Abstract
We measured quantitative cortical mantle cerebral blood flow (CBF) by stable xenon computed tomography (CT) within the first 12 h after severe traumatic brain injury (TBI) to determine whether neurologic outcome can be predicted by CBF stratification early after injury. Stable xenon CT was used for quantitative measurement of CBF (mL/100 g/min) in 22 cortical mantle regions stratified as follows: low (0–8), intermediate (9–30), normal (31–70), and hyperemic (>70) in 120 patients suffering severe (Glasgow Coma Scale [GCS] score ≤8) TBI. For each of these CBF strata, percentages of total cortical mantle volume were calculated. Outcomes were assessed by Glasgow Outcome Scale (GOS) score at discharge (DC), and 1, 3, and 6 months after discharge. Quantitative cortical mantle CBF differentiated GOS 1 and GOS 2 (dead or vegetative state) from GOS 3–5 (severely disabled to good recovery; p<0.001). Receiver operating characteristic (ROC) curve analysis for percent total normal plus hyperemic flow volume (TNHV) predicting GOS 3–5 outcome at 6 months for CBF measured <6 and <12 h after injury showed ROC area under the curve (AUC) cut-scores of 0.92 and 0.77, respectively. In multivariate analysis, percent TNHV is an independent predictor of GOS 3–5, with an odds ratio of 1.460 per 10 percentage point increase, as is initial GCS score (OR=1.090). The binary version of the Marshall CT score was an independent predictor of 6-month outcome, whereas age was not. These results suggest that quantitative cerebral cortical CBF measured within the first 6 and 12 h after TBI predicts 6-month outcome, which may be useful in guiding patient care and identifying patients for randomized clinical trials. A larger multicenter randomized clinical trial is indicated.
Key words: cerebral blood flow, Glasgow Outcome Scale, outcome prediction, quantitative stable xenon CT, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major public health problem in the U.S., and the leading cause of death and disability in young people, involving at least 1.7–2 million people each year. About 52,000 people die, 275,000 are hospitalized, and 83,000 survive with permanent disability (Centers for Disease Control, 2010; Langlois et al., 2006; Zaloshnja et al., 2008). A total of about 3.2 million Americans live with long-term disability (Corrigan et al., 2010). The direct and indirect cost of TBI in the U.S. is approximately $60 billion per year (Centers for Disease Control, 2010).
Early outcome prediction after TBI is desirable for two reasons. First, it could be used to guide treatment and aid decisions on the allocation of resources (Steyerberg et al., 2008; MRC CRASH Trial Collaborators, 2008). Second, it is also important for injury stratification for inclusion in randomized clinical trials (RCTs; Maas et al., 2007,2010). The translation of preclinical studies of neuroprotection into improved outcomes in clinical TBI care has failed despite years of positive outcomes in animal research (Bullock et al., 1999; Jain, 2008; Narayan et al., 2002; Tobias and Bullock, 2004). Thus, presently, physicians have no evidence-based treatment that can ameliorate the devastating neurologic deficits of severe TBI. The reason for this failure in translation is undoubtedly multi-faceted, but we believe a primary reason is the extremely wide range of injury severities in severe clinical TBI trials, compared to the relatively uniform injuries produced in animal models.
The ability to demonstrate efficacy in clinical TBI may depend upon the ability to stratify injury severity and identify patients with salvageable tissue or a “traumatic penumbra” (Coles, 2004; Coles et al., 2004a,2004b; Cunningham et al., 2005; Menon, 2006), or secondary ischemic events (McHugh et al., 2007b). We have, in fact, observed a wide variation in cortical flow values following severe TBI. Within 12 h after severe TBI cortical mantle CBF values ranged from 80% normal to 80% core (unpublished data). This wide variability in injury severity could be part of the basis for the discordance between treatments that are effective in animal models, yet fail in clinical human trials (Jain, 2008; Loane and Faden, 2010). With this degree of variability in injury severity it would be difficult for even the most effective therapies to show efficacy. Central to the premise of neuroprotection is the presence of salvageable tissue that can either be resuscitated or protected from secondary injury. In the absence of salvageable tissue, it may be difficult to demonstrate therapeutic efficacy with any therapy.
The aim of this study was to determine whether stable xenon computed tomography (CT) cerebral blood flow (CBF) measurements in the cortical mantle of patients suffering severe TBI (Glasgow Coma Scale [GCS] ≤8) obtained within 12 h of injury can be used to stratify injury severity based on the Glasgow Outcome Score (GOS) prediction of outcome at 1, 3, and 6 months.
Methods
Research Protocol
This research protocol was approved by the Baylor Institutional Review Board for Human Subject Research. Stable xenon-CT CBF (Xe/CT-CBF) was measured prospectively in 125 patients over 6 years within the first 12 h after TBI (Hlatky et al., 2004). The eligibility criteria for the study included the following: (1) severe TBI with GCS score 8 or less, (2) age ≥15 years, (3) motor GCS score ≤5 at the time of CBF measurement, and (4) measurement of cortical mantle CBF by xenon CT within 12 h after injury. Informed consent was obtained from each patient's nearest relative for participation in the study.
