Abstract
Background
Percutaneous left atrial appendage (LAA) closure can be an alternative to coumadin treatment in patients with atrial fibrillation (AF) at high risk for thromboembolic events and/or bleeding complications. We report the initial experience with this new technique.
Methods
Patients were eligible if they had AF with a high stroke risk (CHADS2 score >1), and/or contraindication for coumadin therapy. The procedure was performed under general anaesthesia, using biplane fluoroscopy and (3D) transoesophageal echocardiography (TEE) guidance. Patients were discharged on coumadin until a TEE was repeated at 45 days after closure to evaluate LAA occlusion. If LAA occlusion was achieved, oral anticoagulation was discontinued and aspirin started.
Results
Percutaneous LAA closure was performed in 10 patients (50% male, age 61.6 ± 9.6 years). The median CHADS2 score was 3 (range 2–4), median CHA2DS2-VASc score 3.5 (range 2–6) and HAS-BLED score 1.5 (range 1–4). Nine patients had a history of stroke and 2 patients had a history of major bleeding while on coumadin. Concomitant pulmonary vein isolation was performed in 9 patients. The device was successfully placed in all patients within a median of 56 min (38–137 min). Asymptomatic catheter thrombus occurred in one patient. At 45-day follow-up, no thromboembolic events occurred, TEE showed minimal residual flow in the LAA in three patients. In one patient the LAA device was dislocated, requiring successful percutaneous retrieval.
Conclusion
Device closure of the LAA may provide an alternative strategy to chronic coumadin therapy in patients with AF and high risk of stroke and/or bleeding complications using coumadin.
Keywords: Left atrial appendage, Percutaneous, Atrial fibrillation, Stroke, Prevention
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting millions of individuals worldwide [1, 2]. Since AF mainly affects elderly people, its prevalence is expected to increase in parallel with the increasing age of the population [1]. The lifetime risk for development of AF is 25% in people over the age of 40 years [3].
AF might cause a reduced cardiac output and formation of atrial thrombi, especially in the left atrial appendage (LAA) [4, 5]. The overall annual stroke risk is 5% in patients with AF [3]. This risk of stroke increases substantially with age, from 1.5% in individuals aged 50–59 years to 23.5% for those aged 80–89 years [3, 6–8].
Although several published controlled trials have shown the effectiveness of oral anticoagulation (OAC) therapy on stroke prevention in patients with AF, it has several disadvantages: (major) bleeding, non-tolerance, non-compliance, interactions with some foods and other medication and a narrow therapeutic range [9–12].
Autopsy and echocardiography studies have shown that the LAA was the source of thrombi in more than 90% of the patients with non-valvular AF [4]. The multiple problems with anticoagulation therapy and predicating on the fact that roughly 90% of the emboli originate from the LAA have led to the strategy of mechanically obliterating the LAA and excluding it from the systemic circulation. We report our initial experience with an LAA closure device in patients with non-valvular AF at high risk for stroke.
Methods
Patient selection
Patients aged 18 years or more with documented paroxysmal, persistent, or permanent non-valvular AF were eligible if they had an increased risk for stroke (CHADS2 score >1) and/or contraindication for coumadin therapy. The CHADS2 score is an overall risk assessment for stroke based on a scale of 0–6. The patient’s score is calculated by adding 1 point if congestive heart failure, (history of) arterial hypertension, age ≥75, or diabetes were present and by adding 2 points if the patient had had a prior stroke or TIA. This score can be used to estimate the patient’s annual risk of stroke. Also the stroke risk according to the CHA2DS2-VASc score and the HAS-BLED score was calculated. The CHA2DS2-VASc extends the CHADS2 score with additional stroke risk factors. In this score 2 points are assigned for a history of stroke, or age ≥75; and 1 point each is assigned for age 65–74 years, a history of hypertension, diabetes, recent cardiac failure, vascular disease (myocardial infarction, complex aortic plaque and peripheral artery disease) and female sex. The HAS-BLED score is a practical risk score based on a scale from 0 to 9 which estimates the 1-year risk for major bleeding, whereby a score of ≥3 indicates “high risk”. The HAS-BLED score can be calculated by assigning 1 point to each risk factor for bleeding: hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalised ratio, elderly patients (> 65 years), and/or drugs/alcohol concomitantly [13].
Prior to the procedure a transoesophageal echocardiography (TEE) was performed to view LAA anatomy and exclude thrombus.
