Abstract
A Ujumqin sheep ear marginal tissue (USEM) fibroblast line, frozen in 147 cryovials with 4 × 106 cell each, was successfully established from 33 Ujumqin sheep ear marginal tissues using explant culture and cryopreservation techniques. The cells were morphologically consistent with fibroblasts. The growth curve was typical S-shape and the cell population passed through a lag phase, a logarithmic phase and a plateau phase. The population doubling time (PDT) was approximately 72 h. Tests for bacteria, fungi, viruses and mycoplasma were all negative. Isoenzyme polymorphism indicated that the genetic characteristics of the cell line were stable in vitro. Karyotyping analysis indicated that the chromosome number of a normal cell was of 2n = 54 and 95.4% of the entire population was diploid. The transfection efficiencies of six fluorescent proteins (pEGFP-N3, pEGFP-C1, pDsRed-N1, pEYFP-N1, pECFP-N1 and pECFP-mito) optimal at 48 h were from 18.5% to 30.1%. The cell line met all criteria from the American Type Culture Collection (ATCC). Not only has the germline of this important sheep breed been preserved at the cell level, but also valuable material had been provided for genome, postgenome and somatic cloning research. Moreover, the establishment of this technical platform may provide both technical and theoretical support for storing the genetic resources of other animals and poultry at the cell level.
Keywords: Ujumqin sheep, Fibroblast line, Biological characteristics, Cryopreservation
Introduction
Livestock and poultry breed resources were an indispensible part of overall biological breed resources and formed a basis for human survival and sustainable development of the society (Wu 1999). The origin of domestic livestock and poultry breeds was reviewed from the first center of domestication in the Middle East during the Neolithic Revolution (Hodges 2006). For about 10,000 years, farmers had been managing cattle, sheep, and goats in a sustainable way, leading to animals that were well adapted to the local conditions (Taberlet et al. 2008). However, about 200 years ago, the situation started to change dramatically. Increased global use of highly productive breeds of farm animals has been coupled to loss of genetic diversity in most species (Woelders et al. 2006). The livestock and poultry breed resource crisis in all of the world became aggravated gradually (Ma et al. 2002). During the last 50 years of the twentieth century, 740 livestock and poultry breeds had become extinct, 531 were endangered, 1092 were critically endangered and only 2395 breeds were safe (Pattison et al. 2007). Ma and Wu (2001) reported the threatened situation of 231 livestock and poultry breeds in China indicating that 16 breeds were seriously endangered, 9 endangered slightly, 17 threatened minimally, 57 endangered potentially, and 132 were safe.
It would be a tremendous loss of genomic resources and life science treasury of the world if these livestock and poultry breeds had not been conserved in any forms before their complete extinction (Guan and Ma 2003). The conservation of endangered breeds as live animals, cryopreservation and banks for sperm, embryos or DNA were the strategy of the governments and breeding organizations to maintain genetic diversity, which could subsequently be used for breeding and production in agriculture (Falge et al. 1996). In addition to the methods outlined above, establishment of somatic cell banks using cryopreservation techniques was a novel and effective approach to conserve and maintain the diversity of domestic animals (Guan and Ma 2003).
Ujumqin sheep, a larger version of the Mongolian sheep, was found in Inner Mongolia, China. These sheep have a type of coarse wool commonly called “carpet wool” and have an ability to deposit fat in the tail (fat-tail sheep). They were adapted to the unfavourable local environmental conditions of the north and northwest pastoral grasslands (http://www.ansi.okstate.edu/breeds/sheep/). The Ujumqin sheep was listed as one of the 78 nationally protected domestic animals by the Chinese government in 2000 (http://cdad-is.org.cn/viewnews/home.cbs?db=sheep).
Our project seeks a different approach to conserving important domestic animals in imminent danger. Our aim is to cryopreserve this nationally protected genomic resource for the purposes of reviving endangered breeds by cloning, and supplying a convenient and effective resource for genomic research using a characteristic Chinese sheep breed to initiate the establishment of cell line and the study of their biological characteristics. Moreover, with the development of science and technology, the roles of limited cell lines will become increasingly prominent and there will be currently unforeseen applications.
Materials and methods
Cell culture
Ear tissues from 33 Ujumqin sheeps were rinsed and cropped into 1 mm3 pieces. These tissue pieces were seeded in flasks and cultured at 37 °C for 4–5 h in incubator with 5% CO2. Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) was added into inverted flasks. The culture was observed every 24 h for the occurrence of a substantial outgrowth of cells from the tissue pieces. Cells logarithmic phase were harvested, split into new flasks under the ratio 1:2 or 1:3 and incubated at 37 °C with 5% CO2 (Doyle et al. 1990).
