Abstract
Human embryonic stem (hES) cells are able to give rise to a variety of cell lineages under specific culture condition. An effective strategy for stable genetic modification in hES cells may provide a powerful tool for study of human embryogenesis and cell-based therapies. However, gene silences are documented in hES cells. In current study, we investigated whether genes controlled under ubiquitin promoter are expressed during hematopoietic-endothelial differentiation in hES cells. Undifferentiated hES cells (H1) were transduced by lentivirus encoding green fluorescent protein (GFP) gene under ubiquitin promoter. GFP-expressing hES cells (GFP-H1) were established after several rounds of mechanical selection under fluorescence microscope. GFP gene was stably expressed in hES cells throughout prolonged (> 50 passages) cultivation, and in differentiated embryo body (EB) and teratoma. Hematopoietic and endothelial markers, including KDR (VEGFR2), CD34, CD31, Tie-2, GATA-1 and GATA-2, were expressed at similar levels during hES cell differentiation in parent hES cells and GFP-H1 hES cells. CD34+ cells isolated from GFP-H1 hES cells were capable to generate hematopoietic colony-forming cells and tubular structure-forming cells. Differentiated GFP-EB formed vasculature structures in a semi-solid sprouting EB model. These results indicated that a transgene under ubiquitin promoter in lentiviral transduced hES cells retained its expression in undifferentiated hES cells and in hES-derived hematopoietic and endothelial cells. With the view of embryonic mesodermal developing events in humans, genetic modification of hES cells by lentiviral vectors provides a powerful tool for study of hematopoiesis and vasculogenesis.
Keywords: Human embryonic stem cells, Differentiation, Hematopoiesis, Vasculogenesis
Introduction
Human embryonic stem (hES) cells are able to differentiate into cell types of all three germ layers. It makes them valuable tools in the study of early development process and a promising source of cells for therapies and gene modification (Thomson et al. 1998). Vectors for efficient transgene expression in hES cells and their progeny are critical to evaluate hES cell self-renewal and directed differentiation into specified lineages (Zaehres et al. 2005). Retroviral vectors derived from MLV were well established and have been a useful tool for gene delivery (Gordon and Anderson 1994). However, it was widely accepted that oncoretroviral transcription resulted in methylation-mediated silencing during long-term passages (Cherry et al. 2000; Pannell et al. 2000).
Lentiviruses are a subclass of retroviruses that are able to infect non-dividing cells and cause chronic illness in the host organisms. The properties of lentiviral vectors make them very attractive for use in gene therapy. For example, HIV-1 based vectors can be transduced into a variety of non-dividing human cells, including hematopoietic stem cells (Akkina et al. 1996; Ramezani et al. 2000), neurons (Blomer et al. 1997; Naldini et al. 1996a), and macrophage (Naldini et al. 1996b). In hES cells, several studies have succeeded in generating genetic modified sublines by using lentiviral vectors (Gropp et al. 2003; Ma et al. 2003; Suter et al. 2006). Transgene expression in hES cells was maintained during differentiation in embryo bodies (EBs) in vitro and in teratomas in vivo (Gropp et al. 2003; Suter et al. 2006). However, lentiviral expressed transgene under control of some ubiquitous promoters, such as CMV, CAG, PGK and EF1α is often suppressed in stem cells (Ramezani et al. 2000; Xia et al. 2007).
The major concern that limits the use of lentiviral vectors as gene delivery vehicles is inactivation of initially active transgene. In hES cells, it happens either immediately after lentiviral transduction and integration of the transgene into the host genome which is termed as “suppression”, or during propagation and differentiation of ES cells termed as “gene silencing” (Xia et al. 2007). Here we established lentiviral transduced hES cells in which intense expression of green fluorescent protein (GFP) transgene was obtained under ubiquitin promoter. Green fluorescence was detected throughout differentiation either via teratoma or via EBs. To specify hemato-endothelial differentiation, we used CD34 as the bilineage marker. CD34+ cells isolated from GFP-H1 hES cells were capable to generate hematopoietic colony-forming cells and tubular structure-forming cells that extensively expressed green fluorescence. Thus, lentiviral transgene modification provided a valuable approach for understanding certain genes of hematopoiesis and vasculogenesis during early development in humans.
