditions (Ferrara 2005; Shibuya 2001).VEGFR-2 seems to be the major transducer of VEGF signals in endothelial cells that lead to cell proliferation, migration, differentiation, tube formation, increase of vascular permeability, and maintenance of vascular integrity (Ferrara 2005; Shibuya 2001; Dvorak 2002). VEGF and VEGFR-2 are frequently up-regulated in a number of clinically important human diseases, including cancer and age-related macular degeneration (AMD) (Ferrara 2005; Shibuya 2001). It has been shown that blockade of the angiogenic pathways by antibody therapy is potentially an effective therapeutic strategy for inhibiting tumor growth and metastasis (Ferrara 2002, 2005; Shibuya 2001; Dvorak 2002; Nakamura et al. 2007) and the inhibition of VEGF or its receptor signaling system is an attractive target for therapeutic intervention (Schenone et al. 2007). Currently, the VEGF/VEGFR pathway is considered to be one of the most important regulators of angiogenesis and a key target in anticancer treatment (Wang et al. 2009; Kiselyov et al. 2007).
Monoclonal antibodies (mAbs) are well-established tools for investigating the proteome and have widespread applicability in biomedical science (Chiarella and Fazio 2008). Inhibition of angiogenesis with anti-VEGFR-2 mAbs has shown some therapeutic efficacy in animal tumor models (Zhu et al. 2003; Paz and Zhu 2005; Roth et al. 2007). One of these mAbs has entered phase I or I/II clinical trials with human leukemia (Zhu et al. 2003). No report has emerged that anti-VEGFR-2 antibody had been used in clinical treatment (Deckert 2009; Calogiuri et al. 2008). These mAbs, which lack human/mouse cross-reactive with VEGFR-2, could not bind to denatured VEGFR-2 antigen. Therefore, they could not be used to detect VEGFR-2 in several kinds of human solid tumor tissues (Witte et al. 1998; Zhu et al. 1999; Stewart et al. 2003; Li et al. 2004). However, two mAbs with human/mouse cross-reactive anti-VEGFR-2 were produced from an immune b9 allotype rabbit antibody library (Popkov et al. 2004). The importance of VEGFR-2 in tumors has encouraged extensive efforts to establish new anti-VEGFR-2 mAbs for cancer in human or mouse models. Until now, no anti-VEGFR-2 mAbs have been developed using hybridoma technology, which have human/mouse cross-reactivity and bind with linear and conformational epitopes of antigen. The present investigation makes up for these short-falls with the development of a mAb for diagnosis and clinical research.
VEGF-2 overexpressing human umbilical vein-derived endothelial cells (HUVECs) and VEGFR-2-positive of NIH-3T3 mouse fibroblast cells (NIH3T3) (Böldicke et al. 2005; Takahashi and Shibuya 1997; Benzinger et al. 2000) were used in preliminary studies and in the present study. A new high-affinity human/mouse cross-reactive monoclonal antibody named A8H1, specific to both VEGFR-2 linear and conformational epitopes, was established by hybridoma technology, confirmed by matrix-assisted laser desorption/ionization–mass spectrometry (MALDI-MS), protein database searching and other immune techniques. Notably, three-bands of VEGFR-2 protein in HUVECs, including a mature form of 230 kDa, an immature incomplete glycosylated form of 200 kDa, and an immature form of 150 kDa, were all seen clearly with A8H1 by immunoprecipitation (IP). Some unexpected and interesting phenomena appeared in the NIH-3T3 cells, which had not been previously reported, and these may be taken as evidence of the expression of VEGFR-2 in NIH-3T3. In addition, the A8H1 could also be used for the immohistochemical detection of VEGFR-2 in several human solid tumor tissues.
It is well known that mAbs have a short survival time in the human body, and a human anti-mouse antibody (HAMA) response may reduce the therapeutic effect, when used in clinical therapy. Though the murine A8H1 could serve as anti-VEGFR-2 for basic and clinical studies, its use was limited to targeted therapy in clinics. The problem of reducing the HAMA response will be summarized in our next study together with further studies about chimeric murine-human fragments from A8H1 for targeted therapy research.
Materials and methods
Cell lines
HUVECs and NIH-3T3 were purchased from the cell bank of the Chinese Academy of Science in Shanghai, China. SP2/0 myeloma cells were stored in our laboratory. They were cultured in DMEM medium (Gibco, Invitrogen, USA) containing 10% fetal calf serum (FCS) at 37 °C, 5% CO2.
