Abstract
In patients with type 2 diabetes mellitus (T2DM), the physiologic glucagon-like peptide-1 (GLP-1) response, which is involved in glucose regulation through several mechanisms, is dysfunctional. GLP-1 receptor agonists can fill an unmet therapeutic need in the treatment of T2DM: improving glycemic control without increasing the risk of hypoglycemia (except with concomitant sulfonylureas) and reducing weight in a substantial proportion of patients. GLP-1 receptor agonists have impacted established disease treatment algorithms for T2DM. For example, in 2009 the American Diabetes Association and European Association for the Study of Diabetes revised their consensus treatment algorithm to incorporate GLP-1 receptor agonists. GLP-1 receptor agonists were originally represented by exenatide BID (ExBID), a short-acting agent requiring twice-daily injections at mealtime. The longer-acting agent liraglutide, requiring once-daily injections, recently received regulatory approval. Several other long-acting agents are in clinical development, one of which is the once-weekly formulation of exenatide (exenatide once weekly [ExQW]). This article reviews the clinical development of ExQW in the DURATION program. Patients in theses clinical trials were receiving various background treatments, ranging from lifestyle therapy to combination oral therapy, although the majority (68%) received metformin monotherapy. Specifically, safety, glycemic control, and weight were compared in patients treated with ExQW versus ExBID, sitagliptin, pioglitazone, or insulin glargine. Moreover, measures of β-cell function, cardiovascular risk, inflammation, and hepatic health were investigated. During ExQW clinical development, consistent clinical efficacy (glycosylated hemoglobin, −1.5% to −1.9%; weight, −2 kg to −4 kg) and safety data were observed in patients with T2DM treated with ExQW.
Introduction
Patients with type 2 diabetes mellitus (T2DM) experience characteristic hyperglycemia, believed to be in response to progressive β-cell dysfunction and/or insulin resistance, leading to relative insulin deficiency and defects in incretin hormone signaling—referred to as the incretin defect. In patients with T2DM, the physiologic response of the incretin hormones (i.e., glucagon-like peptide-1 [GLP-1] and glucose-dependent insulinotropic polypeptide) is dysfunctional, contributing to dysregulation of glucose control through several mechanisms.1–5 GLP-1 is degraded by dipeptidyl peptidase-4 (DPP-4), resulting in a half-life of approximately 2 min in the circulation, thereby limiting its potential as an effective antidiabetes agent.6 The incretin-based therapies have been developed to address this pathophysiologic defect either by inhibiting DPP-4–mediated degradation of endogenous GLP-1 (DPP-4 inhibitors) or by providing pharmacologic GLP-1 agonism via molecules that are resistant to DPP-4 degradation (GLP-1 receptor agonists). Thus, these treatments may fill unmet therapeutic needs by improving glycemic control without increased risk of hypoglycemia and by reducing body weight in many patients.7 The position of incretin-based therapies in the antidiabetes armamentarium was acknowledged in 2009 by the inclusion of GLP-1 receptor agonists in a revision of the American Diabetes Association and European Association for the Study of Diabetes consensus treatment algorithm for T2DM.8 The revised algorithm categorizes GLP-1 receptor agonists and pioglitazone as alternatives to well-validated core therapies (metformin, sulfonylureas, and/or basal insulin), with GLP-1 receptor agonists being recommended for use in circumstances where weight control and hypoglycemia are concerns.8
DPP-4 inhibitors are small molecules, administered orally, with the effect of inhibiting DPP-4–mediated degradation of incretin hormones, increasing endogenous GLP-1 in the circulation by two- to threefold, as well as increasing the concentration of glucose-dependent insulinotropic polypeptide. Each agent in the DPP-4 inhibitor class has unique reversible binding properties via covalent or noncovalent bonds, resulting in potential differences in pharmacologic action. Currently, regulatory approval has been granted in the United States and Europe for sitagliptin and saxagliptin and in Europe for vildagliptin as well.