Materials and methods
Vector plasmid construction and virus production
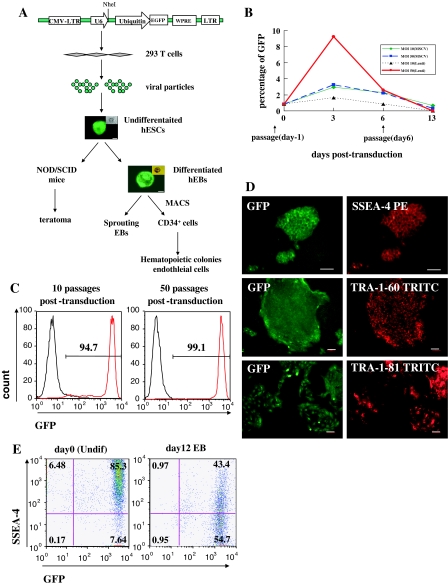
We constructed the lentiviral vector by inserting U6 promoter at a PacI site upstream of a Ubiquitin-EGFP expression cassette to generate pFUGW-U6 vector (Lois et al. 2002). Virus was produced by co-transfection of plasmids into 293T cells as previous described (Dull et al. 1998, Naldini et al. 1996b). Briefly, 0.5 × 106/ml 293T cells were seeded on gelatin-coated plates to 50–70% confluent on the day of transfection with PerFectin Transfection Reagent (Genlantis). A total amount of 8 μg of plasmid DNA: 2 μg of the envelope plasmid harboring the gene encoding VSV-G, 2 μg of the packaging plasmid pCMV ΔR8.91, and 4 μg of the transferring vector was transfected in serum-containing culture medium. 48–60 h after transfection, the medium containing viral particles was collected and concentrated by ultracentrifuge at 4 °C, 65,000 g for 90 min. Viral titrations were determined by transduction of 293T cells with serial dilution of virus in PBS.
Transduction of human ES cells
Human embryonic stem cells (hESCs, H1) were split by 1 mg/ml collagenase type IV and plated on irradiated or mitomycin-C inactivated mouse embryonic fibroblast (MEF) feeder cells 1 day before transduction. At the day of transduction (day 0), viral particles in MEF-condition medium (MEF-CM) were transferred onto hES colonies by spinoculation at 650 g for 30 min. Fresh viruses were added to H1 cells on MEF feeder with the same procedure next day. Transduced H1 cells were split on day 6. Transducing efficiency was carried out by FACS analysis at day 3, day 6 and 1 week after first passage (day 13). GFP-positive colonies were picked mechanically under fluorescence microscope (Nikon) for several rounds of splitting.
Maintenance of undifferentiated hES cells and in vitro differentiation
The hESCs were maintained at undifferentiated stage on irradiated MEF layers. Media, which consisted of DMEM/F-12 (Gibco), 20% knockout serum replacement (Gibco), 0.1 mM MEM non-essential amino acids (Gibco), 1 mM l-glutamine (Mediatech, Inc), 0.1 mM β-mercaptoethanol (Sigma), and 5 ng/ml rhFGF-basic (R&D Systems), were changed every day. Undifferentiated hES colonies were treated by 1 mg/ml collagenase type IV (Gibco) in DMEM/F12 and scraped mechanically at the day of splitting.
To induce hES cells differentiation in vitro, undifferentiated hES cells were cultured in differentiation medium of IMDM with 15% FCS, 0.1 mM MEM non-essential amino acids, 1 mM l-glutamine and antibiotics to form suspended embryoid bodies (EBs). Media were changed every other day.
CD34-positive selection and endothelial culture
After differentiation in EBs for indicated days, the differentiated EBs were digested by 1 mg/ml collagenase type IV and trypsinized in 0.05% trypsin–EDTA, and then passed through 40 μm cell strainer (BD Falcon) to get single cells. CD34-positive cells were selected using MiniMACS immunomagnetic separation systems (Miltenyi Biotec) according to the manufacturer’s instructions. After processed through LS + and MS + column (Miltenyi Biotec), at least 90% of the yielded cells expressed CD34 as determined by fluorescence-activated cell sorting. Sorted CD34+ cells were replated on gelatin in VEGF (50 ng/ml)-containing medium for further endothelial differentiation with the medium change every other day.
Hematopoietic colonies assay
Transduced and nontransduced hEBs were cultured for 12 days and making a single cell suspension as above. After CD34+ cells selection, hematopoietic colonies were demonstrated by growing these cells in Methocult GF + medium (StemCell Technologies) consisting of 1% methylcellulose, 30% fetal bovine serum, 1% bovine serum albumin, 10−4 M 2-mercaptoethanol, 2 mM l-glutamine, 50 ng/ml rhSCF, 20 ng/ml rhGM-CSF, 20 ng/ml rhIL-3, 20 ng/ml rhIL-6, 20 ng/ml rhG-CSF, and 3 U/ml rhEpo. Cells were plated at the density of 1 × 105/well in 6-well plate. Colony-forming units (CFUs) were photographed under microscope (Nikon) for fluorescence and bright field images.