Preparation of antigen
Total RNA from HUVECs was extracted with Trizol reagent (Invitrogen, USA) according to the manufacturer’s instruction. cDNA fragment (GeneBank accession No. NM002253, nucleotides 975-1283) sequence of VEGFR-2 was obtained from the total RNA by reverse transcriptase-polymerase chain reaction (RT-PCR) using the following primers: 5-cgggatccgatgtggttctgagtccgtctcatgg-3 and 5-cggaattctcatttttcatgaactctaacaaaggtg-3. The resulting product were cloned into the expression vector pET28a and pGEX-4T-2, and then cut with restriction enzymes BamHI and XhoI with T4 DNA polymerase separately. The generated plasmids named pET28a-His-VEGFR-2 and pGEX-4T-2-GST-VEGFR-2 were transformed into E. coli strain DH5α separately and the positive clones were confirmed by DNA sequencing. The E. coli strain BL21 was transformed with the two generated plasmids, respectively. The successfully-transformed E. coli was picked up from a single colony and was grown overnight at 37 °C in Luria–Bertani (LB) medium, supplemented with ampicillin. His-tagged VEGFR-2 (His-VEGFR-2) protein and glutathione S-transferase-fusion VEGFR-2 (GST-VEGFR-2) protein in the bacilli ultrasonic supernatant were purified by His-tag and GST-trap affinity chromatography (GE, USA) using the GE purification system. Purified His-VEGFR-2 protein and GST-VEGFR-2 protein were subjected to 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS–PAGE). Then the two proteins were measured with a BioPhotometer Plus (Eppendorf, Germany) and stored at −20° C for further use as immunogen and detective antigen.
Construction of hybridoma cell lines
Anti-VEGFR-2 mAbs were produced by immunization of Balb/c mice (National Rodent Laboratory Animal Resources, Shanghai Branch) with the antigen (His-VEGFR-2 protein) prepared earlier. The mice were immunized intraperitoneally with 100 μg His-VEGFR-2 protein as an immunogen in complete Freund’s adjuvant (Sigma–Aldrich, USA), followed by five boosts with the same amount of antigen with incomplete Freund’s adjuvant (Sigma–Aldrich, USA) at 2-week intervals. Blood samples from the immunized mice were detected by enzyme linked immunosorbent assay (ELISA) described below with GST-VEGFR-2 protein as a detective antigen. The mouse was given the final injection of 200 μg immunogen via intraperitoneal injection 3 days before cell fusion. The spleen was removed and splenocytes were fused with SP2/0 myeloma cells according to previously reported hybridoma technology (Köhler and Milstein 1975). Aliquots of the culture supernatant with growing hybridomas were screened for the presence of antigen-specific antibodies by ELISA with the GST-VEGFR-2 protein. Positive hybrids were immediately subcloned by limiting dilution and further propagated in more flasks and used for the production of ascites fluid in Balb/c mice with pristine (Sigma–Aldrich). Another Balb/c mouse was injected with a non-producing clone as a control, and the ascites fluid was used for SP2/0 blank antibody. These ascites fluid samples were collected and purified with affinity chromatography using protein G (GE, USA) using the manufacturer’s purification system, then measured with a BioPhotometer Plus and stored at −70° C for further use.
Detection of the monoclonal antibody reactivity with recombinant human VEGFR-2/Fc
ELISA plates were coated, respectively with the GST-VEGFR-2 protein (10 μg/ml) or recombinant human VEGFR-2/Fc Chimera (2 μg/ml, R & D, USA) and refrigerated at 4 °C overnight and washed with PBST (phosphate-buffered saline with 0.5% Tween) using an ELx405 microplate washer (Bio Tek, USA), and then blocked with 0.5% skim milk and washed with PBST. Blood samples from the immunized mice, culture supernatants from hybridoma cells which contained antibodies were added to the plates with GST-VEGFR-2 protein. Purified ascites fluid containing antibodies was added (concentration range: 31.25–1,000 ng/ml) to the plates with recombinant human VEGFR-2/Fc chimera. After incubation, the plates were washed, horseradish peroxidase (HRP)-conjugated goat anti-human antibody at 1:2000 dilution (Santa Cruz) was added to each well, followed 1 h later by addition of peroxidase substrate (Thermo Scientific, USA) to the washed plates. The reaction was stopped with 2 M H2SO4 and the absorbance was measured on a Multiskan Spectrum (Thermo Labsystems, USA) at 450 nm with a turbidity reference of 630 nm. In all ELISA assays, the SP2/0 blank antibody was used as the negative control antibody.
Affinity and kinetics assay of monoclonal antibody
Antibody affinity and kinetics assays were analyzed using the Biacore T100 System (GE, USA). Recombinant human VEGFR-2/Fc Chimera (R & D, USA) was diluted to 5 μg/ml with acetate buffer (10 mM NaAc, pH 5.5, GE, USA) and immobilized on the surface of a CM5 sensor chip (GE, USA) to capture purified mAbs. Purified ascites fluid containing mAbs was diluted in HBS-EP buffer (GE, USA) to concentrations ranging from 31.25 to 1,000 nmol/L and the capture was performed at a constant flow rate of 30 μl/min for 3 min at 25 °C. The association time was 180 s and the dissociation time was 600 s, followed by regeneration with 10 mM Glycine–HCl (GE, USA). The sensorgrams were evaluated using the Biacore T100 evaluation software.