Four of the 120 patients included in this study were receiving pentobarbital during their initial Xe/CT-CBF, which represents a small percentage of the total number of subjects. For the management of these patients, the head injury guidelines were followed, including prompt evacuation of mass lesions, control of intracranial pressure (ICP), and maintenance of adequate cerebral perfusion pressure (CPP). All patients received sedation, usually morphine. For intracranial hypertension, CSF drainage, mannitol, and neuromuscular blockade were used. For refractory intracranial hypertension, pentobarbital coma and/or decompressive craniectomy were used.
Quantitative stable xenon-CT cerebral blood flow
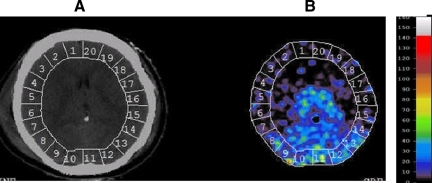
The CT scans were performed in four axial planes with a thickness of 5 mm, with each slice 20 mm apart as previously described (Haltky et al., 2008; Yonas et al., 1991). End-expiratory xenon and CO2, oxygen saturation, electrocardiogram (ECG), arterial blood pressure, and ICP when available, were continuously monitored. A complete CBF study required 13–17 min to complete. The CBF maps were analyzed by a fixed template over the cortical mantle with 20 voxels of interest (VOI) for each of 4–6 planes, for a total of 88–132 VOI in each patient (Fig. 1). CBF values were separated into the following categories as previously published based on xenon the CBF values obtained in the study by Yonas and associates (1991): core=0–8, penumbra=9–30, and normal=31–70 mL/100 g/min. Each of the VOI was separated into the above categories and averaged. To compensate for inequality in the number of VOI, the data were multiplied by percent volume of each voxel, and divided by total volume for the entire cortical mantle. With these numbers, outcome by GOS score was correlated with the CBF categories of our severe TBI patients. This was done at each of the four endpoints.
FIG. 1.
One-slice axial computed tomography (A) and xenon computed tomography cerebral blood flow (CBF) map (B) for a patient with 20–22 voxels of the cortical mantle with a color guide for CBF values. Each patient had 4–6 axial scans 10 mm thick.
Patients with an intracranial mass lesion on the admission CT scan were taken immediately to the operating room for evacuation of the lesion. Patients with evidence of diffuse intracranial hypertension were treated according to the protocol used in the intensive care unit at Baylor College of Medicine, whereby ICP >20 mm Hg was the cut-off for elevated ICP. Factors such as hypotension, fever, hypoxia, and acidosis were treated per protocol in all patients. Mean arterial pressure was kept above 80 mm Hg, and CPP above 60 mm Hg, per standard protocol.
A total of 120 patients were studied. At each time point, the standard GOS score was used to assess outcome. The GOS scale reflects better outcome with a higher score (1–5): GOS 5, good recovery; GOS 4, moderate disability; GOS 3, severe disability; GOS 2, persistent vegetative state; and GOS 1, death. Tabulation of CBF values in the cortical mantle was done blind to the final GOS outcome.
Statistical analysis
Repeated-measures analysis of variance (ANOVA) for percentage CBF for each of low, intermediate, normal, and hyperemia, was done with time as a repeated factor, and GOS score as time-varying factors. p Values were obtained for linear trends across GOS scores. Kruskal-Wallis tests were done across groups of GOS for each of the four tissue types. Post-hoc t-tests were done with a p value <0.05 set to be statistically significant.
A receiver operating characteristic (ROC) curve was also obtained for total normal and hyperemia flow volume (TNHV) in percent at 6-month GOS scores of 3, 4, and 5, using SAS software, and the area under the curve (AUC) with cut-off scores determined.
Multivariate analysis was also performed for age, Marshall CT classification score (Marshall et al., 1991), TNHV10, and emergency room Glasgow Coma Score sum (ERGCSsum). All data analyses were done using SAS Analytics software (Cary, NC).
Results
All 120 patients suffered severe TBI and were studied in a prospective manner regardless of gender or mechanism of injury. The average age was 35±13.7 years (mean±SD), with a range of 15–71 years old. Admission GCS was ≤8 in all patients enrolled in the study. Stable Xe/CT-CBF measurements were made within the first 12 h after injury. The mean time after injury for the Xe/CT-CBF scans was 6.69±3.32 h (mean±SD), and the range was 1–12 h.
Percent total low, intermediate, normal, and hyperemic CBF volumes versus GOS score
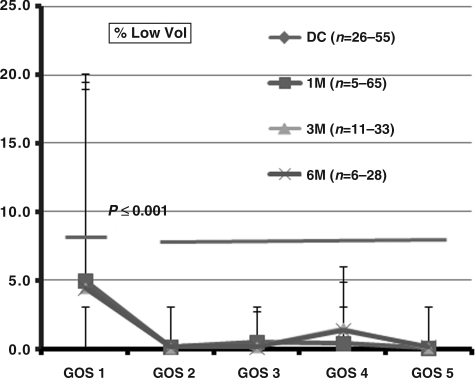
The percent total low volume (%TLV) versus GOS score at discharge (DC), and at 1, 3, and 6 months shows that a mean %TLV of 5% was associated with GOS 1 (Fig. 2). Outcomes at all higher GOS scores 2–5 were statistically significantly lower (p<0.001), compared to the percent core at GOS 1. The relationship between %TLV and GOS scores were similar at DC, and 1, 3, and 6 months.
FIG. 2.