Cryopreservation and recovery
After 3–5 passages, the cultured cells were harvested, and then centrifuged to form a pellet at 168 g for 8 min. The supernatant was removed and the cell pellet was re-suspended in a freezing medium containing 10% dimethylsulfoxide (DMSO), 50% FBS and 40% DMEM to reach a final cell density of 3–5 × 106 viable cells/ml. Cell suspensions were aliquoted into sterile cryovials labeled with breed, gender, passage number and the date. The cryovials kept at 4 °C for 30 min, then put into a programmed cryopreservation system for 12 h, and finally transferred to liquid nitrogen for long-term storage. At recovery, the frozen vials were taken from the liquid nitrogen and thawed in 42 °C water bath, and then transferred into a flask with DMEM containing 10% FBS. The cells were cultured at 37 °C with 5% CO2. The medium was renewed 24 h later (Doyle et al. 1990; Stacey and Masters 2008).
Viability assay
Viabilities before cryopreservation and after recovery were detected using the Trypan blue exclusion test. Cells were seeded in 6-well plates and 1000 cells were counted for calculation (Strober 2001).
Growth kinetics
Cells were plated on 24-well microplates with a density of 4 × 104 cells/well and cultured for 7 days. Cells were counted every 24 h until the plateau phase. Mean values were used to plot a growth curve and to calculate the PDT (Kozak 1992).
Microorganism detection
Cells were thawed and centrifuged to form a pellet at 168 g for 8 min. The supernatant was removed and the cell pellet was re-suspended in DMEM free of antibiotics. The cell suspension was incubated in tryptic soy agar (TSA) and malt extract medium at 37 °C and 26 °C respectively for bacterial and fungal contamination (Stacey 1997).
Under normal culture conditions, cells were selected randomly for cytopathogenic examination using phase-contrast microscopy following Hay’s hemadsorption protocol (Hay 1992).
Mycoplasma was detected by an EZ-PCR mycoplasma test kits (Kibbutz Beit Haemek, Israel). The EZ-PCR mycoplasma test kit uses PCR technology to detect mycoplasma contamination in cell cultures. The primer set is specific to the highly conserved 16S rRNA coding region in the mycoplasma genome and allows various mycoplasma species as well as Acholeplasma and Spiroplasma species to be detected.
Chromosome and karyotype analysis
Cells were grown in a medium containing colcemid for 6 h at 37 °C, and then harvested. Cell chromosomes were prepared using a slight modification of the method of Liang et al. (2006). Chromosomes were counted, and the modal Ujumqin sheep cell karyotype was prepared by the method of Ford et al. (1980). Fifty to 100 spreads were sampled for counting chromosome numbers per initial spread. Ten photographs of metaphase chromosomes were selected, and the long and short arms of 27 pairs of chromosomes were measured. The chromosomal parameters of relative length, centromere index and kinetochore type were calculated according to Lavania and Sharma (1981).
Isoenzyme analysis
The isoenzyme patterns of lactate dehydrogenase (LDH) and malate dehydrogenase (MDH) from Ujumqin sheep ear marginal tissue (USEM) fibroblasts were analyzed using modified polyacrylamide gel electrophoresis (PAGE) as described by Zeng and Chen (1997). Cells were harvested and a protein extraction solution (0.9% Triton X-100, 0.06 mmol NaCl/EDTA in mass ratio 1:15) was added until the cell density was adjusted to 5 × 107 cells/ml. These samples were centrifuged, and after removal of the supernatant, they were stored in aliquots at −80 °C. An equal volume of 40% sucrose liquid and 2.5 μl loading buffer was added to the samples and mixed before performing the PAGE. The mobility of different patterns was represented by the relative mobility fronts, which were calculated as the ratio of the migration distance of the isozyme band to that of the bromchlorphenol blue.
Expression of six fluorescent proteins in USEM fibroblasts
Cells were seeded in 24-well plates and transfected with the plasmid DNA of six fluorescent proteins (pEGFP-N3, pEGFP-C1, pDsRed-N1, pEYFP-N1, pECFP-N1 and pECFP-mito) by Lipofectamine 2000. The plasmid DNA (μg) to Lipofectamine 2000 (μl) ratio was 1:3. The medium was renewed 6 h after transfection, and cells were observed 24, 48, 72 h, 2 weeks and 1 month after transfection using a Nikon TE-2000-E inverted confocal microscope with excitation wavelengths of 405, 488 and 543 nm to determine the transfection efficiency and the morphology of positive cells. For each experimental group, images were captured from 10 visual fields to determine the total and positive cell counts in each field for the calculation of transfection efficiencies (Kain et al. 1995; Guan et al. 2006).