Immunoglobulin isotyping of monoclonal antibodies
The mouse mAb isotyping reagents kit (ISO-2KT, Sigma, USA) was used for the determination of the immunoglobulin class of the hybridoma antibodies from purified mouse ascitic fluids. Goat anti-mouse IgM, IgG1, IgG2a, IgG3, IgA and IgG, with peroxidase labeled rabbit anti-goat IgG were used in the ELISA according to the manufacturer’s instructions.
Matrix-assisted laser desorption/ionization–mass spectrometry and protein database searching
To collect total cellular protein as native antigen from HUVECs and NIH3T3, 1 × 107 cells maintained in culture flasks were washed with phosphate-buffered saline (PBS) and lysed with 500 μl RIPA Lysis Buffer (Santa Cruz Biotechnology Inc) on ice for 20 min. The cell lysates were then centrifuged for 10 min at 12,000 rpm. The total cellular protein in each supernatant was collected, measured with a BioPhotometer Plus and store at −20° C for further use.
Fifty micrograms of total cellular protein from HUVECs and NIH3T3 were used for IP and SDS–PAGE as described below without western blot procedure, while SP2/0 blank antibody was used as the negative control. The SDS–PAGE gel was stained with coomassie brilliant blue and stored in 20% (v/v) ethanol/H2O. The light band in the gel was cut out, washed and digested with trypsin (Promega, USA) for matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS, Bruker Inc, Billerica, USA). The mass analysis was carried out using the SWISS-PROT database of the National Center for Biotechnology Information (NCBI) and the non-redundant database using MASCOT search program (Matrix Science, Oxford, UK) (www.matrixscience.com) to identify the mAb specificity of anti-VEGFR-2.
Western blot
One hundred micrograms of the two kinds of total cellular protein from HUVECs and NIH3T3 mentioned above were subjected to 10% SDS–PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was probed with purified anti-VEGFR-2 mAb at 10 μg/ml and β-actin (Santa Cruz, Biotechnology, USA) at 1:1000 dilution, followed by a secondary HRP-conjugated goat anti-human antibody (Dako, USA). The reaction was detected by ECL Western Blot Substrate (Pierce, USA) per the manufacturer’s instruction.
Immunoprecipitation
Twenty micro liters of resuspended volume of Protein A/G Plus-Agarose (Santa Cruz Biotechnology) with 5 μg anti-VEGFR-2 mAb were added into Eppendorf tubes and incubated at 4° C on a rotating device for 3 h. Precipitates were collected by centrifugation at 12,000 rpm for 30 s and washed three times with PBS. Then 30, 70, and 100 μg total cellular proteins of HUVECs and 100 μg total cellular protein of NIH3T3 cells were transferred to these tubes and incubated at 4° C on a rotating device overnight. The precipitate was then washed 5 times with PBS, and agarose beads were added with 40 μl 2× electrophoresis sample loading buffer and boiled to release immunocomplexes. After an additional centrifugation, proteins in the supernatant were resolved on a 10% SDS–PAGE. The SDS–PAGE gel band was used in a western blot as described previously, using another positive clone of purified ascites fluid as primary antibody without β-actin.
Immunofluorescence assay
HUVECs and NIH3T3 were cultured on Lab-Tek® chamber slide (Electron Microscopy Sciences, USA) and fixed in 1% (v/v) formaldehyde/PBS. After being washed with PBS, the two kinds of cells were added with the purified ascites fluid of anti-VEGFR-2 mAb at 20 μg/ml at 4 °C overnight with SP2/0 blank antibody as the negative control, and incubated with 0.01% Rhodamine-conjugated antibodies against mouse IgG (Sigma–Aldrich) for 2 h at room temperature. The cell nuclei were stained with 1 mg/ml DAPI (Dojindo, Japan) for 20 min at 37 °C. After washing, the samples were analyzed by fluorescence microscopy (ZEISS Axioskop 40, Germany). As mentioned above, the immunofluorescence experiment were done using cells from different passage numbers and repeated at least three times.
Fluorescence-activated cell sorter analysis
Approximately 1 × 106 cells of HUVEC and 1 × 106 NIH3T3 were treated with 150 μl purified ascites fluid of anti-VEGFR-2 mAb, 20 μg/ml, and incubated in Eppendorf tubes at 4 °C for 12 h. After being washed with PBS, these cells were allowed to react with 100 μl of 0.01% Rhodamine-conjugated antibodies against mouse IgG for 30 min at 37 °C. These cells were centrifuged and washed, then analyzed on the BD FACS Calibur using the Cell Quest program. Background staining was obtained by SP2/0 blank antibody at the same volume.