Illustration of percent total low volume versus Glasgow Outcome Scale (GOS) score 1–5 at discharge (DC), and 1 (1 M), 3 (3 M), and 6 months (6 M). Percent core volume at GOS 1 was significantly higher than the percent core volumes between GOS 2 through GOS 5 (p<0.001). Error bars show standard deviations. The range of n values reflects the variability in the numbers falling into the various cerebral blood flow classifications, as well as patient attrition over time. The same applies to Figures 3 and 4.
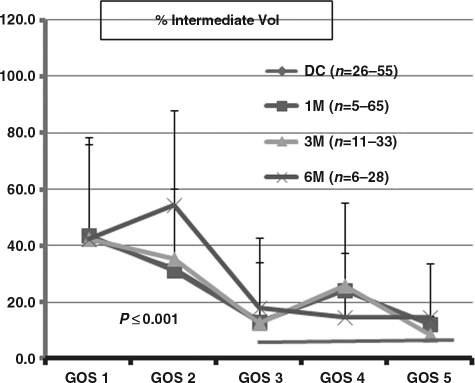
The percent total intermediate volume (%TIV) shows that %TIV of 40–30 % was associated with GOS 1 and GOS 2, which were significantly different (p<0.001) from the %TIV of 20% for GOS 3 to GOS 5 (Fig. 3). The relationship between GOS and %TIV were similar at all time points after injury, from DC to 6 months, as well.
FIG. 3.
Illustration of percent total intermediate volume (%TIV) versus Glasgow Outcome Scale (GOS) score at discharge, and 1 (1 M), 3 (3 M), and 6 months (6 M). Percent penumbra volumes at GOS 1 and GOS 2 were significantly different than percent penumbra volume at GOS 3 through GOS 5 (p<0.001). The number of patients at each point differed, which is reflected in the range of numbers in parentheses.
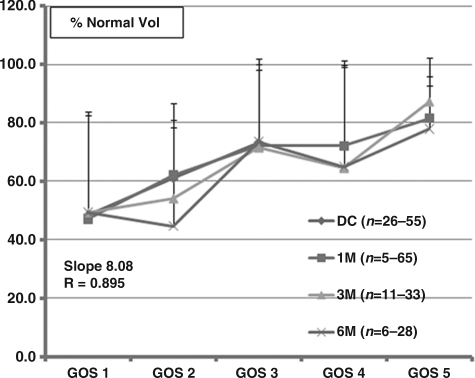
The percent total normal volume (%TNV) versus GOS scores showed a significant direct positive linear correlation to %TNV of 40% at GOS 1 to 80% at GOS 5 (Fig. 4). GOS 3 to GOS 5 were correlated with %TNV of 70–80%, and GOS 1 and GOS 2 with %TNV of 50–60%. The relationship between %TNV and GOS scores was statistically significant (R=0.895). Unlike the dichotomous distribution of outcome by GOS score and %TLV and %TIV, there was a linear relationship between %TNV and outcome at all time points post-injury.
FIG. 4.
Illustration of percent total normal volume (%TNV) versus Glasgow Outcome Scale (GOS) score at discharge (DC), and 1 (1 M), 3 (3 M), and 6 months (6 M). There was a linear relationship between the %TNV and GOS score, with a slope of 8.09 and a correlation coefficient of 0.895 (p<0.001). The number of patients at each point differed, which is reflected in the range of numbers in parentheses.
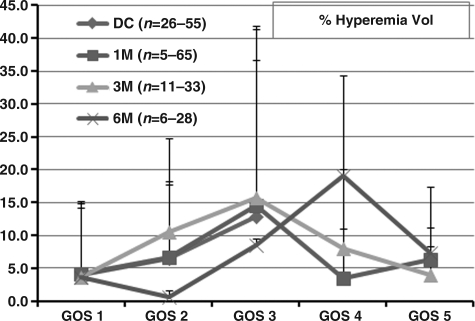
The percent total hyperemic volume (%THV) versus GOS scores (Fig. 5) shows that although there was wide variability, at DC, 1, and 3 months, %THV increased with increasing GOS score, from GOS 1 through GOS 3, followed by a decrease at GOS 4 and GOS 5. At 6 months, %THV increased progressively from GOS 1 through GOS 4, then fell at GOS 5, as did %THV at 1 and 3 months.
FIG. 5.
Illustration of percent total hyperemia volume (%THV) versus Glasgow Outcome Scale (GOS) scores at discharge (DC), and 1 (1 M), 3 (3 M), and 6 months (6 M). Note that %THV is reduced at GOS 5. The number of patients at each point differed, which is reflected in the range of numbers in parentheses.