Results
Cell morphology
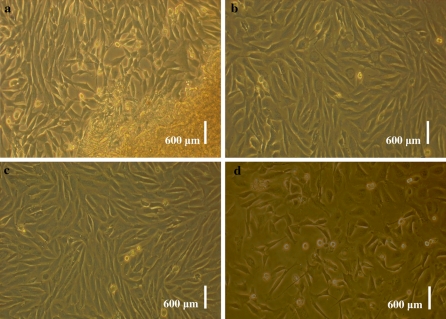
Epithelial-like and fibroblast-like cells migrated from tissue pieces 5–12 day after explanting (Fig. 1a). As time passed, cells continued to proliferate and were subcultured when they reached 90% confluences (Fig. 1b). After subculture, the fibroblasts grew rapidly, gradually outgrew and excluded their epithelial counterparts. After 2–3 passages, we obtained purified fibroblasts (Fig. 1c). The cells were morphologically consistent with fibroblasts. They were viable and grew well after thawing (Fig. 1d).
Fig. 1.
Morphology of USEM fibroblasts. a primary cultured cells from Ujumqin sheep ear marginal tissue; b subcultured fibroblasts cells of Ujumqin sheep; c fibroblasts before cryopreservation; d fibroblasts after thawing and cultured at 12 h. Scale bar: a–d 600 μm
Cell viability
A viable USEM fibroblasts have a clear cytoplasm whereas a nonviable cell has a blue cytoplasm when stained with Trypan blue. The mean viability before cyropreservation was 96.3 and 90.5% after recovery. The results indicated that cells were viable, and the culture conditions were appropriate and cells were not significantly affected by cryopreservation.
Growth kinetics
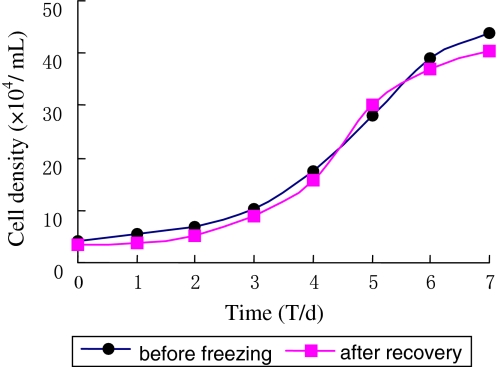
The growth curve of USEM fibroblast line before cyropreservation and after recovery displayed typical “S” shape (Fig. 2). Lag phase of around 48 h was observed after the cells were seeded, which was needed for recovery corresponding to the recovery period after trypsin damage. After this, they proliferated rapidly and entered the exponential growth phase until the stationary phase after about 6–7 days. Then growth plateaued and the population began to degenerate. The PDT calculated from the curve was about 72 h.
Fig. 2.
The growth curve of USEM fibroblast line. The growth curve was typical S-shape. A lag phase of around 48 h was observed after cells were seeded. Then, cells proliferated and entered the logarithmic phase until they reached the stationary phase after about 6–7 days. The cells reached the plateau phase
Microbial analysis
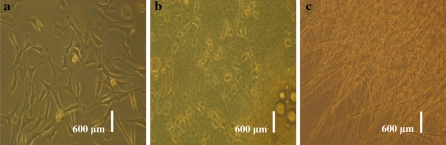
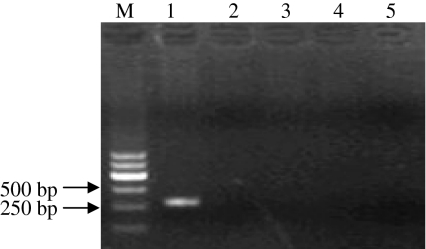
Tests for contamination with bacteria and fungi were negative, and no microorganisms were observed in the culture media (Fig. 3). No viruses were indicated by the cytopathogenic evidence or by the hemadsorption test. Mycoplasma testing by the EZ-PCR test kits was negative, showing that the established USEM fibroblast line was not contaminated by mycoplasma. In contrary, an −270 bp fragment was present in agarose gel electrophoresis for the positive control groups (Fig. 4).
Fig. 3.
Bacteria and fungi contamination for the USEM fibroblast line. a USEM fibroblasts subjected to bacteria and fungi contamination were negative; b positive control, cells contamined by bacteria; c positive control, cells contamined by fungi. Scale bar: a–c 600 μm
Fig. 4.