Immunohistochemistry
Human tissues, including liver cancer, prostate cancer, colon cancer and stomach cancer, were obtained from the Department of Pathology, Affiliated Hospital of Nantong University, Jiangsu, China. All tissues were formalin fixed and paraffin embedded. Sections were cut at 4 μm, deparaffinized and peroxidase was quenched with methanol and H2O2 3% for 15 min. Microwaving for antigen retrieval was used for 3 × 5 min. The anti-VEGFR-2 mAb was used as primary antibody applied at 4 °C overnight. Following washing with PBS, sections were incubated with HRP-conjugated goat anti-human antibody (Dako-cytomation, USA) for 15 min and washed. The color was developed by 15 min incubation with DAB solution (Kem-En-Tec Diagnostics, Denmark) and sections were weakly counterstained with haematoxylin. The mAb against rabies virus (isotype Ig2a, self-made in our laboratory) was used as the appropriate negative control.
Results
Expression of two recombinant VEGFR-2 proteins
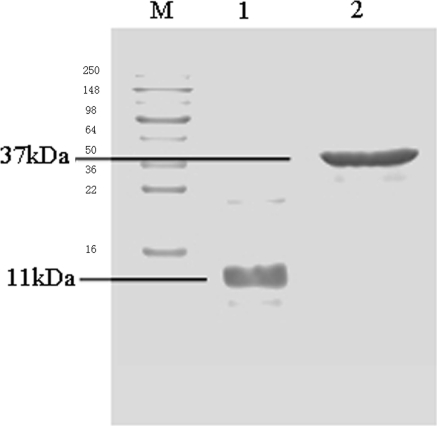
Two recombinant E. coli strain BL21 were constructed successfully. Sufficient quantities of His-VEGFR-2 protein and GST-VEGFR-2 protein were produced from those two recombinant E. coli strains, and these were purified by His-tag and GST-trap affinity chromatography, respectively using the purification system. The two types of VEGFR-2 purified recombinant proteins were 11 kDa and 37 kDa, determined individually by SDS–PAGE (Fig. 1). The concentrations of purified proteins were determined as 2 mg/ml. Both the His-VEGFR-2 and GST-VEGFR-2 proteins were stored at −20 °C for subsequent use as immune antigen with mice and detective antigen in ELISAs.
Fig. 1.
SDS–PAGE: Purified recombinant VEGFR-2 proteins of 11 kDa and 37 kDa. M marker, 1 His-VEGFR-2 protein, 2 GST-VEGFR-2 protein
Production of the anti-VEGFR-2 mAbs
Balb/c mice were immunized with His-VEGFR-2 protein as immunogen and the presence of anti-VEGFR-2 was checked by ELISA with GST-VEGFR-2 protein. Two stable hybridoma clones, named as 8H1 and 8H2, were found that had good affinity and specificity for VEGFR-2. MAbs from the two clones, named as A8H1 and A8H2 in order, were found to be IgG2a by immunoglobulin isotyping. They were purified by protein G chromatography and analyzed by SDS–PAGE (results not shown). The concentrations of purified mAbs were measured approximately 3 mg/ml and stored at 4 °C for further use. A8H1 antibody, from the most stable hybridoma clone of 8H1, was used for further study.
The A8H1 reactivity, affinity and kinetics assay with recombinant human VEGFR-2/Fc chimera
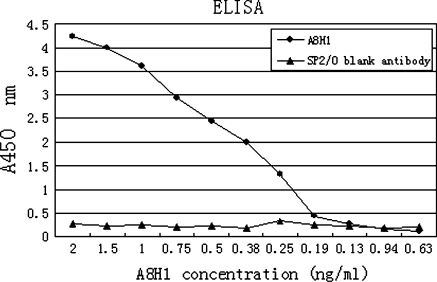
The mAb reactivity was assessed by ELISA with recombinant human VEGFR-2/Fc chimera at 2 μg/ml. Results indicated that the purified A8H1 could detect a level of 0.25 ng/ml (Fig. 2). Antibody affinity and kinetics assay using the BiacoreT100 System with evaluation software showed that the equilibrium dissociation constant (KD) of A8H1 was 6.952 × 10−9 M with recombinant human VEGFR-2/Fc chimera at 5 μg/ml.
Fig. 2.