Percent total normal volume (%TNV) and percent total low volume (%TLV) + percent total intermediate volume (%TIV)
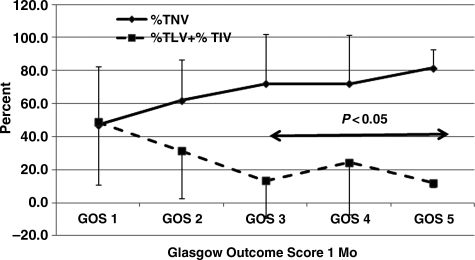
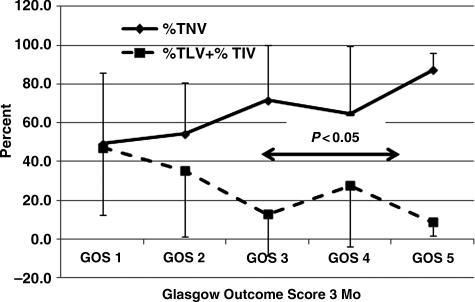
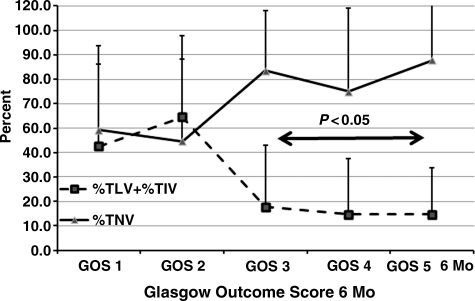
Based upon the notion that a larger %TNV was significantly and positively correlated with GOS scores as shown in Figure 6, and a larger %TLV and %TIV might be expected to correlate with poor outcome, these variables were related to GOS scores at 1 (Fig. 6), 3 (Fig. 7), and 6 months (Fig. 8). The data show that there was a significant relationship between %TNV and outcome by GOS score, which distinguished GOS 1 and GOS 2 from GOS 3 to GOS 5, as did the relationship for %TLV + %TIV. Differences between these variables and GOS scores were significant (p<0.05) between GOS 1and GOS 2 compared to GOS 3 to GOS 5.
FIG. 6.
Percent total normal volume (%TNV) and percent total low volume (%TLV) + percent total intermediate volume (%TIV) with respect to Glasgow Outcome Scale (GOS) score at 1 month post-injury. GOS 3 through GOS 5 were significantly (p<0.05) different compared to GOS 1 and GOS 2. Error bars indicate standard deviation. Numbers of patients for GOS 1, GOS 2, GOS 3, GOS 4, and GOS 5, were 25, 23, 42, 5, and 3, respectively. The total number of patients was 98.
FIG. 7.
Percent total normal volume (%TNV) and total core volume (TLV) + percent total intermediate volume (%TIV) with respect to Glasgow Outcome Scale (GOS) score at 3 months post-injury. GOS 3 through GOS 5 were significantly (p<0.05) different compared to GOS 1 and GOS 2. Error bars indicate standard deviation. Numbers of patients for GOS 1, GOS 2, GOS 3, GOS 4, and GOS 5, were 28, 11, 33, 11, and 11. The total number of patients was 94.
FIG. 8.
Percent total normal volume (%TNV) and percent total low volume (%TLV) + percent total intermediate volume (%TIV) with respect to Glasgow Outcome Scale (GOS) score at 6 months post-injury. GOS 3 through GOS 5 were significantly (p<0.05) different compared to GOS 1 and GOS 2. Error bars indicate standard deviation. Numbers of patients for GOS 1, GOS 2, GOS 3, GOS 4, and GOS 5, were 29, 6, 21, 13, and 16. The total number of patients was 85.
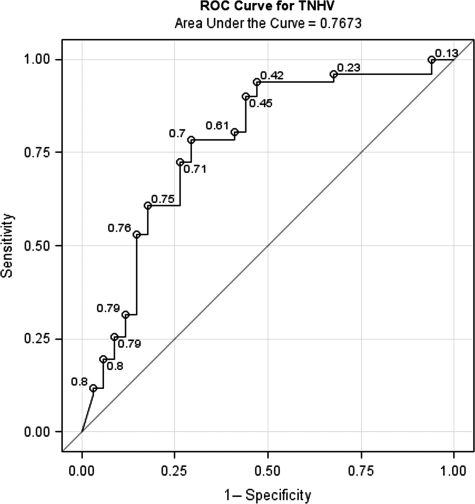
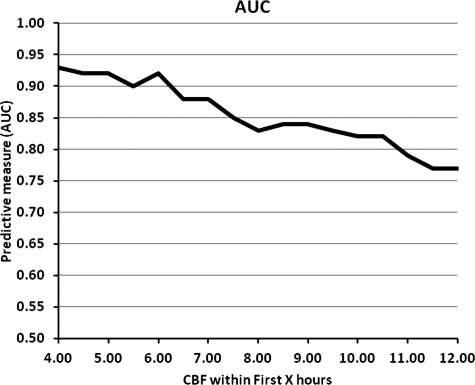
ROC analysis for %TNV + %TNHV measured within 12 h after injury and outcome at 6 months for GOS 3 to GOS 5, relating sensitivity (true-positives) versus 1 − specificity (false-positives; Fig. 9) had an AUC of 0.7673. There was a progressive decrease in AUC cut-scores between 6 and 12 h (Fig. 10). At 6, 9, and 12 h the AUC cut-scores were 0.92, 0.84, and 0.77, respectively.
FIG. 9.
Receiver operating characteristic (ROC) curve for percent total normal plus hyperemic flow volume (TNHV) measured <12 h and prediction of Glasgow Outcome Scale (GOS) scores for GOS 3, GOS 4, and GOS 5 at 6 months in 120 severe traumatic brain injury patients. Numbers adjacent to the curve indicate cut-scores for the area under the curve.
FIG. 10.