Flow cytometry analysis
The percentage and intensity of transgene expression were analyzed on a FACSCalibur system (Becton–Dickinson) by comparing with nontransduced control hES-H1 cells. The proportion of undifferentiated cells among the transduced ES cells or in EBs was determined by immunostaining with mouse anti-human SSEA-4 antibody (Developmental Studies Hybridoma Bank) followed by incubation with secondary Phycoerythrin (PE) conjugated rat anti-mouse IgG (BD PharMingen). For the detection of hemato-endothelial markers after EBs induction, direct fluorochrome-conjugated anti-human monoclonal antibodies including CD31-PE, CD34-allophycocyanin (APC) (all from BD PharMingen) were stained on transduced cells and examined by co-expression with GFP. Isotype-matched controls (BD PharMingen) and nontransduced cells were used to determine the background staining. Data analysis was carried out using FlowJo Software.
Reverse transcription-polymerase chain reaction analysis
At different time points, the total RNAs from undifferentiated hESCs and from differentiated EBs were extracted using TRIzol (Invitrogen). One μg of RNA was used for each reverse transcription (RT) reaction. To eliminate DNA contamination, the RNA samples were treated with DNase (Invitrogen) before RT reaction (SuperScript II RNase H-Reverse Transcriptase, Invitrogen). Oligonucleotide primers used are listed in Table 1.
Table 1.
oligonucleotide primers used for RT–PCR analysis
| Genes | 5′ Primer sequence (5′–3′) | 3′ Primers sequence (5′–3′) | Products size (bp) |
|---|---|---|---|
| Oct-4 | GACAACAATGAAAATCTTCAGGAGA | TTCTGGCGCCGGTTACAGAACCA | 217 |
| Nanog | ACTAACATGAGTGTGGATCC | TCATCTTCACACGTCTTCAG | 929 |
| GATA-1 | TCA ATT CAG CAG CCT ATT CC | TTC GAG TCT GAA TAC CAT CC | 378 |
| GATA-2 | AGCAAGGCTCGTTCCTGTTC | TCGGTTCTGCCCATTCATCT | 141 |
| CD34 | GCCATTCAGCAAGACAACAC | AAGGGTTGGGCGTAAGAGAT | 152 |
| CD31 | CAACGAGAAAATGTCAGA | GGAGCCTTCCGTTCTAGAGT | 260 |
| KDR | ATG CAC GGC ATC TGG GAA TC | GCT ACT GTC CTG CAA GTT GCT GTC | 537 |
| Tie-2 | ATCCCATTTGCAAAGCTTCTGGCTGGC | TGTGAAGCGTCTCACAGGTCCAGGATG | 512 |
Immunofluorescence staining
The undifferentiated colonies cultured on MEF feeder layers were fixed in 4% paraformaldehyde/PBS for 15 min. After blocking nonspecific binding by 4% normal goat serum for 30 min, the colonies were stained indirectly with mouse anti-human SSEA-4 (Chemicon), TRA-1-60, TRA-1-81 (Santa Cruz Biotechnology) and secondary rat anti-mouse IgG-PE (BD PharMingen) or goat anti-mouse IgM-TRITC (Santa Cruz Biotechnology) for 30 min and were analysed under fluorescence microscope (Nikon).
Karyotyping
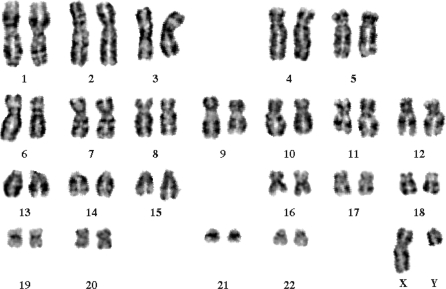
Undifferentiated colonies were cultured on Matrigel coated 6-well plates to deplete MEF feeder layer. Exponentially growing cultures were arrested in metaphase by using 10 μg/ml colchicine (Sigma) for 1 h at 37 °C and were resuspended in 0.7% sodium citrate (Sigma) for 45 min at room temperature. After fixed with 3:1 methanol:acetic acid (Sigma) for two times, cells in fixative were dropped onto a slide and dried for 2 h at 74 °C. Resulting chromosome spreads were digested with 0.05% trypsin (invitrogen) for 1 min and detected under microscope (Nikon).
Sprouting EB formation in collagen matrix
11-day-old EBs were resuspended in 15%FCS, 100 ng/ml rhVEGF, 200 ng/ml rhFGF-basic (all from R&D Systems), mixed with an isovolume of 15%FCS, collagen type-I from rat tail (BD Biosciences) in IMDM. One milliliter of the mixture was plated in 2-well chamber slides (Nalge Nunc), maintained at 37 °C without CO2 for 30 min to allow gelation, and then incubated for 3 days at 37 °C in humidity 5%CO2.
Matrigel capillary structure formation
The assay was carried out in 24-well plates coated with 200 μl/well matrigel matrix (BD Biosciences) at room temperature for more than 30 min. 50,000 VEGF-cultured CD34+ cells were trypsinized and replated on matrigel-coated plates in differentiation medium at 37 °C in 5%CO2. The structures were photographed under fluorescence and phase-contrast microscope (Nikon) after 16 h incubation.