ELISA: The reactivity of A8H1 with recombinant human VEGFR-2/Fc chimera at 2 μg/ml. The A8H1 could detect up to a level of 0.25 ng/ml
Cross-reactivity with human and mouse VEGFR-2 linear epitope
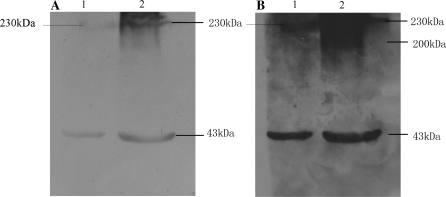
WB and IP were used to examine the binding ability of A8H1 to denatured native antigen proteins from total cellular protein. The WB showed that there were two bands at approximately 230 kDa for mature VEGFR-2 and a 43 kDa β-actin band in HUVECs, while only the 43 kDa β-actin band was clearly visible in NIH3T3 cells with a short exposure time. With sufficient exposure time, a 230 kDa VEGFR-2 band appeared in NIH3T3 cell samples and a 200 kDa band of the glycosylated intermediate form of VEGFR-2 appeared in the HUVEC samples (Fig. 3).
Fig. 3.
Western blot: a With 2 min exposure time, there were two bands of mature VEGFR-2 (230 kDa) and β-actin (43 kDa) visible in HUVEC, and only the β-actin was visible in NIH3T3 total protein samples. b With 10 min exposure time, the mature VEGFR-2 bands appeared in NIH3T3 and a glycosylated intermediate VEGFR-2 band (200 kDa) appeared in the HUVEC sample. M marker, 1 NIH3T3, 2 HUVECs
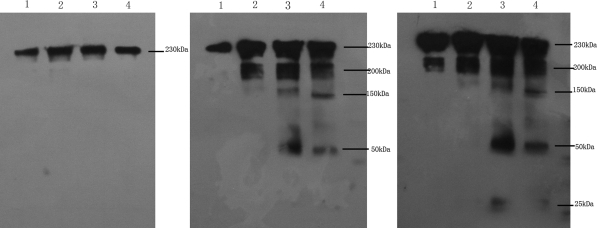
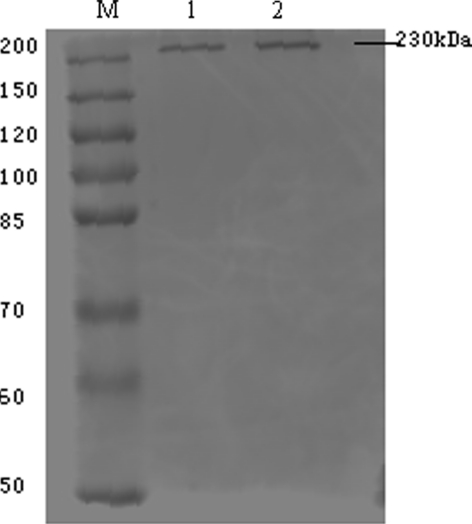
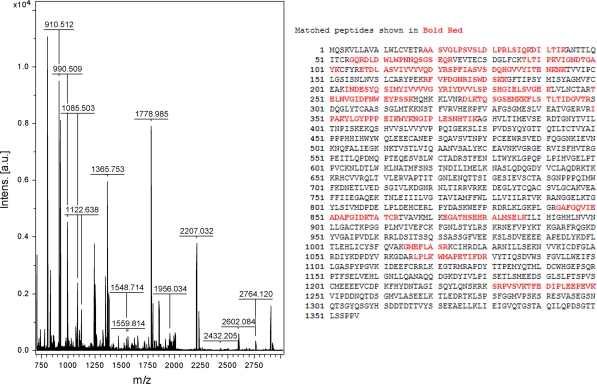
The IP results displayed a total cellular protein concentration- and exposure time-dependent pattern (Fig. 4). With increasing HUVEC total cellular protein and increasing exposure time, 230 and 200 kDa glycosylated intermediate forms, and a 150 kDa nonglycosylated form of VEGFR-2 were evident, and A8H1 heavy and light chain (50 and 25 kDa) antibodies appeared. With an even longer exposure time and sufficient NIH3T3 total cellular protein two VEGFR-2 bands of 230 and 200 kDa could be seen, while the other three bands (150 kDa, heavy and light chain of A8H1) were not seen. As shown in Fig. 5, the coomassie brilliant blue staining results indicated that the A8H1 were reactive with total cellular protein antigen from the above mentioned cell lines by SDS–PAGE. The one band clearly showed at 230 kDa in HUVECs was cut out and assessed by MALDI-TOF MS using protein database searching. The results confirmed the antibody’s specificity toward VEGFR-2 (Fig. 6).
Fig. 4.
Immunoprecipitation: With increasing the total cellular protein of HUVECs (line 2: 30 μg, line 2: 70 μg and line 3: 100 μg) and increasing exposure time (a, 1 min; b, 3 min; c, 6 min), mature form (230 kDa), glycosylated intermediate form (200 kDa) and nonglycosylated form (150 kDa) of VEGFR-2, and the heavy and light chain (50 and 25 kDa) of A8H1 appeared. With even 6 min of exposure time and sufficient amounts of total cellular protein (100 μg) from NIH3T3 (line 1), two bands (mature and glycosylated intermediate) also could be seen, while the other three bands (nonglycosylated form of VEGFR-2, heavy and light chain of A8H1) could not be seen. M marker, 1 100 μg cell total protein of NIH3T3, 2–4 30, 70 and 100 μg cell total protein of HUVECs
Fig. 5.