Mycoplasma contamination for the USEM fibroblast line. Cells were cultured and detected by EZ-PCR mycoplasma test kits. Panel 1, positive control; Panel 2, negative control; Panel 3–5, USEM fibroblasts samples. Mycoplasma testing result was negative
Chromosome and karyotype analysis
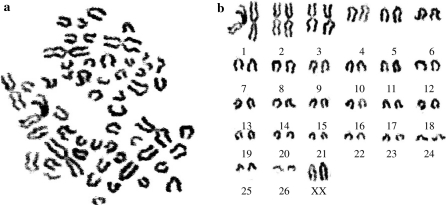
The chromosome number of Ujumqin sheep is 2n = 54, consisting of 26 pairs of euchromosomes and two sex chromosomes, XY (♂) or XX (♀) (Fig. 5a, b). Among 26 pairs of autosomes, three largest pairs are metacentric, 4–26 pairs are telocentric (Table 1). In size, X chromosome is the biggest telocentric chromosome, and Y chromosome is the smallest submetacentric chromosome. Chromosomes were counted at first and third passage and 95.4% of the cells analyzed were diploid (2n = 54) in 100 cell spreads. The results supported the conclusion that the USEM fibroblast line was reproducibly diploid in vitro.
Fig. 5.
Chromosome at metaphase and karyotype of Ujumqin sheep (♀). a cells were grown in a medium containing colcemid for 6 h at 37 °C, then chromosomes were prepared; b the karyotype of Ujumqin sheep consisting of 26 pairs of euchromosomes and 2 sex chromosomes, XX, the chromosome number was 2n = 54
Table 1.
Chromosome parameters of Ujumqin sheep
| Chromosome number | Raletive length | Centromere morphology | Chromosome number | Raletive length | Centromere morphology |
|---|---|---|---|---|---|
| 1 | 19.4 ± 0.36 | M | 15 | 5.1 ± 0.15 | T |
| 2 | 19.0 ± 0.12 | M | 16 | 4.9 ± 0.39 | T |
| 3 | 18.3 ± 0.12 | M | 17 | 4.3 ± 0.37 | T |
| 4 | 9.3 ± 0.28 | T | 18 | 4.5 ± 0.33 | T |
| 5 | 9.0 ± 0.03 | T | 19 | 4.1 ± 0.11 | T |
| 6 | 8.8 ± 0.16 | T | 20 | 3.9 ± 0.9 | T |
| 7 | 8.2 ± 0.03 | T | 21 | 3.6 ± 0.15 | T |
| 8 | 8.1 ± 0.22 | T | 22 | 3.3 ± 0.12 | T |
| 9 | 7.8 ± 0.16 | T | 23 | 3.3 ± 0.26 | T |
| 10 | 7.2 ± 0.21 | T | 24 | 3.4 ± 0.17 | T |
| 11 | 7.3 ± 0.16 | T | 25 | 3.1 ± 0.11 | T |
| 12 | 6.2 ± 0.18 | T | 26 | 3.2 ± 0.27 | T |
| 13 | 5.3 ± 0.17 | T | X | 9.7 ± 0.22 | T |
| 14 | 5.0 ± 0.22 | T | X | 9.4 ± 0.19 | T |
This Table shows the chromosome parameters of Ujumqin sheep from ten metaphase chromosomes, the results are expressed as % raletive length of chromosomes. M represent metacentric chromosome, T represent telocentric chromosome
Isoenzyme polymorphism
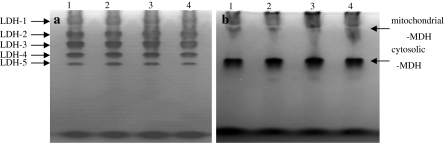
LDH and MDH isoenzyme patterns were obtained from USEM fibroblasts and compared with those of Ganjia goat, Duolang sheep and Alatai sheep ear tissue fibroblasts. Five bands were apparent representing LDH1, LDH2, LDH3, LDH4 and LDH5 from anode to cathode (Fig. 6a). The patterns of MDH showed two bands (Fig. 6b). One band of cytosolic MDH was located near the anode and another bands of mitochondrial MDH was found near the cathode. The relative mobility fronts of LDH (Table 2) and MDH (Table 3) was calculated. Different species and breeds have distinctive isoenzyme band patterns and each band has a different relative mobility. Those results indicated that the genetic characteristics of USEM fibroblasts were stable in vitro.
Fig. 6.
LDH and MDH isoenzymes for different sheep breeds. a LDH zymotype for Ganjia goat, Duolang sheep, Ujumqin sheep and Alatai sheep presented in lanes 1–4; b MDH zymotype for Alatai sheep, Ujumqin sheep, Duolang sheep and Ganjia goat presented in lanes 1–4
Table 2.