Illustration of the relationship between area under the curve (AUC) of receiver operating characteristic curves based on percent total normal plus hyperemic flow volume (TNHV) prediction of Glasgow Outcome Scale (GOS) scores for GOS 3, GOS 4, and GOS 5, at 6 months post-injury as a function of time of cerebral blood flow (CBF) measurement after injury.
Multivariate analysis of outcome GOS 3 to GOS 5 at 6 months revealed that the predictors %TNHV, ERGCSsu, age, and Marshall CT score had odds ratios of 1.460 (per 10 percentage point increase), 1.090, 0.978, and 0.274, with p values <0.001, <0.26, <0.42, and <0.03, respectively. %TNHV remained the best predictor in this study of limited sample size.
Discussion
Outcome prediction for individual TBI patients based on physiological monitoring rather than probabilities derived from large epidemiological databases provides the advantage of predicting outcome based on patient-specific pathophysiology. Our data show that quantitative cortical mantle CBF measurements stratified into low, intermediate, normal, and hyperemic flow values within 12 h after severe TBI can predict outcome dichotomously between bad (death [GOS 1] and vegetative state [GOS 2]) and better (severe disability [GOS 3] to good [GOS 5]) outcome. Based on %TNV (normal CBF volume) it also predicts outcome in a linearly defined relationship. ROC analysis with AUC cut-scores showed that the best prediction is obtained if Xe/CT-CBF is measured within 6 h, but has a good cut-score of 0.77 if measured within 12 h. These observations suggest a quantitative basis for predicting outcome that could influence care and utilization of resources for the individual patient. It may also allow the selection of patients for clinical trials that have the greatest possibility of benefiting from intervention.
Stratification of CBF of 0–8 mL/100 g/min for low, 9–30 mL/100 g/min for intermediate, 30–70 mL/100 g/min for normal, and >70 mL/100 g/min for hyperemia, was based on the 50±10 mL/100 g/min (mean±SD) of the CBF values obtained in normal volunteers (Yonas et al., 1991). Normal CBF was defined as between 30 and 70 mL/100 g/min (mean±2 SD). In an earlier study of patients with acute stroke with CBF measured by stable xenon-CT (Jovin et al., 2003), the researchers used CBF categories of: core=0–8 mL/100 g/min, penumbra=8–20 mL/100 g/min, and non-core/non-penumbra ≥20 mL/100 g/min, and reported that core rather than penumbra volume predicted outcome. Our results showed that %TLV could only differentiate death (GOS 1) from all other outcomes.
Pre-clinical studies on the thresholds of ischemia support our selection of CBF thresholds in this study. The earliest studies in baboons by Symon and associates (1977), and Astrup and colleagues (1977), suggested that CBF levels consistent with infarction based on potassium release and histology were <10 mL/100 g/min, and on the order of 6–8 mL/100 g/min, respectively, whereas the loss of function occurred at a CBF of about 15 mL/100 g/min. Thus, the CBF of 0–8 mL/100 g/min for the low volume is generally agreed upon. The intermediate volume (penumbra) we defined was 9–30 mL/100 g/min, which differed from the 9–20 mL/100 g/min defined by Jovin and colleagues (2003). Thus our stratification in this study may also include a component of benign oligemia, with CBF values between 20 and 30 mL/100 g/min (Bandera et al., 2006). Positron emission tomography (PET) studies (Baron et al., 2001; Marchal et al., 1996) also indicate that the penumbra CBF values in stroke fall in the range we used, but again, may also include regions of benign oligemia.
Bouma and associates (1991) used radioactive Xe-133 with external detection to measure cortical hemispheric CBF in 186 adult TBI patients as early as 6 h after injury to seek evidence of cerebral ischemia. They reported that there was a significant correlation with GCS motor scores and CBF in the first 8 h after injury, which was lost by 12 h. In a subsequent study (Bouma et al., 1992), the researchers used stable Xe/CT-CBF with measurement of regional CBF between 4 and 12 h of injury in 35 patients, and reported ischemia in 11/35 (31.4%). However, in neither of these studies was the objective the prediction of long-term outcome.
Using quantitative CBF to predict outcome in TBI, Kelly and colleagues (1997) studied 54 moderately-to-severely head-injured patients with 184 measurements of 133Xe-CBF measurements, from day 0 to day 5. The lowest flows were observed at day 0, and the highest flows between days 1 and 5. The patients were divided into groups based upon their CBF values. Group I had CBF <33 mL/100 g/min at all sites; group II had CBF <33> mL/100 g/min; and group III had CBF ≥33 mL/100 g/min in all circumstances. Based on this stratification, at 6 months, favorable neurological outcomes in groups 1, 2, and 3, were 0%, 46.2%, and 58.8%, respectively (p<0.05). Increasing CBF values between days 0 and 5 post-injury positively correlated with predicted outcome at 6 months. Our results are consistent with these observations in that the higher cortical mantle flows of %TNV in the first 12 h after injury better predicted outcome, whereas lower cortical CBF values of percent total low volume (%TVC) + percent total intermediate volume (%TIV) indicated worse outcome.