Coomassie brilliant blue staining after immunoprecipitation: The A8H1 was reactive with antigen of total cellular protein from HUVECs and NIH3T3. The one clearly mature form band in HUVECs was cut off for MALDI-TOF MS. M marker, 1 NIH3T3, 2 HUVECs
Fig. 6.
MALDI-TOF MS with protein database search: Matched peptides are shown in bold red. Database: IPI_human (71983 sequences; 30123581 residues). Top Score: 109 for IPI00021396, Tax_Id = 9606. Gene_Symbol = KDR Vascular endothelial growth factor receptor 2 precursor
Cross-reactivity with human and mouse VEGFR-2 conformational epitope
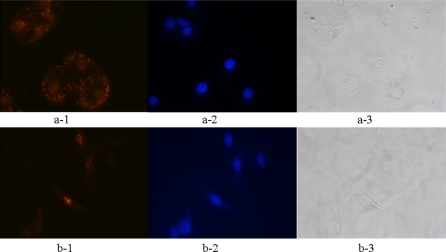
An immunofluorescence technique was used with the A8H1 to visualize the VEGFR-2 antigen–antibody interaction in HUVECs and NIH3T3 cells. The results showed that in HUVECs A8H1 identified antigen (red) in the intracellular and plasma membranes, and as expected A8H1 labeling was less in the NIH3T3 cells, and was seen unexpectedly in the nuclei of these cells (Fig. 7). Cell nuclei were stained blue with DAPI.
Fig. 7.
Immunofluorescence: A8H1 reactivity was higher in HUVECs (red) in the plasma membrane than in NIH3T3 cells in which nuclear labeling was apparent. Cell nuclei were stained with DAPI (blue). a: HUVECs, b: NIH3T3. 1: A8H1, 2: DAPI, 3: white light
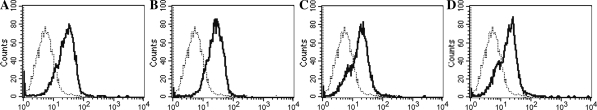
FACS analysis showed that different concentrations of A8H1 bound differently with HUVECs and NIH3T3 cells (Fig. 8). At the same concentration of A8H1, labeling of HUVECs was much higher than labeling of NIH3T3 cells. With decreasing A8H1 concentrations, there was a modest decrease in labeling.
Fig. 8.
Fluorescence-activated cell sorter analysis: A8H1 binding differed with HUVECs and NIH3T3 cells and is shown as a bold line. Background staining (shown as dashed line) was obtained by SP2/0 blank antibody. Gated values were calculated as following, a 20 μg/ml A8H1 with 35.55% HUVECs, b 10 μg/ml A8H1 with 31.33% HUVECs, c 20 μg/ml A8H1 with 10.18% NIH3T3, d 10 μg/ml A8H1 with 10.12% NIH3T3
Further analysis of the A8H1 in human cancer tissues by immunohistochemistry
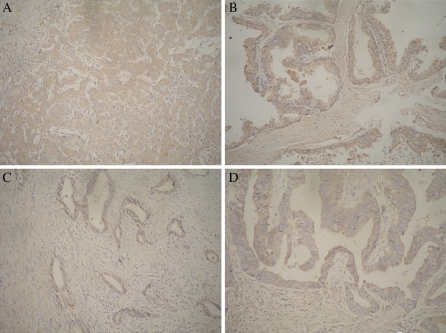
Several human cancer tissue samples, including liver cancer, prostate cancer, colon cancer and stomach cancer, were labeled immunohistochemically using A8H1 as the primary antibody. The experiments showed that the overexpression of VEGFR-2 by cancer tissues could clearly be detected by A8H1. Part of these solid tumor cells of these tissues, which were overexpressing VEGFR-2, were dyed dark brown in the plasma membrane of tumor cells and their nuclei did not react (Fig. 9).
Fig. 9.
Immunohistochemistry: Overexpressed VEGFR-2 cancer tissues were dyed brown in tumor cells. Notice that these nuclei did not react with A8H1. a human liver cancer tissue, b human prostate cancer tissue, c human colon cancer tissue, d human stomach cancer tissue
Discussion
There are some reports in the literature of the production of mAbs used against VEGFR-2. These antibodies had been produced by such methods as the hybridoma technique, phage display technique, gene engineering technology and so on (Witte et al. 1998; Zhu et al. 1999; Stewart et al. 2003; Li et al. 2004). Because A8H1 was broadly successful in identifying VEGFR-2, this mAb might be better than those other mAbs used against VEGFR-2 for basic and clinical studies.