Relative mobility fronts of LDH in different sheep breeds
| Breed name | LDH1 | LDH2 | LDH3 | LDH4 | LDH5 |
|---|---|---|---|---|---|
| Ujumqin sheep | 20.31 | 32.81 | 39.18 | 46.72 | 50.08 |
| Ganjia goat | 21.96 | 29.58 | 34.38 | 46.79 | 49.81 |
| Duolang sheep | 21.88 | 29.69 | 35.94 | 46.88 | 50.12 |
| Alatai sheep | 18.75 | 31.25 | 39.06 | 44.09 | 49.97 |
This Table presents the results as % relative mobility fronts
Table 3.
Relative mobility fronts of MDH in different sheep breeds
| Breed name | Mitochondrial MDH | Cytosolic MDH |
|---|---|---|
| Ujumqin sheep | 15.25 | 45.76 |
| Ganjia goat | 20.34 | 44.13 |
| Duolang sheep | 15.63 | 47.46 |
| Alatai sheep | 16.95 | 44.07 |
This Table presents the results as % relative mobility fronts
Expression of six fluorescent proteins in cells
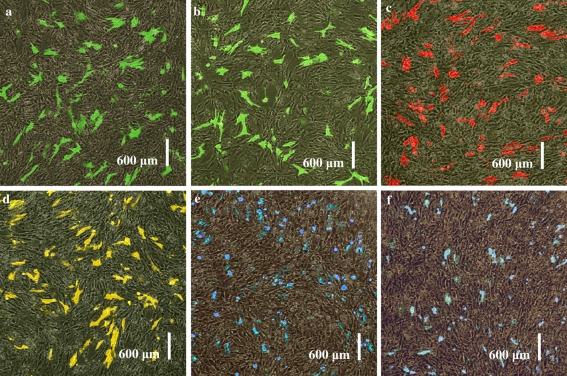
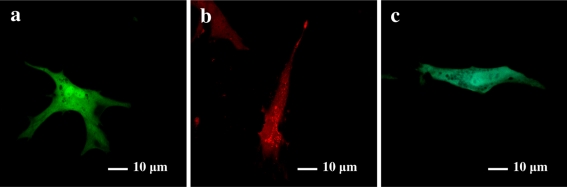
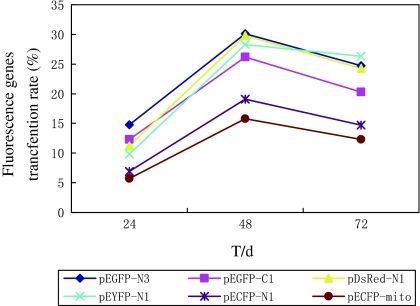
Six fluorescent proteins pEGFP-N3, pEGFP-C1, pDsRed-N1, pEYFP-N1, pECFP-N1 and pECFP-mito were highly expressed in USEM fibroblasts (Fig. 7a, b, c, d, e, f). Positive cells were observed at 24, 48, 72 h, 2 weeks and 1 month after transfection. The green and cyan fluorescences could be observed throughout the cytoplasm and nuclei of control cells except in cryptomere vesicle (Fig. 8a, c), whereas, red fluorescence was mostly seen in cytoplasm with a perforated pattern (Fig. 8b). The strongest fluorescent intensities and the highest transfection efficiencies of the six fluorescent proteins appeared at 48 h after transfection between 18.5 and 30.1% (Fig. 9). The transfer efficiency of the green fluorescent proteins (pEGFP-N3 and pEGFP-C1) were maximal, whilst, the cyan fluorescent proteins (pECFP-N1 and pECFP-mito) were significantly lower than those of the green, yellow and red fluorescent proteins (p < 0.01). Most positive cells shrunk and shed 72 h after transfection. The number of cells expressing fluorescent proteins decreased and the fluorescence intensity gradually faded, and disappeared 7 days after transfection, though some cells still expressed fluorescent proteins after 2 weeks and even after 1 month. These results showed that the expression of exogenous genes has no obvious effect on the growth and proliferation of the USEM fibroblasts.
Fig. 7.
Comparative figures of expression of six fluorescent protein transgenes in USEM fibroblasts. a expression of pEGFP-N3 in cells at 48 h; b expression of pEGFP-C1 in cells at 48 h; c expression of pDsRed-N1 in cells at 48 h; d expression of pEYFP-N1 in cells at 48 h; e expression of pECFP-N1 in cells at 48 h; f expression of pECFP-mito in cells at 48 h. Scale bar: a–f 600 μm
Fig. 8.