Procedure
Electrophysiological and ablation procedure
Electrophysiological procedures were performed under general anaesthesia with patients in a drug-free state. Mapping with the pulmonary vein ablation catheter (PVAC; Ablation Frontiers, Inc., Carlsbad, CA, USA) was performed with an electrophysiological recording system (Prucka, GE Medical, Waukesha, WI, USA) using filter settings of 100–500 kHz and a signal amplification set at 5000. The PVAC is a 9Fr, over-the-wire, circular, decapolar mapping and ablation catheter with a 25-mm-diameter array at the distal tip. No additional nonfluoroscopic guiding or steering systems were used. A 7Fr sheath was introduced through the right femoral vein. A quadripolar catheter was introduced in the coronary sinus (CS) for pacing purposes. A standard transseptal puncture was performed using a Brockenbrough needle with either a 10Fr nonsteerable sheath (SL1, St. Jude Medical, Inc., Minnetonka, MN, USA) or a 12.5Fr steerable sheath (Channel, Bard, Lowell, MA, USA). Both sheaths have a 9.5Fr or larger inner lumen diameter to accommodate the PVAC. Angiography via the sheath was performed to delineate the pulmonary veins. A single heparin bolus of 5000 international units was administered IV through the sheath and repeated every 90 min after transseptal puncture when necessary without activated clotting time measurements [14].
Left atrial appendage closure
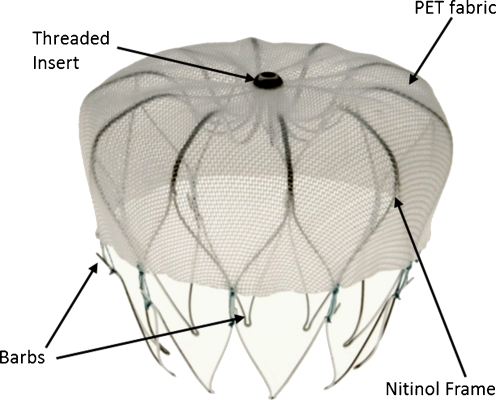
The procedure was performed when necessary after the PVAC procedure with the patient still under general anaesthesia, using biplane fluoroscopy and (3D) TEE guidance. The device was implanted via femoral venous access and a transseptal puncture into the LAA. Standard transseptal puncture techniques were used. After the transseptal puncture, heparin was given to achieve an active clotting time of at least 250 s. After the transseptal puncture a 14 F transseptal access sheath was positioned in the left atrial appendage. This access sheath serves as a conduit for the delivery catheter which contains the device (WATCHMAN Left Atrial Appendage Occlusion Device, Atritech Inc., Plymouth, Minnesota), a self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover (Fig. 1). The device comes in 5 sizes (21, 24, 27, 30 and 33 mm) to accommodate the varying anatomy and size of the LAA. A device size 10–20% larger than the largest diameter of the LAA body as measured by angiography and echocardiography was chosen to have sufficient compression for stable positioning of the device. By retracting the access sheath the device deploys. Before releasing it from the delivery catheter several release criteria had to be fulfilled. First of all, proper device positioning was confirmed by angiography and echocardiography. Secondly, residual flow was checked by echocardiography. Thirdly, a tug test was performed by gently retracting and releasing the delivery catheter while observing proximal movement of the closure device to check the stability. The device was deemed stable when it moved in unison with the LAA on both TEE and fluoroscopy. Finally, the compression of the device was measured. When the device is properly sized, the maximum device diameter is 80–92% of its original size. If all these device release criteria were confirmed the device was released. Patients were typically hospitalised overnight and OAC was started as standard of care (INR between 2 and 3) or aspirin and/or clopidogrel in case of contraindications. Patients used bridging low-molecular-weight heparin (LMWH) until the INR was >2.0.
Fig. 1.
The WATCHMAN device, a self-expanding nitinol frame structure with fixation barbs and a permeable polyester fabric that covers the atrial facing surface of the device
Post-procedure
Patients were seen in the outpatient clinic 45–60 days post-procedure. Prior to this visit a TEE was repeated to evaluate LAA occlusion, thrombus formation, device position and residual flow. If the echocardiographic criteria for successful sealing of the LAA were met (LAA completely sealed or a minimal residual flow (<5 mm jet) around the device) the physician was allowed to discontinue the coumadin while the aspirin was continued indefinitely and clopidogrel was started (75 mg daily) for 6 months. If these echocardiographic criteria for successful sealing where not met, TEE was repeated at 6 months.