The GLP-1 receptor agonists are peptides administered by subcutaneous injection, sharing some glucoregulatory effects with GLP-1 but with resistance to degradation by DPP-4. The first GLP-1 receptor agonist to receive regulatory approval in the United States and Europe was exenatide BID (ExBID), a short-acting peptide molecule with resistance to DPP-4, requiring twice-daily injection prior to mealtime.7 More recently, the longer-acting agent liraglutide (once-daily injection) received regulatory approval in the United States and Europe; liraglutide is a modified human GLP-1 molecule that binds to serum albumin in the circulation, thereby conferring resistance to DPP-4 degradation and slow consistent release for GLP-1.9,10 Several other long-acting agents are in clinical development, with the sustained-release formulation of exenatide (exenatide once weekly [ExQW]), requiring once-weekly injection, being the furthest in clinical developed at the time of writing.11
Overall, the GLP-1 receptor agonists achieve pharmacologic activation of the GLP-1 receptor, whereas DPP-4 inhibitors increase endogenous glucose-dependent insulinotropic polypeptide and GLP-1 concentrations.10 The GLP-1 receptor agonists are generally regarded as having greater efficacy, evidenced by more robust improvements in glycemic control and weight loss than DPP-4 inhibitors.12–14 The greater effects of GLP-1 receptor agonist therapy on glycemic control and weight are due to the more potent mode of action achieved with pharmacologic concentrations of GLP-1 receptor agonists on (1) stimulation of glucose-dependent insulin secretion, (2) suppression of inappropriate glucagon secretion, (3) regulation of gastric emptying, (4) suppression of appetite, and (5) trophic effects on β-cell function; this compares with some stimulation of insulin secretion and suppression of glucagon secretion as the likely mode of action of DPP-4 inhibitors.10–14 However, GLP-1 receptor agonists are associated with worse gastrointestinal tolerability (nausea, vomiting) than DPP-4 inhibitors.10–14
ExQW consists of exenatide (the same molecule used with ExBID) encapsulated in microspheres made of poly(d,l-lactic-co-glycolic acid), a biodegradable medical polymer similar to the material in reabsorbable sutures, that allows for controlled drug delivery over an extended period of time,15 thereby facilitating once-weekly administration and continuous exposure to the drug above the therapeutic threshold. Early clinical work demonstrated that ExQW had greater effects on glycemic control and similar effects on weight compared with placebo.11 The clinical development of ExQW has proceeded with the Phase 3 DURATION (Diabetes therapy Utilization: Researching changes in A1C, weight and other factors Through Intervention with exenatide ONce weekly) program, examining the safety and efficacy of ExQW versus ExBID, sitagliptin, pioglitazone, and titrated insulin glargine in patients with T2DM on background treatment of diet and exercise, metformin monotherapy, or combination oral therapy (predominant background therapy was metformin).14,16–18
At the time of writing this article, the U.S. Food and Drug Administration requested a thorough QT study to assess the effects of exenatide on QT interval.19 The QT interval is defined as the start of the Q wave to the end of the T wave in the electrical cycle of the heart and is a measure of the electrical depolarization and repolarization of the left and right ventricles. As such, thorough QT studies are used to assess potential arrhythmia liability in new pharmaceuticals. In addition, the Food and Drug Administration requested safety and efficacy data from the DURATION-5 trial (ExQW vs. ExBID) as part of its approval review process.19 The purpose of this article is to review the safety and efficacy, including measures of glycemic control, weight loss, β-cell function, cardiovascular risk, inflammation, and hepatic health, in patients with T2DM who were treated with ExQW in the DURATION trials.
Review of Clinical Efficacy
The DURATION clinical trial program comprises controlled clinical trials of 24–30 weeks in duration that compared the safety and efficacy of ExQW with those of ExBID (DURATION-1 and DURATION-5), maximum daily doses of sitagliptin or pioglitazone on metformin background (DURATION-2), and titrated insulin glargine (DURATION-3) (Table 1). In the DURATION program to date, 68% of patients (1,021 of 1,494 patients) were treated with metformin monotherapy (specifically, 36% in DURATION-1, 100% in DURATION-2, 70% in DURATION-3, and 41% in DURATION-5),14,16–18 and at least 73% of patients in each trial received concomitant metformin therapy (alone or in combination). Overall, 91% of patients in the DURATION clinical program received concomitant metformin therapy.
Table 1.