The expression and distribution of six fluorescent proteins in USEM fibroblasts. a was the transfection result of pEGFP-N3 at 48 h after transfection; b was the transfection result of pDsRed1-N1 at 72 h after transfection; c was the transfection result of pECFP-mito at 72 h after transfection. Scale bar: a–c 10 μm
Fig. 9.
Transfection efficiencies of six fluorescence genes in the USEM fibroblasts. The strongest fluorescence intensity and the highest transfection efficiency of the six fluorescent proteins appeared 48 h after transfection between 18.5 and 30.1%. The transfer efficiency of the green fluorescent proteins (pEGFP-N3 and pEGFP-C1) were maximal, whilst, the cyan fluorescent proteins (pECFP-N1 and pECFP-mito) were significantly lower than those of the green, yellow and red fluorescent proteins (p < 0.01)
Discussion
The cryopreservation in vitro comprises materials like semen, embryos and oocytes. However, there are a number of reasons, why these techniques cannot generally be used for endangered breeds world wide. Deep freezing of semen and embryos can only be done for a number of species, and furthermore requires species specific techniques. Moreover, it needs substantial infrastructure in the countries which is not generally available. Instead, storage of somatic cells may open an option as pointed out by Corley-Smith and Brandhorst (1999). Somatic cells can be collected from livestock and poultry tissues, including ear marginal tissues, embryos or internal organs, making the collection of samples cheap and fast. Collecting a sufficiently large number of samples is thus also possible in countries with little infrastructure. Thus, somatic cell cryopreservation is proposed as the method of choice for the rapid creation of emergency gene banks.
In primary culture, fibroblasts were usually mixed with epithelial cells, which may grow either in groups or scattered (Guan et al. 2005). Epithelial cells and fibroblasts have different tolerances to trypsin, so fibroblasts can be collected at appropriate digestion time. The fibroblasts were first detached from the flask wall while the epithelial cells remained attached when digested with trypsin (Wu et al. 2008; Liu et al. 2008). We can obtained purified USEM fibroblasts by 2–3 passages.
The dye exclusion test was used to determine the number of viable cells present in a cell suspension. It was based on the principle that live cells possess intact cell membranes that exclude certain dyes, such as trypan blue, eosin, or propidium, whereas dead cells do not (Strober 2001). In this test, a cell suspension was mixed with trypan blue and then examined to determine whether cells take up or exclude dye. A viable USEM fibroblasts have a clear cytoplasm whereas a nonviable cell have a blue cytoplasm. The viability before cyropreservation and after recovery was about 96.3 and 90.5% repectively, indicated that cytopreservation had little influence on the viability of the freezing cells. It was likely that Ujumqin sheep genetic materials could be conserverd by freezing their fibroblasts in liquid nitrogen for long term storage.
Normal cells possess stable chromosome numbers, shape and structure. Therefore, karyotype analysis is an effective method for distinguishing normal cells from variants. Karyotype analysis also identifies the gender of the animal from which a cell line is derived (Stacey 1997; Ansari et al. 1999). The biological and genetic characteristics of the cells may be affected by in vitro culture conditions after many passages, so only a minimal number of passage should be performed in order to conserve them (Guan et al. 2005; Liu et al. 2008). USEM fibroblasts were cryopreserved within five passages. 100 spreads of the first and third passages were subjected to karyotype analysis. The numbers of hypodiploid, diploid and hyperdiploid chromosomes were counted, and the diploid rate was 95.4%, indicating that the USEM fibroblast line was genetically stable. The Ujumqin sheep karyotyping confirmed the karyotype characters of sheep (Shen and Guo 1980; Liang et al. 2006). A small percentage of cells displayed abnormal chromosomes, presumably as a result of chromosome loss or overlap during preparation, or chromosomal damage during culture and passage in vitro (Guan et al. 2005).
Isoenzyme polymorphism is commonly selected to distinguish between normal and tumor cells, act as biochemical index of animal classification and confirm the species origin of the cells (Steube et al. 1995). ATCC considered the biochemical analysis of isoenzyme polymorphism to be a routine method for detecting intercellular contamination (Hay 1992). In almost all vertebrate tissues examined the enzyme LDH has heen shown to consist of five distinct isoenzymes (Coffin and Hall 1974). Using polyacrylamide gel electrophoresis, a good and clear separation of all five LDH isoenzymes from heart, liver, lung and muscle in Small Tail Han sheep and German Merino was achieved by Li et al. (2002). Five LDH isoenzymes were also obtained in Qinghai native goats serum (Xu 2003). In this study, we obtained isoenzyme patterns for LDH and MDH, and improved the ATCC starch gel electrophoresis method. The isoenzyme bands of LDH and MDH in USEM fibroblasts were clear and the isoenzyme activities in vitro was similar to that in the original tissues. The results indicated that the genetic characteristics of USEM fibroblasts were stable. It was thus demonstrated that the Ujumqin sheep genetic materials could be conserved by freezing their fibroblasts in liquid nitrogen for a long term.