Endpoints
The primary endpoint of this study was successful device implantation and successful sealing of the LAA as measured by TEE at 45 days post-procedure without major adverse events. Major adverse events were defined as death, stroke, systemic embolism, and major bleedings requiring invasive treatment or blood transfusion.
Statistical analysis
Descriptive statistics were used to report patient characteristics. Continuous variables with normal distribution are reported by mean ± standard deviation. Median and range were used when normal distribution was absent. Percentages were used to report categorical variables. All statistical analyses were performed using SPSS software (SPSS Inc., version 17.0 for Windows).
Results
Patient characteristics
Between September 2009 and June 2010, 10 patients (mean age 61.6 ± 9.6 years, 50% female) were treated. All patients had documented AF and a CHADS2 score of >1 that necessitated the use of oral anticoagulation. The median CHADS2 score was 3 (range 2–4), median CHA2DS2-VASc score was 3.5 (range 2–6) and HAS-BLED score was 1.5 (range 1–4). All patients but one had a history of stroke. The indication for LAA closure as alternative to OAC was a history of stroke under OAC in 3 of 10 patients, severe bleeding under OAC in 2 patients and patients preference in 5 patients due to several drawbacks of the OAC therapy. All patients used coumadin pre-procedure. The average maximum LAA diameter was 20.2 ± 2.2 mm and the depth was 27.9 ± 5.4 mm. In 40% of the patients there was a multilobular atrial appendage (Table 1).
Table 1.
Baseline characteristics
| Number, n | 10 |
| Age (years) | 61.6 ± 9.6 |
| Sex (%) | |
| Male | 5 (50) |
| Female | 5 (50) |
| Median CHADS2 | 3 (2-4) |
| 2 | 4 (40) |
| 3 | 4 (40) |
| 4 | 2 (20) |
| CHA2DS2-VASc | 3.5 (2-6) |
| HAS-BLED | 1.5 (1-4) |
| Coumadin, n(%) | 10 (100) |
| Indication, n (%) | |
| Bleeding with OAC | 2 (20) |
| Stroke using OAC | 3 (30) |
| Patient preference | 5 (50) |
| LAA, mm | |
| LAA width | 20.2 ± 2.2 |
| LAA length | 27.9 ± 5.4 |
| Multilobular | 4 (40) |
All data are presented as mean ± standard deviation, as number with percentage (n (%)) or median with lower and upper range, N = number,% = percentage, mm = millimetres, OAC = oral anticoagulation, LAA = left atrial appendage
Procedural and in-hospital results
The LAA closure device was successfully placed in all patients within a median time of 56 min (range 38–137 min). Concomitant pulmonary vein ablation was performed in 9 patients. The median size of the LAA device was 24 mm. A median of 2 devices per patient (range 1–3) was required to obtain optimal LAA closure. At the end of the procedure one patient had minimal residual flow; in all other cases complete closure of the LAA was achieved. During the implant an asymptomatic catheter thrombus occurred in one patient. The thrombus was successfully aspirated and an extra bolus of heparin was administered. This patient developed a groin haematoma due to prolonged femoral vein bleeding for which a blood transfusion and longer hospitalisation were necessary. All other patients were discharged the next day (Table 2).
Table 2.
Procedural characteristics
| PVAC, n(%) | 9 (90) |
| Size device (mm) | 24 (21–27) |
| 21 | 1 (10) |
| 24 | 8 (80) |
| 27 | 1 (10) |
| Duration (min) | 56 (38–137) |
| Devices, n | 2 (1-3) |
| Complications, n (%) | |
| Catheter thrombus | 1 (10)* |
| Inguinal bleeding | 1 (10)* |
| Pericardial effusion | 0 (0) |
| TEE | |
| Successful implantation | 10 (100) |
| Residual flow | 1 (10) |
| Hospitalisation, days | 2 (2–7) |
All data are presented as mean ± standard deviation, as number with percentage (n (%)) or median with lower and upper range, N = number, mm = millimetres, min = minutes. PVAC = pulmonary vein ablation catheter, TEE = transoesophageal echocardiography
*Both in the same patient
Follow-up results
At 45-day follow-up no recurrent major adverse events and especially no thromboembolic events occurred. TEE showed minimal residual flow in the LAA in three patients (30%). None of the patients had thrombus formation on the surface of the device. In one patient the LAA device was found to have asymptomatically dislocated to the abdominal aorta. The device was successfully retrieved transfemorally by means of a percutaneous procedure. Another patient had haematuria under OAC during the follow-up period. After a single procedure 5 of the 10 patients (50%) did not have any documented recurrence of AF at 3-month follow-up, while the other 5 patients needed one or multiple direct current cardioversions to remain in sinus rhythm. A redo-ablation procedure with successful pulmonary vein isolation was performed in 1 patient. The LAA device was not affected and did not interfere with the redo catheter ablation. In 3 of the 10 patients warfarin was discontinued at 45 days of follow-up (Table 3).