Baseline Demographics in the DURATION Clinical Development Program
| |
DURATION-1 (30 weeks) |
DURATION-5 (24 weeks) |
DURATION-2 (26 weeks) |
DURATION-3 (26 weeks) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| ExQW | ExBID | ExQW | ExBID | ExQW | Sitagliptin | Pioglitazone | ExQW | Glargine | |
| n | 148 | 147 | 129 | 123 | 160 | 166 | 165 | 233 | 223 |
| Women (%) | 45 | 49 | 40 | 45 | 44 | 48 | 52 | 48 | 45 |
| Race (%) | |||||||||
| White | 83 | 73 | 63 | 55 | 33 | 30 | 39 | 82 | 85 |
| Black | 6 | 13 | 5 | 7 | 12 | 12 | 8 | 1 | <1 |
| Hispanic | 11 | 14 | 29 | 33 | 31 | 30 | 27 | 12 | 9 |
| Asian | 0 | 1 | 4 | 4 | 23 | 25 | 24 | 6 | 6 |
| Other | 0 | 0 | 0 | 0 | 1 | 3 | 2 | 0 | 0 |
| Age (years) | 55 (10) | 55 (10) | 56 (11) | 55 (10) | 52 (10) | 52 (11) | 53 (10) | 58 (10) | 58 (9) |
| Body weight (kg) | 102 (19) | 102 (21) | 97 (21) | 94 (19) | 89 (20) | 87 (20) | 88 (20) | 91 (19) | 91 (16) |
| BMI (kg/m2) | 35 (5) | 35 (5) | 34 (5) | 33 (5) | 32 (5) | 32 (5) | 32 (6) | 32 (5) | 32 (5) |
| Duration of diabetes (years) | 7 (6) | 6 (5) | 7 (5) | 7 (5) | 6 (5) | 5 (4) | 6 (5) | 8 (6) | 8 (6) |
| HbA1c (%) | 8.3 (1.0) | 8.3 (1.0) | 8.5 (1.1) | 8.4 (1.2) | 8.6 (1.2) | 8.5 (1.2) | 8.5 (1.1) | 8.3 (1.1) | 8.3 (1.0) |
| FG (mg/dL) | 173 (43) | 166 (41) | 173 (47) | 168 (47) | 166 (52) | 164 (45) | 164 (43) | 178 (45) | 175 (49) |
Data are mean (SD) unless otherwise noted.
BMI, body mass index; ExBID, exenatide twice daily; ExQW, exenatide once weekly; FG, fasting glucose; HbA1c, glycosylated hemoglobin.
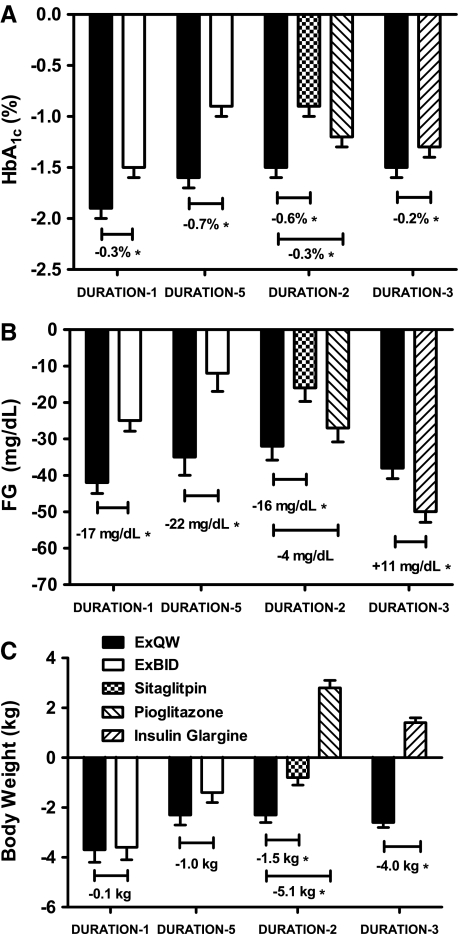
Improvements in glycemic control, as measured by glycosylated hemoglobin (HbA1c) and fasting glucose (FG), were achieved by all treatments in these studies, with ExQW demonstrating mean HbA1c reductions of −1.5% to −1.9% and mean FG reductions of −32 to −41 mg/dL (Fig. 1A and B).14,16–18 ExQW resulted in significantly greater reductions in HbA1c at study end point compared with all comparators (ExBID, sitagliptin, pioglitazone, and titrated insulin glargine).14,16–18 Overall, 58–71% of patients receiving ExQW achieved an HbA1c of <7% in these four DURATION trials, which was significantly more than each comparator in these head-to-head trials (Table 2).14,16–18 Consistent with the greater effects of longer-acting GLP-1 receptor agonists on FG, ExQW resulted in significantly greater reductions in FG than ExBID and the DPP-4 inhibitor sitagliptin (Fig. 1B);14 however, FG reduction was greater with insulin glargine (there was no difference in FG reduction between ExQW and pioglitazone) (Fig. 1B).14,16–18 Of note is that self-monitored blood glucose tests in DURATION-1, DURATION-2, and DURATION-3 suggest that, although all treatments improved mean postprandial glucose (PPG), the reduction with ExQW was greater than it was with sitagliptin or titrated insulin glargine but less robust than it was with ExBID (an effect that was supported by 2-h glucose measurements during a meal tolerance test in DURATION-1).14,16,17
FIG. 1.