Fluorescent proteins were rapidly becoming an important reporter molecules for monitoring gene expression and protein localization in vivo, in situ and in real time (Shaner et al. 2007; Kain et al. 1995; Baird et al. 2000; Rizzo et al. 2004). In this study, The USEM fibroblasts were transfected with plasmid DNA coding for fluorescent protein, a significant fluorescent signal was observed, suggesting gene expression and in vitro protein localization in the cells monitored for. Transfection had no significant effects (p > 0.05) on cell morphology, growth and proliferation, apoptosis rate or reduplication status compared with untransfected control under optimal conditions. The number of fluorescent cells decreased 1 week after transfection but a few scattered cells remained positive after 1 month, indicating that the exogenous gene expression in the fibroblast was modified. The fluorescent protein positive cell strains observed here will provide donor cells for transgenic sheep cloning. The USEM fibroblasts can be widely used as tools for investigating the functions of exogenous genes. This new resource will be important for future identification of breedspecific genetic markers and for nuclear transplantation and transgenic cloning investigations.
Acknowledgments
This research was supported by the “863” National Major Research Program (2006AA10Z198, 2007AA10Z170), National Key Technology R&D Program (2006BAD13B08, 2008BADB2B01) and a project (2008ZX08009-003) from the Ministry of Agriculture of China for transgenic research.
Footnotes
The authors Ri Su Na and Qian Jun Zhao as made the equivalent contribution to this paper.
Contributor Information
Wei Jun Guan, Phone: +86-10-62815992, FAX: +86-10-6281-3463, Email: wjguan86@iascaas.net.cn.
Yue Hui Ma, Phone: +86-10-62813463, FAX: +86-10-62813463, Email: yuehuima@yahoo.com.cn.
References
- Ansari HA, Bosma AA, Broad TE, Bunch TD, Long SE, Maher DW, Pearce PD, Popescu CP. Standard G-, Q-, and R-banded ideograms of the domestic sheep (Ovis aries): homology with cattle (Bos taurus). Report of the committee for the standardization of the sheep karyotype. Cytogenet Cell Genet. 1999;87:134–142. doi: 10.1159/000015380. [DOI] [PubMed] [Google Scholar]
- Baird GS, Zacharias DA, Tsien RY. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci USA. 2000;97:11984–11989. doi: 10.1073/pnas.97.22.11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PA, Hall BK. Isozymes of lactate dehydrogenase (LDH) in skeletal tissues of the embryonic and newly hatched chick. J Embryol Exp Morphol. 1974;31:169–181. [PubMed] [Google Scholar]
- Corley-Smith GE, Brandhorst BP. Preservation of endangered species and populations: a role for genome banking, somatic cell cloning, and androgenesis? Mol Reprod Dev. 1999;53:363–367. doi: 10.1002/(SICI)1098-2795(199907)53:3<363::AID-MRD12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Doyle A, Hay R, Kirsop BE. Animal cells, living resources for biotechnology. New York: Cambridge; 1990. [Google Scholar]
- Falge R, Ehling C, Niemann H. Preservation of genetic variation in domestic animals using biotechnical methods. Dtsch Tierarztl Wochenschr. 1996;103:336–340. [PubMed] [Google Scholar]
- Ford CE, Pollock DL, Gustavsson I (1980) Proceedings of the First International Conference for the Standardisation of Banded Karyotypes of Domestic Animals. University of Reading Reading, England. 2nd–6th August 1976. Hereditas 92:145–162 [DOI] [PubMed]
- Guan WJ, Ma YH. The construction and evaluation of the cell bank of species of domestic animals on the brink of extinct. Rev China Agric Sci Technol. 2003;5:65–70. [Google Scholar]
- Guan WJ, Ma YH, Ding H, Yu TY, Zhang HY, Liang HQ. The establ ishment of fibroblast cell line and its biological characteristic research in small tail han sheep. Acta Veterinaria et Zootechnica Sinica. 2005;36:511–516. [Google Scholar]
- Guan WJ, Liu CQ, Na RS, Lu TF, Ma YH. Research on the six fluorescent protein expression in the fibroblast cells of Mongolia Sheep. Acta Veterinaria et Zootechnica Sinica. 2006;37:1141–1148. [Google Scholar]