Teratoma formation in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice
Clumps consisting of about ~1,000 transduced hES cells with an undifferentiated morphology were mechanically sliced and injected into the testis of 6–8 weeks old male NOD/SCID mice (Gropp et al. 2003; Thomson et al. 1998). The resulting tumors were removed 8–12 weeks later, frozen and sectioned by a cryostat. GFP expression by the tumor tissue and staining with hematoxylin and eosin (HE) on sequential slides was detected under fluorescence microscope (Nikon).
Results
Establishment of GFP-expressing hES cells (GFP-H1 hES cells) for prolonged cultivation
Undifferentiated hES cells (H1) were plated in 24-well plates on mitomycin-C treated MEF feeder cells. One day after splitting, cells were transduced with lentiviruses that expressed GFP transgene under control of ubiquitin promoter at a multiplicity of infection (MOI) of 10 or 50 on 2 successive days. Seventy-two hours post-transduction, the transducing efficiency was determined by GFP expression. At an MOI of 50, nearly 10% of lentiviral transduced cells were GFP positive, whereas less than 2% cells were GFP positive at an MOI of 10. The transduction efficiency of MSCV retroviruses for GFP expression was used as a control, resulting in 2.9% or 3.2% positive cells at an MOI of 10 or 50, respectively. GFP expression in MSCV transduced hES cells declined after 5 days and was almost undetectable after 2 weeks (Fig. 1b).
Fig. 1.
Establishment of a stable GFP-expressing hES subline by lentivirus transduction. a Schematic representation of the procedure to establish a stable GFP+ hES subline. After generating pFUGW-U6 vector by introducing the U6 promotor into an Ubiquitin-EGFP expression cassette, the vector was pseudotyped with VSV-G into 293T cells to produce viruses. The concentrated viral particles were transduced into undifferentiated H1 on MEF feeder cells. To generate stable GFP-expressing colonies, green fluorescent colonies were picked under microscope for several rounds of passage. GFP+ undifferentiated hESCs were afterwards induced to differentiate via teratoma in vivo or EBs in vitro. b Transducing efficiencies of MSCV and lentiviral vectors on human ES cells were determined at an MOI 10 and 50, respectively. The undifferentiated H1 cells were split 1 day before transduction. The day of first transduction was dated as day 0. The percentages of GFP+ cells were analyzed by FACS before transduction and at 3, 6, 13 days post-transduction. Data were representative from 3 independent experiments. c GFP expression in undifferentiated GFP-H1 hES cells at 10 and 50 passages after transduction was analyzed by FACS. Background fluorescence was assessed by nontransduced hES-H1 cells. Data are representative from 3 independent experiments. d Undifferentiated GFP-expressing colonies on MEF layers were stained by anti-human SSEA-4, TRA-1-60 and TRA-1-81 and PE- or TRITC-conjugated secondary antibodies. Scale bar = 100μm. e FACs analysis of SSEA-4 expression of transduced undifferentiated cells (day 0) and differentiated day 12 EB cells. Fluorescence background was assessed by nontransduced H1 cells with isotype-matched antibody. Data were representative from 3 independent experiments
Because hES cells are highly sensitive to dissociation and poorly survivable in single cells (Verfaillie et al. 2002), we enriched GFP+ hES cells by mechanically selection at weekly passage. At the day of passage, hES cells were fed with fresh MEF-CM media. GFP+ colonies were scraped and dissected into small clusters under fluorescence microscope. The GFP-H1 hES cell clusters were transferred onto new MEF feeders with CM medium. Two subclones were picked out from transduced hES-H1 cells. After five rounds of manual selection, the expression of GFP in GFP-H1 hES cells was analyzed by flow cytometry (Fig. 1c) or under microscope (Fig. 1d). After 50 passages, approximately >95% cells were GFP+ cells, suggesting that transgene expression was sustained in prolonged cultivation. In addition, GFP expression in GFP-H1 hES cells was stable during freezing-thawing cycles (Fig. 1c–e), and GFP-H1 hES cells showed a normal 46xy karyotype (Fig. 2).
Fig. 2.