Least squares mean (SE) changes from baseline for (A) glycosylated hemoglobin (HbA1c), (B) fasting glucose (FG), and (C) body weight. (A) Reduction of HbA1c from baseline with exenatide once weekly (ExQW) treatment was greater than with any other agent. (B) Reduction of FG from baseline with ExQW treatment was greater than with exenatide twice daily (ExBID), sitagliptin, or pioglitazone but less than the reduction seen with insulin glargine. (C) Reduction of body weight with ExQW was similar to that seen with ExBID and greater than that seen with sitagliptin. In contrast, pioglitazone and insulin glargine were both associated with weight gain. *P<0.05.
Table 2.
Efficacy Parameters in the DURATION Clinical Development Program
| |
|
DURATION-1 |
DURATION-5 |
DURATION-2 |
DURATION-3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ExQW range | ExQW | ExBID | ExQW | ExBID | ExQW | Sitagliptin | Pioglitazone | ExQW | Glargine | |
| Glycemic control | ||||||||||
| HbA1c (%) to goal | ||||||||||
| <7% | 58–71 | 71a | 51ab | 58 | 30b | 59 | 31b | 44b | 60 | 48b |
| ≤6.5% | 39–45 | 45a | 38a | 41 | 16b | 39 | 16b | 27b | 43a | 28ab |
| End point mean HbA1c (%) | 6.4–7.2 | 6.4 | 6.8 | 7.1 | 7.7 | 7.2 | 7.7 | 7.4 | 6.8 | 7.0 |
| Markers of CV risk | ||||||||||
| Δ SBP (mm Hg) | −2.9 to −4.7 | −4.7c | −3.4c | −2.9c | −1.2 | −3.6c | +0.2b | −1.6 | −3.0c | −1.0 |
| ΔTotal cholesterol (mg/dL) | −0.6 to−15 | −11.9c | −3.8b | −15c | +0.6b | −0.6 | +3.1 | +6.2c | −4.6c | −1.5 |
| Δ LDL cholesterol (mg/dL) | −1 to −6 | −4.9c | +1.2b | −6.4c | +2.8b | −1.0 | +1.8 | +1.8 | −1.9 | +1.5 |
| Δ HDL cholesterol (mg/dL) | −1 to +2 | −0.9 | −1.3c | 0 | +1.3 | +2.0c | +2.0c | +6.2bc | 0 | +0.4 |
| Δ Triglycerides (%) | −4 to −15 | −15c | −11c | −6 | −1 | −5 | −5 | −16bc | −4 | −11c |
Data on file at Amylin Pharmaceuticals.
Treatment difference versus ExQW, P<0.05.
Significant difference versus baseline, P<0.05.
CV, cardiovascular; ExBID, exenatide twice daily; ExQW, exenatide once weekly; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Weight loss with ExQW treatment was consistent across the DURATION clinical development program, ranging from 2 kg to 4 kg (Fig. 1C).14,16–18 Weight loss with ExQW was significantly greater than the weight loss observed with sitagliptin and resulted in treatment differences of 4–5 kg compared with the weight gain associated with pioglitazone and glargine treatment (Fig. 1C).14,16–18 Weight loss with ExQW was shown to be similar (DURATION-1 and DURATION-5) to the weight loss achieved with ExBID (Fig. 1C).16,17 Overall, 70–79% of ExQW patients experienced reductions in both HbA1c and weight loss compared with 51–74% of ExBID patients, 46% of sitagliptin patients, 14% of pioglitazone patients, and 31% of insulin glargine patients.14,16–18
Measures of cardiovascular risk were also investigated in the DURATION trials. Mean systolic blood pressure reductions of −3 to −5 mm Hg were observed across trials with ExQW, whereas the only comparator to reduce blood pressure was ExBID in DURATION-1 (−3.4 mm Hg) (Table 2).14,16–18 In DURATION-3, ExQW significantly increased heart rate by a mean of 4 beats/min.18 Exenatide once weekly demonstrated a significant improvement in mean total cholesterol in three of the four DURATION trials; the improvement in total cholesterol was significantly greater for ExQW than it was for ExBID in both comparator trials (Table 2).14,16–18 In the single trial in which total cholesterol with ExQW was unchanged (DURATION-2), pioglitazone was associated with a significant increase in total cholesterol (Table 2).14 Low-density lipoprotein (LDL) cholesterol was improved with ExQW in two trials, and no other drug was associated with improvements in LDL cholesterol (Table 2).14,16–18 Increases in high-density lipoprotein (HDL) cholesterol and decreases in triglycerides were greater with pioglitazone than they were with ExQW (Table 2).14 ExQW reduced total cholesterol in three trials (DURATION-1, DURATION-3, and DURATION-5), significantly reduced LDL cholesterol in two of these trials (DURATION-1 and DURATION-5), and had no significant effect on HDL cholesterol in any of these