- Hay RI. Cell line preservation and characterization. New York: Oxford; 1992. [Google Scholar]
- Hodges J. Conservation of genes and culture: historical and contemporary issues. Poult Sci. 2006;85:200–209. doi: 10.1093/ps/85.2.200. [DOI] [PubMed] [Google Scholar]
- Kain SR, Adams M, Kondepudi A, Yang TT, Ward WW, Kitts P. Green fluorescent protein as a reporter of gene expression and protein localization. Biotechniques. 1995;19:650–655. [PubMed] [Google Scholar]
- Kozak RW. Introduction to the issues: recently developed methods for characterizing cell lines. Dev Biol Stand. 1992;76:21–24. [PubMed] [Google Scholar]
- Lavania UC, Sharma AK. An interphase model for mitotic chromosome organization in eukaryota. Biosystems. 1981;14:171–178. doi: 10.1016/0303-2647(81)90066-6. [DOI] [PubMed] [Google Scholar]
- Li TJ, Duan XP, Wu QD, Song GM. Study on LDH isoenzymes in tissues of sheep. Anim Sci Vet Med. 2002;19:16–17. [Google Scholar]
- Liang HQ, Guan WJ, Yue WB. Analysis of chromosomal karyotype of fibroblast cell lines in Menggu Sheep. Chinese Qinghai J Anim Vet Sci. 2006;36:9–10. [Google Scholar]
- Liu CQ, Guo Y, Guan WJ, Ma YH, Zhang HH, Tang XX. Establishment and biological characteristics of Luxi cattle fibroblast bank. Tissue Cell. 2008;40:417–424. doi: 10.1016/j.tice.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Ma YH, Wu CX. Evaluation of threatening degree of the animal genetic resources in China. Ecol Domest Anim. 2001;22:8–13. [Google Scholar]
- Ma YH, Xu GF, Wang DY, Liu HL. Study on dynamic information of animal genetic resources in China. Scientia Agricultura Sinica. 2002;35:552–555. [Google Scholar]
- Pattison J, Drucker AG, Anderson S. The cost of conserving livestock diversity? Incentive measures and conservation options for maintaining indigenous Pelon pigs in Yucatan, Mexico. Trop Anim Health Prod. 2007;39:339–353. doi: 10.1007/s11250-007-9022-4. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- Shen CJ, Guo AL. Chromosomes of Tan-sheep and Mongolia sheep. Chin J Anim Vet Sci. 1980;1:83–86. [Google Scholar]
- Stacey GN. Standardization in animal cell technology. Folia Microbiol (Praha) 1997;42:113–116. doi: 10.1007/BF02898717. [DOI] [PubMed] [Google Scholar]
- Stacey GN, Masters JR. Cryopreservation and banking of mammalian cell lines. Nat Protoc. 2008;3:1981–1989. doi: 10.1038/nprot.2008.190. [DOI] [PubMed] [Google Scholar]
- Steube KG, Grunicke D, Drexler HG. Isoenzyme analysis as a rapid method for the examination of the species identity of cell cultures. In Vitro Cell Dev Biol Anim. 1995;31:115–119. doi: 10.1007/BF02633971. [DOI] [PubMed] [Google Scholar]
- Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3: Appendix 3B. doi: 10.1002/0471142735.ima03bs21 [DOI] [PubMed]
- Taberlet P, Valentini A, Rezaei HR, Naderi S, Pompanon F, Negrini R, Ajmone-Marsan P. Are cattle, sheep, and goats endangered species? Mol Ecol. 2008;17:275–284. doi: 10.1111/j.1365-294X.2007.03475.x. [DOI] [PubMed] [Google Scholar]
- Woelders H, Zuidberg CA, Hiemstra SJ. Animal genetic resources conservation in the Netherlands and Europe: poultry perspective. Poult Sci. 2006;85:216–222. doi: 10.1093/ps/85.2.216. [DOI] [PubMed] [Google Scholar]
- Wu CX. The theory and technology of the conservation of animal genetic resources-the specy foundation of animal agricultural continuing development in 21 century. J Yunnan Univ. 1999;21:7–10. [Google Scholar]
- Wu HM, Guan WJ, Li H, Ma YH. Establishment and characteristics of white ear lobe chicken embryo fibroblast line and expression of six fluorescent proteins in the cells. Cell Bio Int. 2008;32:1478–1485. doi: 10.1016/j.cellbi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Xu SC. Studies on activity and isozymes of serum lactate dehydrogenase in Qinghai native goats. J Qinghai Univ. 2003;21:1–3. [Google Scholar]
- Zeng KW, Chen FY. LDH isoenzyme of vertebrate animals. Harbin: Harbin; 1997. [Google Scholar]