Table 3.
Follow-up characteristics at 45 days
| Number, n (%) | 10 |
| TEE, n (%) | |
| Residual flow | 3 (33) |
| Device embolisation | 1 (10) |
| Thrombus on device | 0 (0) |
| Coumadin, n (%) | 6 (67) |
| Complications during follow-up, n (%) | |
| Death | 0 (0) |
| Stroke or TIA | 0 (0) |
| Bleeding | 1 (10) |
All data are presented as mean ± standard deviation, as number with percentage (n (%)) or median with lower and upper range, N = number, TEE = transoesophageal echocardiography, TIA = transient ischaemic attack.
Discussion
We report the safety, feasibility and short-term follow-up of an LAA closure device in a population with a moderate to severe risk of stroke and non-valvular AF. Our results indicate that a high procedural success rate with a relatively low complication rate can be obtained.
The risk of stroke is increased fivefold in patients with AF compared with those with sinus rhythm, but also the stroke recurrence and post-stroke mortality is higher among patients with AF [7]. According to the guidelines, anticoagulation should be given to prevent thromboembolic events [13]. However, only a small number of patients with an indication for anticoagulation, especially elderly patients, are currently under treatment [12, 15]. The Euro Heart Survey showed that 28% of the high-risk patients were undertreated and this was associated with a higher chance of thromboembolism and the combined endpoint of cardiovascular death, thromboembolism, or major bleeding [16]. Contraindications for coumadin therapy are a recent history of major bleeding, frequent falls and the inability to comply with treatment. Nonadherence to warfarin is also a problem with 20–30% of the patients not taking the proper warfarin dose [17]. Another problem is the narrow therapeutic range of OAC with fewer than 60% of those treated having therapeutic INR levels.
Thrombi in patients with non-valvular AF are found in the LAA in more than 90% of the cases [4]. This finding and the multiple problems with anticoagulation therapy have led to a search for alternative approaches for stroke prevention in patients with AF. The WATCHMAN Left Atrial Appendage Occlusion device was developed to prevent embolisation of thrombus from the LAA by sealing the orifice of the LAA.
The initial experience with the WATCHMAN device was successful in 66 of the 75 patients (mean age 68.5 years, 64% male) [18]. The average CHADS2 score of the 66 patients with implants was 1.8 ± 1.1 (range 0–5). TEE at 45 days showed successful sealing of the LAA in 93%. No strokes occurred during a mean follow-up of 740 days despite 92% of patients discontinuing anticoagulation. During follow-up two patients experienced device embolisation; both the devices were successfully retrieved percutaneously. This incidence is generally comparable with that of the Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device which was the first investigated for obliteration of the LAA [19].
This device was further investigated in the PROTECT AF trial. This multicentre, non-inferiority trial randomised 707 patients with non-valvular AF in a 2:1 schema to either the WATCHMAN device or the control (conventional) treatment with warfarin [20, 21]. In this study both groups had to be eligible for warfarin therapy. The device was successfully implanted in 88% of the patients assigned to this intervention group. The primary endpoint of this study was a composite efficacy endpoint of freedom from all stroke, cardiovascular death, and systemic embolisation. Safety endpoints were major bleeding, pericardial effusion, and device embolisation. Using an intention-to-treat non-inferiority analysis at 1065 patient-years of follow-up, the event-free probability was better in the device group (3.0% per 100 patient-years vs. 4.9% per 100 patient-years) and met noninferiority criteria. The event rate of all ischaemic and haemorrhagic strokes was lower in the intervention group than in the control group (2.3% per 100 patient-years vs. 3.2% per 100 patient-years) and also all-cause mortality was lower in the intervention group (3.0% per 100 patient-years vs. 4.8% per 100 patient-years). Primary safety endpoints were more frequent in the intervention group than in the control group (7.4% per 100 patient-years vs. 4.4% per 100 patient-years). The majority of these were periprocedural, predominantly caused by pericardial effusion and procedural stroke related to air embolism due to the inexperience of the operator with transseptal punctures and working with large sheaths. The recent published data from the Continued Access Registry showed a significant decrease in procedural events from 7.7% in the first half of the PROTECT AF to 5.5% in the second half and 3.7% in the Continued Access Registry [22].