Transduced GFP+-H1 cells preserve normal karyotype. Undifferentiated GFP-expressing colonies were cultured on Matrigel and were arrested in metaphase for karyotype analysis. The cells showed a normal 46xy karyotype
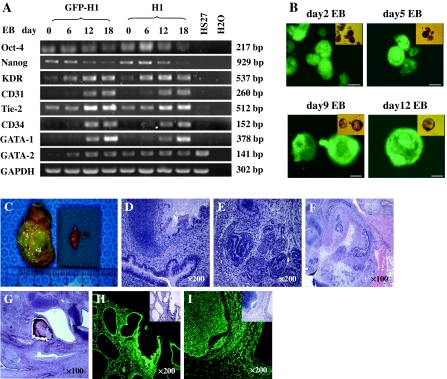
To determine whether GFP-H1 hES cells retained pluripotency, we analyzed the expression of pluripotent genes. Immunohistochemistry was used to determine the expression of SSEA-4, TRA-1-60 and TRA-1-81 in GFP-H1 hES cells. As shown in Fig. 1, GFP-H1 hES cells were GFP positive and expressed SSEA-4, TRA-1-60 and TRA-1-81 (Fig. 1c–e). RT–PCR analysis demonstrated that undifferentiated GFP-H1 hES cells (day 0) expressed Oct-4 and Nanog as control hES cells (Fig. 3a). These data suggested that lentiviral-transduced hES cells remain pluripotent.
Fig. 3.
Lentiviral transduced H1 cells retained their pluripotent property. a RNA samples were isolated at different time points of EBs and analyzed by RT–PCR. Primers used are shown in Table 1. None of the samples showed genomic DNA amplification (data not shown). b GFP-H1 hEBs were formed over 12 days into multicellular aggregates, which developed a cystic morphology from day 9. The EBs in fluorescence images are identical to those in bright-field images. Scale bar = 100 μm. c Teratoma was acquired in the testis of NOD/SCID mice 8 weeks after implantation of GFP-H1 hES cells. The tumor (2.5 cm × 3.5 cm) was compared with the normal testis (0.5 cm × 1 cm) from the same mouse. d–i Histological images of GFP-H1 hES-derived teratoma. Hematoxylin-eosin staining images of cartilage (d, g), primitive bronchus (d), glandular structures (e), gastrointestinal tract epithelium (f), muscle (f), hair follicle and bone (g). The cells within these structures expressed GFP as demonstrated on the sequential slides under fluorescence microscope (h, i). Original magnification:100× (f, g), 200× (d, e, h, i)
Stable transgene expression throughout differentiation of EB in vitro and teratoma in vivo
Transgene inactivation is the major concern that limits the use of lentiviral vectors. Gene silences were demonstrated in hES cells under some ubiquitous promoters, including CMV, CAG, PGK and EF1α promoters (Wang et al. 2008; Xia et al. 2007). To determine whether GFP transgene is silenced during hES cell differentiation under ubiquitin promoter, GFP-H1 hES cells were examined for transgene expression during in vitro EB differentiation or in vivo teratoma formation. After removal from feeder layers and transfer to suspension culture, GFP-H1 hES cells began to differentiate into multicellular aggregates, and acquired a cystic morphology after 9–12 days of differentiation (Fig. 3b), which was accompanied by the reduction of SSEA-4 expression (Fig. 1e). Fluorescence microscopy and flow cytometry analyses revealed that 12-day differentiated EBs retained intensive GFP expression (Fig. 1e, 3b).
To further examine the pluripotency of transduced hES cells, undifferentiated GFP-H1 cells (40–45 passages after transduction) were injected into the testis of NOD/SCID mice. Teratomas were developed at 8 weeks after injection (Fig. 3c). Histological analysis of the teratomas after 12 weeks of engraftment demonstrated that teratomas from GFP-H1 hES cells contained cells derived from all three germ layers, including gastrointestinal tract epithelium (endoderm); muscle, cartilage, bone (mesoderm); and hair follicle (ectoderm) (Fig. 3d–g). Moreover, GFP was expressed in the tissues within the teratomas (Fig. 3h–i).
GFP-H1 hES cells maintained the capability to differentiate into hematopoietic and endothelial commitment with expression of GFP gene
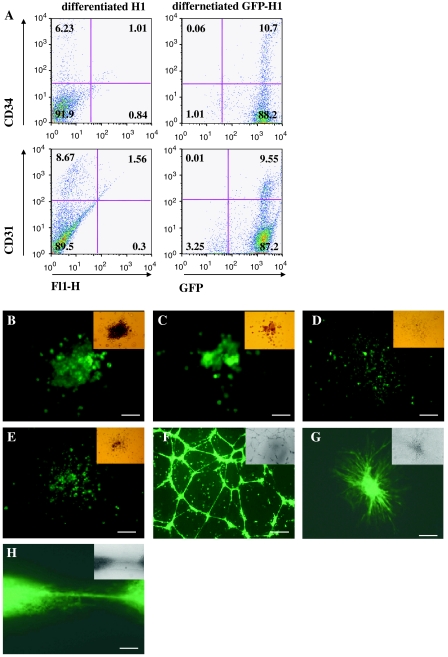
To determine whether GFP transgene under control of ubiquitin promoter is sustained in hematopoietic and endothelial cells, GFP-H1 hES cells were induced to differentiate into hematopoietic and endothelial lineages. Similar to the progression of hES cell differentiation, GFP-H1 hES cells showed a reduction in the transcription of the pluripotent genes, Oct-4 and Nanog, whereas the expression of hemato-endothelial markers, VEGF-R2, Tie-2, CD31, CD34, GATA-1 and GATA-2, were increased during hES cell differentiation (Fig. 3a, 4a). CD34+ cells in adult human peripheral blood, bone marrow, and cord blood contain progenitors for both hematopoietic and endothelial cells (Andrews et al. 1986; Asahara et al. 1997), whereas CD34 was not expressed in undifferentiated hES cells. We and others showed that hES cell-derived CD34+ cells were able to give rise to hematopoietic and endothelial cells in vitro (Narayan et al. 2006; Wang et al. 2007; Woll et al. 2008). In our previous study, we determined that CD34+ cell populations increased and peaked around day 12 upon hESC differentiation (Chen et al. 2007). After 12 days of differentiation, approximately ~10% differentiated cells were CD34+ cells that also expressed GFP (Fig. 4a). CD34+ cells were isolated by magnetic sorting. After two rounds of selection, the purity of the magnetic isolated CD34+ cells was greater than 90%, most of which expressed green fluorescence (data not shown).