Based on the above observations, the A8H1 murine anti-VEGFR-2 mAb was produced and isotyped as IgG2a, and was confirmed by MALDI-TOF MS with protein database searching. The high affinity was verified with recombinant human VEGFR-2/Fc chimera by ELISA and the Biacore T100 System. After A8H1 was purified by affinity chromatography, the mAb was characterized by several immunological methods in this study.
The total cellular proteins from HUVECs and NIH-3T3 cells were used and the denatured natural antigen produced to identify the immunological function of A8H1 using WB and IP. Fortunately, all three VEGFR-2 protein bands were clearly seen in samples from HUVECs when high concentrations of antibody were used and the exposure time was increased in the IP assay. These VEGFR-2 bands were the mature form of 230 kDa, the immature incomplete glycosylated form of 200 kDa, and the immature form of 150 kDa (Takahashi and Shibuya 1997). The immature form of 150 kDa was not detected in total protein samples from NIH-3T3 cells. When WB was used only the mature form and immature incompletely glycosylated VEGFR-2 form obtained from HUVECs and a small amount of the mature form of VEGFR-2 from NIH-3T3 cells could be seen when the exposure time was long. These observations, together with the human/mouse cross-reactivity characterized the anti-VEGFR-2 A8H1 mAb and indicated which kinds of VEGFR-2 exist in HUVECs and NIH-3T3 cells. Further studies using IF and FACS showed that, as with the WB and IP results, A8H1 binding and labeling of HUVECs were much higher than for NIH-3T3 cells.
NIH-3T3, which was regarded as a cell line that did not express VEGFR-2 in past studies (Takahashi and Shibuya 1997; Benzinger et al. 2000), showed some A8H1 nuclear labeling in the immunofluorescence assay, which was a surprising observation. Perhaps the prior studies that found that NIH-3T3 cells were non-expressive of VEGFR-2 used an antibody with a lower affinity than A8H1 or used a different experimental design to the one used here. Only one report (Gitay-Goren et al. 1992) indicated that VEGFR could be weakly detected in NIH3T3 cells. Though the purpose of the study was designed to certify the role of A8H1 in basic studies, we were able to verify the expression of VEGFR-2 in NIH-3T3 cells by the above methods. This observation provides a basis for future research of the relationship between VEGFR-2 and NIH-3T3 cells, and certifies that A8H1 has a high affinity for VEGFR-2.
To identify the binding of A8H1 in human solid tumor tissues by IHC, VEGFR-2 over-expressing cancer cells of human solid tumor tissues from liver cancer, prostate cancer and colorectal cancer showed staining of the cell membrane and cytoplasm. Nuclear proteins did not react with the mAb. The results suggest that A8H1 could not only be used in IHC for basic research, but the mAb might also be used for detecting over-expressed VEGFR-2 tissues in clinical research.
From these results it appears that A8H1 can recognize not only linear epitope of VEGFR-2 antigen measured by WB and IP, but can also recognize conformational epitope of VEGFR-2 antigen tested by IF and FACS. Together with the IHC results in human solid tumors, the A8H1 mAb is unique when compared to other anti-VEGFR-2 antibodies reported to date.
In conclusion, this study produced and characterized a high affinity anti-VEGFR-2 mAb with human/mouse cross-reactivity, and provides a useful tool not only for the in-depth study of the molecular structure and biological effects of VEGFR-2 protein, but also for testing VEGFR-2 distribution in various tissues and organs, and its role in the physiological and pathological changes in humans or mice. Further studies on the mAb could be continued by gene engineering techniques, additional animal models or preclinical experiments.
Acknowledgments
This study was supported by Natural Science Foundation (BS2007019) of Jiangsu Province and grant (H200938) from Health Department of Jiangsu Province, China.
Glossary
- VEGFR-2
Vascular endothelial growth factor receptor 2
- mAb
Monoclonal antibody
- HUVECs
Human umbilical vein endothelial cells
- NIH3T3
NIH-3T3 mouse fibroblast cells
- MALDI-TOF MS
Matrix-assisted laser desorption/ionization–mass spectrometry
Contributor Information
Zhenqing Feng, Phone: +86-138-51412491, FAX: +86-25-86862799, Email: fengzhenqing@njmu.edu.cn.
Jin Zhu, Phone: +86-139-51799321, FAX: +86-25-84540749, Email: zjsimmons@yahoo.com.cn.