It is important that measurements of CBF are made as soon as possible after TBI, in order to avoid the disassociation between CBF and the cerebral metabolic rate for oxygen (CMRO2) (uncoupling of flow and metabolism), which may occur after the first day following TBI. Some investigators were unable to observe cerebral ischemia days to weeks after TBI, and were therefore of the opinion that low CBF levels after TBI were due to contusion-related depression of CMRO2 rather than cerebral ischemia (Vespa et al., 2005; Xu et al., 2010). Ischemia as defined by increased oxygen extraction fraction (OEF) by PET has, however, been demonstrated very early after ischemia (Coles, 2004; Coles et al., 2004a,2004b). The observation that quantitative CBF values obtained in the initial hours post-TBI predict outcome is most consistent with low flow being a measure of the severity of the initial diffuse brain injury. This type of injury is often associated with a prolonged period of hypoxia and/or ischemia at the time of injury.
The prediction of outcome on an individual basis after TBI was first addressed by the development of the GCS and the GOS (Teasdale and Jennett, 1974,1976; Jennett et al., 1976). These neurologic scoring systems predicted outcome after TBI with 98% accuracy based on scores obtained at 3 days. Since then various methods of predicting outcome after TBI have been explored, including statistical epidemiological methods (McHugh et al., 2007a; Murray et al., 2007); admission characteristics (Steyerberg et al., 2008); pathoanatomic imaging by CT (Nelson et al., 2010) and MRI (Legares et al., 2009; Levine et al., 2006); serum laboratory values and biomarkers (Van Beek et al., 2007); and physiological measurements (McHugh et al., 2007b). All of these methods of predicting outcome used GCS or GOS as the basis for comparison.
Outcome predictions based on admission characteristics could enable immediate treatment decisions derived from large patient populations through analysis of databases such as the IMPACT (Murray et al., 2007) and CRASH (MRC CRASH Trial Collaborators, 2008) studies combined. Univariate and multivariate analyses of IMPACT data (Murray et al., 2007) showed that the most powerful independent prognostic variables were age, GCS motor score, CT characteristics, and pupillary response. Validation of the IMPACT outcome prediction score was studied in 1061 patients (Yeoman et al., 2011) in the Nottingham Head Injury Registry obtained over 10 years. Based on the IMPACT model, mortality and unfavorable outcome were predicted with ROC AUC cut-scores of 0.835 and 0.823, respectively. Similarly, Panczykowski and associates (2011) validated the IMPACT model using age, motor score, and pupillary reactivity (core model), Marshall grade on head CT and secondary insults (extended), and laboratory values as prediction tools in 587 patients, and reported good prediction of unfavorable outcome and mortality. However, whether the IMPACT model by default can predict favorable outcome was not determined. Despite the calculation of odds ratios, exactly how the combination of the various odds ratios for prognostic variables can be used to predict outcome for an individual patient have yet to be resolved. The individual physiological variable obtained by quantitative Xe/CT-CBF is also capable of predicting favorable outcome. Multivariate analysis in this study of limited size also shows that TNHV is an independent predictor of favorable outcome at 6 months. The changes seen in THV flow that affect outcome were notable. Whereas the increase in THV between GOS 1/GOS 2 and GOS 3/GOS 4 may be an indication of recovery, the decrease in THV at GOS 5 from that at GOS 3 and GOS 4 might reflect a normalization of flow with improved outcome.
Part of the difficulty in prognostication is that secondary injury processes serve to modify morbidity and mortality, apart from the severity of the initial insult. Following TBI, multiple factors may be involved in secondary injury, such as hypoxia (Dunham et al., 2004; Hattori et al., 2003; Hlatky et al., 2002; Menon et al., 2004; Hlatky et al., 2008), arterial hypotension, and high ICP (Martin et al., 1997). Mazzeo and associates (2006) analyzed 172 severe TBI patients and proposed a simple ischemia scoring system, taking into account hemodynamic status as well as herniation, which is able to provide a more quantitative approach toward determining outcome at 3 and 6 months. They found a negative relationship between ischemia score and GOS score. Greater ischemia-inducing insults were correlated with a worse outcome at 3 and 6 months. While there is some evidence that hyperoxia is beneficial in severe TBI (Rockswold et al., 2010) the lack of effect of hyperoxia in others suggest otherwise (Dringer et al., 2007; Magnoni et al., 2003).
In summary, our observations show that quantitative CBF measured within the first 6 and 12 h after severe TBI may be used a predictor of unfavorable (GOS 1 and GOS 2) and favorable (GOS 3 to GOS 5) outcomes. Although this study was prospective and performed under well-defined guidelines, these results should be re-examined in a larger, multicenter clinical trial.
Acknowledgments
We wish to acknowledge the xenon CT technicians at Baylor College of Medicine as well as the nursing staff for their dedication to this project. This work was supported in part by National Institutes of Health grants no. NS061216, NS051639, and P01-NS38660.
Author Disclosure Statement
No competing financial interests exist.