Fig. 4.
Hematopoietic and endothelial differentiation of transduced hES cells. a FACS analysis for CD31 and CD34 expression of day 12 GFP-H1 hEBs. Background fluorescence was assessed by nontransduced H1 cells with isotype control. Data are representative from 3 independent experiments. (b-e) Photographs of hematopoietic colonies derived from GFP-H1 hEB-selected CD34+ cells. The cells within CFU-GEMM (b), CFU-E (c), and CFU-GM (d, e) were expressing GFP in fluorescence image. Scale bar = 50 μm (b, c), 125 μm (d, e). f CD34+ cells were sorted by magnetic beads from day 11 GFP-H1 hEBs and cultured further in presence of VEGF (50 ng/ml) on gelatin for 10 days for maturation. The mature GFP-expressing hES-derived endothelial cells formed tubular structure after having been replated on Matrigel. Scale bar = 100 μm. g–h Day 11 GFP-H1 hEBs were cultured on collagen type-I with VEGF (50 ng/ml) and bFGF (100 ng/ml) for 72 h. Sprouting GFP+ outgrowth were photographed under fluorescence microscope. Scale bar = 100 μm (g), 50 μm (h)
The CD34+ cells were further induced to differentiate into hematopoietic cells in methylcellulose to generate hematopoietic colonies (so-called CFUs or CFCs). As shown in Fig. 4b–e, CFCs from hES cell-derived CD34+ cells were essentially all GFP+ colonies, indicating that GFP transgene is expressed during hematopoietic differentiation of hES cells.
After the isolated CD34+ cells were cultured on gelatin-coated wells in the presence of VEGF (50 ng/ml) for 10–14 days, they became morphologically similar to mature human umbilical vein endothelial cells which were uniform flat, adherent, stellate-appearing with specific endothelial markers, including CD31, CD34, Flk-1, vWF and Dil-AcLDL uptaken (data not shown). It is a well-documented characteristic that endothelial cells rapidly form capillary-like networks in Matrigel with several aspects of new vessel formation, including endothelial cell migration, attachment, and cell–cell contact (Passaniti et al. 1992; Salvucci et al. 2002). After being replated on Matrigel for 16 h, the hES cell-derived endothelial cells from GFP-H1 hES cells formed capillary-like structures with GFP expression (Fig. 4f).
The sprouting EB model was used to assess embryonic endothelial outgrowth of vascular development in mouse ES cells (Feraud et al. 2001; Wang et al. 2004) and in human ES cells (Chen et al. 2007). In sprouting structure, the capillaries emerged from the initial primitive endothelial structures, branched, and then gave rise to an endothelial network. After cultivation on collagen I matrix for 72 h, sprouts from individual GFP+ EBs grew to connect and form vascular network-like structures (Fig. 4g, h).
Our study demonstrated that the differentiated hemato-endothelial cells, including selected CD34+ cells, hematopoietic colonies, endothelial networks, and sprouting outgrowth structures, expressed green fluorescence under microscope, suggesting that transgene expression under ubiquitin promoter was retained during hematopoietic-endothelial differentiation.
Discussion
Efficient, long-term gene transfer is essential for profound study of gene function. Viral vectors are proven to be able of efficiently transducing genes into target cells because they can bind to host cell receptors and exert viral mechanisms for efficient gene expression (Chang et al. 1999).