Recently the direct thrombin inhibitor, dabigatran, became available. In the RE-LY study, dabigatran was associated with lower rates of stroke and systemic embolism with similar rates of major haemorrhage, compared with OAC. Advantages of dabigatran over OAC include: no need for routine laboratory monitoring, a fixed-dose regimen, and potentially fewer clinically important drug interactions. Although the results are promising, there are some concerns including higher incidences of dyspepsia and gastrointestinal bleeding and lack of effective antidote. Additional drawbacks include higher drug costs, accumulation in case of renal impairment, and high discontinuation rates due to adverse events [23, 24]. In patients with a high risk of bleeding (for example patients with Rendu-Osler-Weber syndrome) or contraindication for OAC, LAA closure might still be a better option.
We are the first to combine the LAA closure with a pulmonary vein ablation. As mentioned before, patients with AF have a fivefold risk of stroke compared with those who have sinus rhythm. This combination can be an elegant way to reduce both symptomatology and the risk of stroke and bleeding in selected patients. In addition pulmonary vein ablation to maintain sinus rhythm combined with the placement of the WATCHMAN LAA closure device may further reduce stroke risk. Although with a successful pulmonary vein isolation, the benefit of an additional LAA closure might be less. However, in a meta-analysis the success rate of ablation in patients with all types of atrial fibrillation with a mean follow-up of 14 months was 57% (95% CI, 50–64%) showing that several patients need more than one ablation procedure to remain in sinus rhythm and have recurrent episodes of AF after the first ablation with an increased risk for stroke and potentially benefit from additional LAA closure [25].
Limitations of our study are the small number of patients and the short follow-up time, but we are continuing to select patients for this treatment and will perform mid- and long-term follow-up regarding safety and efficacy. Secondly, it is an observational study, not comparing the percutaneous technique with conventional treatment. Also important is the risk-benefit ratio, in which the periprocedural risk must be weighed against the risk of long-term OAC therapy (either bleeding or embolic stroke from lack of efficacy or poor therapeutic control).
Secondly, the cost-benefit ratio, in which the substantial costs of the device must be weighed against the total cost of OAC therapy. At this point there are insufficient data to draw firm conclusions about benefit ratios. Recently a second prospective, randomised, multicentre trial (PREVAIL trial) has started in the USA to provide additional information on the safety and efficacy of the WATCHMAN LAA closure device and studies the performance of this device in patients with AF for whom long-term warfarin therapy is contraindicated.
Conclusion
Closure of the LAA with the Watchman device may provide an alternative strategy to chronic coumadin therapy in patients with AF and high risk of stroke or bleeding complications using coumadin. Long-term follow-up will further reveal the risk and benefits of this therapy.
Acknowledgments
Disclosure
The Cardiology Department receives proctoring fees for training/educational services
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 4.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 5.Goldman ME, Pearce LA, Hart RG, et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study) J Am Soc Echocardiogr. 1999;12:1080–1087. doi: 10.1016/S0894-7317(99)70105-7. [DOI] [PubMed] [Google Scholar]
- 6.Garcia DA, Hylek E. Reducing the risk for stroke in patients who have atrial fibrillation. Cardiol Clin. 2008;26:267–275. doi: 10.1016/j.ccl.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The framingham study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.STR.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.STR.31.4.822. [DOI] [PubMed] [Google Scholar]
- 11.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 12.Sudlow M, Thomson R, Thwaites B, Rodgers H, Kenny RA. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352:1167–1171. doi: 10.1016/S0140-6736(98)01401-9. [DOI] [PubMed] [Google Scholar]
- 13.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the european society of cardiology (ESC) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq160. [DOI] [PubMed] [Google Scholar]
- 14.Boersma LV, Wijffels MC, Oral H, Wever EF, Morady F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008;5:1635–1642. doi: 10.1016/j.hrthm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. The Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153:1006–1012. doi: 10.1016/j.ahj.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Parker CS, Chen Z, Price M, et al. Adherence to warfarin assessed by electronic pill caps, clinician assessment, and patient reports: results from the IN-RANGE study. J Gen Intern Med. 2007;22:1254–1259. doi: 10.1007/s11606-007-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490–1495. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Bayard YL, Omran H, Neuzil P, et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010;6:220–226. doi: 10.4244/EIJV6I2A35. [DOI] [PubMed] [Google Scholar]
- 20.Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am Heart J. 2006;151:956–961. doi: 10.1016/j.ahj.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 22.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 23.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 25.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]