References
- Astrup J. Symon L. Branston N.M. Lassen N.A. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1997;8:51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Bandera E. Botteri M. Minelli C. Sutton A. Abrams K.R. Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37:1334–1339. doi: 10.1161/01.STR.0000217418.29609.22. [DOI] [PubMed] [Google Scholar]
- Baron J.C. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc. Dis. 2001;11:2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Choi S.C. Newlon P.G. Young H.F. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J. Neurosurg. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Stringer W. Choi C. Fatouros P. Young H.F. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J. Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- Bullock M.R. Lyeth B.G. Muizelaar J.P. Current status of neuroprotection trials for traumatic brain injury: lessons from animal models and clinical studies. Neurosurgery. 1999;45:207–217. doi: 10.1097/00006123-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) http://www.cdc.gov/TraumaticBrainInjury/statistics.html http://www.cdc.gov/TraumaticBrainInjury/statistics.html
- Coles J.P. Fryer T.D. Smielewski P. Chatfield D.A. Steiner L.A. Johnston A.J. Downey S.P. Williams G.B. Aigbirhio F. Hutchinson P.J. Rice K. Carpenter T.A. Clark J.C. Pickard J.D. Menon D.K. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J. Cereb. Blood Flow Metab. 2004a;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Fryer T.D. Smielewski P. Rice K. Clark J.C. Pickard J.D. Menon D.K. Defining ischemic burden after traumatic brain injury using 15O PET imaging of cerebral physiology. J. Cereb. Blood Flow Metab. 2004b;24:191–201. doi: 10.1097/01.WCB.0000100045.07481.DE. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Regional ischemia after head injury. Curr. Opin. Crit. Care. 2004;210:120–125. doi: 10.1097/00075198-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Corrigan J.D. Selsassie A.W. Orman J.A. The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- Cunningham A.S. Salvador R. Coles J.P. Chatfield D.A. Bradley P.G. Johnston A.J. Steiner L.A. Fryer T.D. Aigbirhio F.I. Smielewski P. Williams G.B. Carpenter T.A. Gillard J.H. Pickard J.D. Menon D.K. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Brain. 2005;128:1931–1942. doi: 10.1093/brain/awh536. [DOI] [PubMed] [Google Scholar]
- Diringer M.N. Aiyagari V. Zazulia A.R. Videen T.O. Powers W.J. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J. Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- Dunham C. Random K. Flowers L. Siegal J. Kohli C. Cerebral hypoxia in severely brain injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. J. Trauma. 2004;56:482–491. doi: 10.1097/01.ta.0000114537.52540.95. [DOI] [PubMed] [Google Scholar]
- Hattori N. Huang S.C. Wu H.M. Yeh E. Glenn T.C. Vespa P.M. McArthur D. Phelps M.E. Hovda D.A. Bergsneider M. Correlation of regional metabolic rates of glucose with Glasgow Coma Scale after traumatic brain injury. J. Nucl. Med. 2003;44:1709–1716. [PubMed] [Google Scholar]
- Hlatky R. Furuya Y. Valadka A.B. Gonzalez J. Chacko A. Mizutani Y. Contant C.F. Robertson C.S. Dynamic autoregulatory response after severe head injury. J. Neurosurg. 2002;97:1054–1061. doi: 10.3171/jns.2002.97.5.1054. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Gopinath S.P. Robertson C.S. Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J. Neurosurg. 2008;108:53–58. doi: 10.3171/JNS/2008/108/01/0053. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Conant C.E. Diaz-Marchan P. Valadka A.B. Robertson C.S. Significance of reduced cerebral blood flow during the first twelve hours after traumatic brain injury. Neurocrit. Care. 2006;1:69–83. doi: 10.1385/NCC:1:1:69. [DOI] [PubMed] [Google Scholar]
- Jain K.K. Neuroprotection in traumatic brain injury. Drug Discovery Today. 2008;13:1082–1089. doi: 10.1016/j.drudis.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Jennett B. Teasdale G. Braakman R. Minderhoud J. Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;15:1031–1034. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- Jovin T.G. Yonas H. Gebel J.M. Kanal E. Chang Y.F. Grahovac S.Z. Goldstein S. Wechsler L.R. The cortical ischemic core and not the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke. 2003;34:2426–2433. doi: 10.1161/01.STR.0000091232.81947.C9. [DOI] [PubMed] [Google Scholar]
- Kelly D.F. Martin N.A. Kordestani R. Counelis G. Hovda D.A. Bergsneider M. McBride D.Q. Shalmon E. Herman D. Becker D.P. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J. Neurosurg. 1997;86:633–641. doi: 10.3171/jns.1997.86.4.0633. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Legares A. Ramos A. Perez-Nunez Ballenilla F. Alday F. Gomez P.A. Lobato A.K. The role of MR imaging in assessing prognosis after severe and moderate head injury. Acta Neurochir. 2009;151:341–356. doi: 10.1007/s00701-009-0194-8. [DOI] [PubMed] [Google Scholar]
- Levine B. Fujiwara E. O'Connor C. Richard N. Kovacevic N. Mandic M. Restagno A. Easdon C. Robertson I.H. Graham S.J. Cheung G. Gao F. Schwartz M.L. Black S.E. In vivo characterization of traumatic brain injury neuropathology with structural and functional neuroimaging. J. Neurotrauma. 2006;23:1396–1411. doi: 10.1089/neu.2006.23.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane D.J. Faden A.I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.I.R. Marmarou A. Murray G.D. Teasdale G.M. Steyerberg E.W. Prognosis and clinical trial design in traumatic brain injury: The IMPACT Study. J. Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R. Roozenbeek B. Manley G.T. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoni S. Ghisoni L. Locatelli M. Caimi M. Colombo A. Valeriani V. Stochetti N. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J. Neurosurg. 2003;98:952–958. doi: 10.3171/jns.2003.98.5.0952. [DOI] [PubMed] [Google Scholar]