The successful isolation of stem cells from human embryos brings new relevance to understand gene functions which reinforce hematopoiesis and vasculogenesis during early human development. Retroviral vectors are appealing vehicles for gene transfer and was demonstrated capable of efficient transduction of CD45negPFV hematopoietic progenitors derived from hESCs (Menendez et al. 2004). However, long-term expression mediated by integrated retro-proviruses in primary cells has been difficult to achieve (Cherry et al. 2000). Retroviral regulatory elements are repressed in numerous cell types which is associated with methylation-related silicing (Challita and Kohn 1994). In particular, the lack of significant provirus transcription in ES cells and their differentiated descendants has hampered the use of retroviral vectors-modified ES cells in the research of embryogenesis (Gautsch 1980). Tissues of transgenic animals, which was derived from ES cells carrying the MSCV-based provirus, were proven to lack of GFP expression (Cherry et al. 2000). Recent incremental improvement in viral-mediated gene transfer into ES cells has been achieved by using vectors derived from the HIV-1 lentivirus (Kosaka et al. 2004) which can transduce a variety of non-dividing cells (Mostoslavsky et al. 2005; Terskikh et al. 2005). Lentiviruses have the distinguishing property of being able to infect both dividing and nondividing cells, and this ability has led to their development as gene delivery vehicles (Naldini et al. 1996b). However, lentiviral expressed transgene under CMV promoter is often suppressed in stem cells.
Promoter choice has been shown to be important for durable cellular transgene expression (Wang et al. 2008). RNA polymerase III (pol III) promoters, most commonly the human U6 small nuclear promoter and the human H1 promoter, have been incorporated into the lentiviral vectors for stable expression of short hair pin RNA for gene silencing (An et al. 2006). They feature a relatively simple structure and a well-defined transcription start-site enabling the straightforward use of U6 and H1 promoters compared with other pol II/pol III promoters. U6 promoter is more efficient than H1 promoter in gene silencing. It has been shown that a long-term inhibition of target-gene expression was retained either in vitro or in vivo under the control of U6 promoter (An et al. 2006, Makinen et al. 2006). Here we transduced hES cells by using a lentiviral vector contained U6 promoter to test whether U6 promoter interferences the function of ubiquitin promoter. Our data indicated that the expression of GFP transgene controlled by ubiquitin promoter was not interrupted by upstream U6 promoter in hES cells.
We modified FUGW vector by insertion of U6 promoter for future siRNA study at a PacI site upstream of an ubiquitin-eGFP expression cassette. The lentiviral vectors were pseudotyped with VSVG to facilitate concentration of vector particles and to provide broad target cell tropism (Miyoshi et al. 1998). Transducing efficiency of hES colonies in our study was relatively low at an MOI of 10 and of 50. Taking into account the viral uptake by feeder cells and colony-depending survival condition of hES cells, we increased MOI to get high GFP-expressing colony. After several rounds of mechanical selection for GFP+ colonies, transgene was expressed at a stable level during long-term passages which indicated the integration of lentiviral vector into the target hES cells with undifferentiated fate.
As hES cells offer a scalable source for basic biology, drug discovery, and transplantation medicine, genetic modification of hES cells may have far-reaching applications (Gropp et al. 2003). It is important to determine whether viral transduction per se would exert effect upon hESC differentiation (Pfeifer et al. 2002). After differentiation via EB in vitro and teratoma in vivo, we found that the transgene was not “shut off” during differentiation. Transduced hES cells retained the ability to differentiate into progeny of all three germ layers while expressing high levels of GFP. Moreover, it was fully demonstrated in our study that GFP-H1 hES-derived CD34+ cells were endowed with the features of hematopoietic and endothelial progenitors. After culture for 10 days in the presence of hematopoietic and endothelial growth factors, CD34+ cells from differentiated GFP-H1 hES cells could grow into two distinct lineages showing hematopoietic or endothelial property. Hematopoietic colonies, endothelial capillary-network and sprouting EBs expressed intensively green fluorescence.
Pluripotent hES cells have driven great attention to their therapeutic potential from both a biologic and a medical view. Efficient genetic manipulation is one of the essential techniques to establish human ES cells as a widely used research source (Ma et al. 2003). Here we proved that lentivirus provide a powerful tool for genetic manipulation, and demonstrated that lentiviral transgene can be expressed stably in hESC-derived hematopoietic and endothelial progenitors, allowing the permanent and enduring benefit desired in basic research and clinical trials of gene therapy and regenerative technology.
Acknowledgments
This study was partially supported by National Natural Science Foundation of China (30771093), Shanghai Pujiang Program (07pj14020) and Foundation from Science and Technology Commission of Shanghai Municipality (08DJ1400500) to Tong Chen.
Disclosures
The authors indicate no potential conflict of interest.
Footnotes
Hua Jiang and Xiaolong Lin contributed equally to this work.