References
- Benedetta Donati M, Gozdzikiewicz J. Angiogenesis and the progress of vascular and tumor biology: a tribute to Judah Folkman. Thromb Haemost. 2008;99:647–650. doi: 10.1160/TH08-02-0096. [DOI] [PubMed] [Google Scholar]
- Benzinger P, Martiny-Baron G, Reusch P, Siemeister G, Kley JT, Marmé D, Unger C, Massing U. Targeting of endothelial KDR receptors with 3G2 immunoliposomes in vitro. Biochim Biophys Acta. 2000;1466:71–78. doi: 10.1016/S0005-2736(00)00172-3. [DOI] [PubMed] [Google Scholar]
- Böldicke T, Weber H, Mueller PP, Barleon B, Bernal M. New highly efficient intrabody mediates complete inhibition of cell surface expression of the human vascular endothelial growth factor receptor-2 (VEGFR-2/KDR) J Immunol Methods. 2005;303:153–154. doi: 10.1016/j.jim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Calogiuri G, Ventura MT, Mason L, Valacca A, Buquicchio R, Cassano N, Vena GA. Hypersensitivity reactions to last generation chimeric, humanized [correction of humanized] and human recombinant monoclonal antibodies for therapeutic use. Curr Pharm Des. 2008;14:2883–2891. doi: 10.2174/138161208786369786. [DOI] [PubMed] [Google Scholar]
- Chiarella P, Fazio VM. LinksMouse monoclonal antibodies in biological research: strategies for high-throughput production. Biotechnol Lett. 2008;30:1303–1310. doi: 10.1007/s10529-008-9706-5. [DOI] [PubMed] [Google Scholar]
- Deckert PM. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr Drug Targets. 2009;10:158–175. doi: 10.2174/138945009787354502. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;94:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992;267:6093–6098. [PubMed] [Google Scholar]
- Kiselyov A, Balakin KV, Tkachenko SE. VEGF/VEGFR signalling as a target for inhibiting angiogenesis. Expert Opin Investig Drugs. 2007;16:83–107. doi: 10.1517/13543784.16.1.83. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature . 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Li R, Xiong DS, Shao XF, Liu J, Xu YF, Xu YS, Liu HZ, Zhu ZP, Yang CZ. Production of neutralizing monoclonal antibody against human vascular endothelial growth factor receptor II. Acta Pharmacol Sin. 2004;25:1292–1298. [PubMed] [Google Scholar]
- Nakamura K, Zen Y, Sato Y, Kozaka K, Matsui O, Harada K, Nakanuma Y. Vascular endothelial growth factor, its receptor Flk-1, and hypoxia inducible factor-1alpha are involved in malignant transformation in dysplastic nodules of the liver. Hum Pathol. 2007;38:1532–1546. doi: 10.1016/j.humpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Paz K, Zhu Z. Development of angiogenesis inhibitors to vascular endothelial growth factor receptor 2. Current status and future perspective. Front Biosci. 2005;10:1415–1439. doi: 10.2741/1629. [DOI] [PubMed] [Google Scholar]
- Popkov M, Jendreyko N, Gonzalez-Sapienza G, Mage RG, Rader C, Barbas CF., 3rd Human/mouse cross-reactive anti-VEGF receptor 2 recombinant antibodies selected from an immune b9 allotype rabbit antibody library. J Immunol Methods. 2004;288:149–164. doi: 10.1016/j.jim.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Roth P, Hammer C, Piguet AC, Ledermann M, Dufour JF, Waelti E. Effects on hepatocellular carcinoma of doxorubicin-loaded immunoliposomes designed to target the VEGFR-2. J Drug Target. 2007;15:623–631. doi: 10.1080/10611860701502723. [DOI] [PubMed] [Google Scholar]
- Schenone S, Bondavalli F, Botta M. Antiangiogenic agents: an update on small molecule VEGFR inhibitors. Curr Med Chem. 2007;14:2495–2516. doi: 10.2174/092986707782023622. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Func. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- Stewart M, Turley H, Cook N, Pezzella F, Pillai G, Ogilvie D, Cartlidge S, Paterson D, Copley C, Kendrew J, Barnes C, Harris AL, Gatter KC. The angiogenic receptor KDR is widely distributed in human tissues and tumours and relocates intracellularly on phosphorylation. An immunohistochemical study. Histopathology. 2003;43:33–39. doi: 10.1046/j.1365-2559.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen K, Niu G, Chen X. Site-specifically biotinylated VEGF(121) for near-infrared fluorescence imaging of tumor angiogenesis. Mol Pharm. 2009;6:285–294. doi: 10.1021/mp800185h. [DOI] [PubMed] [Google Scholar]
- Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Böhlen P. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155–161. doi: 10.1023/A:1006094117427. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Lu D, Kotanides H, Santiago A, Jimenez X, Simcox T, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor induced mitogenesis of human endothelial cells by a chimeric anti-kinase insert domain-containing receptor antibody. Cancer Lett. 1999;136:203–213. doi: 10.1016/S0304-3835(98)00324-3. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Hattori K, Zhang H, Jimenez X, Ludwig DL, Dias S, Kussie P, Koo H, Kim HJ, Lu D, Liu M, Tejada R, Friedrich M, Bohlen P, Witte L, Rafii S. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604–611. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]