- Marchal G. Beaudouin V. Rioux P. de la Sayette V. Le Doze F. Viader F. Derlon J.M. Baron J.C. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke. 1996;27:599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. van Berkum Clark M. Eisenberg H.M. Jane J.A. Luerssen T.G. Marmarou A. Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991;75:S14–S20. [Google Scholar]
- Martin N.A. Patwardhan R.V. Alexander M.J. Africk C.Z. Lee J.H. Shalmon E. Hovda D.A. Becker D. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J. Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- Mazzeo A. Kunene N. Choi S. Gilman C. Bullock R. Quantitation of ischemic events after severe traumatic brain injury in humans: A simple scoring system. J. Neurosurg. Anesth. 2006;18:170–178. doi: 10.1097/01.ana.0000210999.18033.f6. [DOI] [PubMed] [Google Scholar]
- McHugh G.S. Butcher I. Steyerbert E.W. Lu J. Mushkudiani N. Marmarou A. Maas A.I. Murray G.D. Statistical approaches to the univariate prognostic analysis of the IMPACT database on traumatic brain injury. J. Neurotrauma. 2007a;24:251–258. doi: 10.1089/neu.2006.0026. [DOI] [PubMed] [Google Scholar]
- McHugh G.S. Engel D.C. Butcher I. Steyerberg E.W. Lu J. Mushkudiani N. Hernández A.V. Marmarou A. Maas A.I. Murray G.D. Prognostic value of insults in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007b;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Brain ischemia after traumatic brain injury: Lessons from 15O2 positron emission tomography. Curr. Opin. Crit. Care. 2006;12:85–89. doi: 10.1097/01.ccx.0000216572.19062.8f. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Coles J.P. Gupta A.K. Fryer T.D. Smielewski P. Chatfield D.A. Aigbirhio F. Skepper J.N. Minhas P.S. Hutchinson P.J. Carpenter T.A. Clark J.C. Pickard J.D. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004;32:1384–1389. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- MRC CRASH Trial Collaborators. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;23:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G.D. Butcher I. McHugh G. Lu J. Mushkudiani N.A. Maas A.I.R. Marmarou A. Steyerberg E.W. Multivariable prognostic analysis in traumatic brain injury. Results from the IMPACT Study. J. Neurotrauma. 2007;24:329–327. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. the Clinical Trials in Head Injury Study Group. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.W. Nyström H. MacCallum R.M. Thornquist B. Lilja A. Bellander B.M. Rudehill A. Wanecek M. Weitzberg E. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J. Neurotrauma. 2010;27:51–64. doi: 10.1089/neu.2009.0986. [DOI] [PubMed] [Google Scholar]
- Panczykowski D. Puccio A. Scruggs B. Bauer J. Hricik A. Beers S. Okonkwo D. Prospective independent validation of IMPACT modeling as a prognostic tool in severe traumatic brain injury. J. Neurotrauma. 2011;29:47–52. doi: 10.1089/neu.2010.1482. [DOI] [PubMed] [Google Scholar]
- Rockswold S.B. Rockswold G.L. Zaun D.A. Zhang X. Cerra C.E. Bergman T.A. Liu J. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J. Neurosurg. 2010;112:1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W. Mushkudiani N. Perel P. Butcher B. Lu J. McHugh G.S. Murray G.D. Marmarou A. Roberts I. Habbema J.D. Maas A.I. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:1251–1261. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon L. Branston N.M. Strong A.J. Hope T.D. The concepts of thresholds of ischaemia in relation to brain structure and function. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 1997;11:149–154. doi: 10.1136/jcp.s3-11.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir. (Wien.) 1976;34:45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tobias R.M. Bullock M.R. Critical appraisal of neuroprotection trials in head injury: What have we learned? J. Amer. Soc. Exp. Neurotherapeutics. 2004;1:71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek J. Mushkuidani N.A. Steyerberg E.W. Butcher I. McHugh G.S. Lu J. Marmarou A. Murray G.D. Maas A.I. Prognostic value of admission laboratory parameters in traumatic brain injury: Results from the IMPACT Study. J. Neurotrauma. 2007;24:315–328. doi: 10.1089/neu.2006.0034. [DOI] [PubMed] [Google Scholar]
- Vespa P. Bergsneider M. Hattori N. Wu H.M. Huang S.C. Martin N.A. Glenn T.C. McArthur D.L. Hovda D.A. Metabolic crisis with brain ischemia is common after traumatic brain injury. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. McArthur D.L. Alger J.R. Etchepare M. Hovda D.A. Glenn T.C. Huang S. Dinov I. Vespa P.M. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J. Cereb. Blood Flow Metab. 2010;30:883–894. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman P. Pattani H. Silcocks P. Owens V. Fuller G. Validation of the impact outcome score using the Nottingham Head Injury Register dataset. J. Trauma. 2011;71:387–392. doi: 10.1097/TA.0b013e31820ceadd. [DOI] [PubMed] [Google Scholar]
- Yonas H. Darby J.M. Marks E.C. Durham S.R. Maxwell C. CBF measured by Xe-CT: approach to analysis and normal values. J. Cereb. Blood Flow Metab. 1991;11:716–725. doi: 10.1038/jcbfm.1991.128. [DOI] [PubMed] [Google Scholar]
- Zaloshnja E. Miller T. Langlois J.A. Selassie A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]