References
- Akkina RK, Walton RM, Chen ML, Li QX, Planelles V, Chen IS. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An DS, Qin FX, Auyeung VC, Mao SH, Kung SK, et al. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006;14:494–504. doi: 10.1016/j.ymthe.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RG, Singer JW, Bernstein ID. Monoclonal antibody 12–8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood. 1986;67:842–845. [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challita PM, Kohn DB. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci USA. 1994;91:2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Urlacher V, Iwakuma T, Cui Y, Zucali J. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 1999;6:715–728. doi: 10.1038/sj.gt.3300895. [DOI] [PubMed] [Google Scholar]
- Chen T, Bai H, Shao Y, Arzigian M, Janzen V, et al. Stromal cell-derived factor-1/CXCR4 signaling modifies the capillary-like organization of human embryonic stem cell-derived endothelium in vitro. Stem Cells. 2007;25:392–401. doi: 10.1634/stemcells.2006-0145. [DOI] [PubMed] [Google Scholar]
- Cherry SR, Biniszkiewicz D, Parijs L, Baltimore D, Jaenisch R. Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol Cell Biol. 2000;20:7419–7426. doi: 10.1128/MCB.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraud O, Cao Y, Vittet D. Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab Invest. 2001;81:1669–1681. doi: 10.1038/labinvest.3780380. [DOI] [PubMed] [Google Scholar]
- Gautsch JW. Embryonal carcinoma stem cells lack a function required for virus replication. Nature. 1980;285:110–112. doi: 10.1038/285110a0. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Anderson WF. Gene therapy using retroviral vectors. Curr Opin Biotechnol. 1994;5:611–616. doi: 10.1016/0958-1669(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gropp M, Itsykson P, Singer O, Ben-Hur T, Reinhartz E, et al. Stable genetic modification of human embryonic stem cells by lentiviral vectors. Mol Ther. 2003;7:281–287. doi: 10.1016/S1525-0016(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Kosaka Y, Kobayashi N, Fukazawa T, Totsugawa T, Maruyama M, et al. Lentivirus-based gene delivery in mouse embryonic stem cells. Artif Organs. 2004;28:271–277. doi: 10.1111/j.1525-1594.2004.47297.x. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- Makinen PI, Koponen JK, Karkkainen AM, Malm TM, Pulkkinen KH, et al. Stable RNA interference: comparison of U6 and H1 promoters in endothelial cells and in mouse brain. J Gene Med. 2006;8:433–441. doi: 10.1002/jgm.860. [DOI] [PubMed] [Google Scholar]
- Menendez P, Wang L, Chadwick K, Li L, Bhatia M. Retroviral transduction of hematopoietic cells differentiated from human embryonic stem cell-derived CD45(neg)PFV hemogenic precursors. Mol Ther. 2004;10:1109–1120. doi: 10.1016/j.ymthe.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Narayan AD, Chase JL, Lewis RL, Tian X, Kaufman DS, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006;107:2180–2183. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell D, Osborne CS, Yao S, Sukonnik T, Pasceri P, et al. Retrovirus vector silencing is de novo methylase independent and marked by a repressive histone code. EMBO J. 2000;19:5884–5894. doi: 10.1093/emboj/19.21.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci USA. 2002;99:2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A, Hawley TS, Hawley RG. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther. 2000;2:458–469. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.V99.8.2703. [DOI] [PubMed] [Google Scholar]
- Suter DM, Cartier L, Bettiol E, Tirefort D, Jaconi ME, et al. Rapid generation of stable transgenic embryonic stem cell lines using modular lentivectors. Stem Cells. 2006;24:615–623. doi: 10.1634/stemcells.2005-0226. [DOI] [PubMed] [Google Scholar]
- Terskikh AV, Ershler MA, Drize NJ, Nifontova IN, Chertkov JL. Long-term persistence of a nonintegrated lentiviral vector in mouse hematopoietic stem cells. Exp Hematol. 2005;33:873–882. doi: 10.1016/j.exphem.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Verfaillie CM, Pera MF, Lansdorp PM (2002) Stem cells: hype and reality. Hematology (Am Soc Hematol Educ Program): 369–391 [DOI] [PubMed]
- Wang Z, Cohen K, Shao Y, Mole P, Dombkowski D, Scadden DT. Ephrin receptor, EphB4, regulates ES cell differentiation of primitive mammalian hemangioblasts, blood, cardiomyocytes, and blood vessels. Blood. 2004;103:100–109. doi: 10.1182/blood-2003-04-1063. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- Wang R, Liang J, Jiang H, Qin LJ, Yang HT. Promoter-dependent EGFP expression during embryonic stem cell propagation and differentiation. Stem Cells Dev. 2008;17:279–289. doi: 10.1089/scd.2007.0084. [DOI] [PubMed] [Google Scholar]
- Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zhang Y, Zieth CR, Zhang SC. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev. 2007;16:167–176. doi: 10.1089/scd. 2006.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]