three trials (Table 2).16–18 The effects of pioglitazone on lipids in the DURATION-2 study (decreased triglycerides, increased HDL cholesterol and total cholesterol) are consistent with earlier investigations and are likely a result of direct effects of pioglitazone treatment on the transcription of genes involved in the regulation of lipid metabolism.14,20 There were no significant differences in changes to fasting lipids between ExQW and sitagliptin or insulin glargine treatment (Table 2).14,18 Several additional measures of cardiovascular risk were assessed in DURATION-2 (ExQW vs. sitagliptin vs. pioglitazone). All therapies significantly reduced high-sensitivity C-reactive protein (ExQW, −24%; sitagliptin, −14%; and pioglitazone, −33%; no significant difference between treatments).14 The mean high-sensitivity C-reactive protein concentrations of patients in this trial were in the normal range (2–3 mg/dL); after treatment concentrations remained in the average risk range. In addition, all therapies in DURATION-2 significantly increased adiponectin (ExQW, 14%; sitagliptin, 15%; and pioglitazone, 187% from baseline; P<0.0001 for pioglitazone vs. ExQW).14 ExQW was the only therapy that significantly reduced the albumin-to-creatinine ratio and the concentration of B-type natriuretic peptide from baseline (B-type natriuretic peptide was also significantly reduced vs. sitagliptin and pioglitazone).14 Pioglitazone was the only treatment that reduced plasminogen activator inhibitor-1 from baseline and was associated with a significantly greater increase in adiponectin than was ExQW.14 In DURATION-3, insulin glargine was reported to reduce alanine aminotransaminase concentration (P<0.05 vs. baseline), compared with no change with ExQW.18
In the DURATION trials, ExQW improved some measures of β-cell function. Indeed, long-term treatment (3 years) with ExBID is reported to sustain improvements in β-cell function through at least 4 weeks after treatment termination.21 In DURATION-1, ExQW significantly increased β-cell sensitivity from baseline after 14 weeks of treatment, as measured by the insulin-to-glucose ratio.16 In DURATION-3, both ExQW and insulin glargine significantly improved homeostasis model assessment of β-cell function, and the improvement with ExQW was significantly greater than that associated with insulin glargine treatment.18 Glargine significantly improved, whereas ExQW had no significant effect, on homoeostasis model assessment of insulin sensitivity.18
Review of Clinical Safety
Eighty-six percent of patients in the intention-to-treat populations completed the DURATION clinical trials (DURATION-1, 87%; DURATION-5, 81%; DURATION-2, 82%; DURATION-3, 92%).14,16–18 The most common adverse event with ExQW and ExBID in DURATION-1 and DURATION-5 was nausea, although incidence was lower with ExQW than it was with ExBID (9% lower in DURATION-1 and 21% lower in DURATION-5) (Table 3).16,17 In DURATION-2, the most common adverse events were nausea with ExQW and sitagliptin and upper respiratory tract infection with pioglitazone (Table 3).14 Nausea, nasopharyngitis, and injection site reactions were the most common adverse events with ExQW in DURATION-3, compared with nasopharyngitis and headache with insulin glargine (Table 3).18 In DURATION-1 and DURATION-5, injection site reactions were more common with ExQW treatment than they were with ExBID treatment (Table 3).16,17 In DURATION-2 injection site reactions with ExQW (10%) were comparable to injection site reactions with placebo microsphere injections (7%) (Table 3).14 The relative incidences of mild- to moderate-intensity nausea, which was reported to decrease over time with both exenatide formulations,17,22 and injection site reactions in ExQW and ExBID were consistent with earlier clinical trials.11
Table 3.
Incidence of Selected Adverse Events During Each DURATION Clinical Trial
| |
DURATION-1 |
DURATION-5 |
DURATION-2 |
DURATION-3 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| ExQW | ExBID | ExQW | ExBID | ExQW | Sitagliptin | Pioglitazone | ExQW | Glargine | |
| Nausea (%) | 26 | 35 | 14 | 35 | 24 | 10 | 5 | 13 | 1 |
| Vomiting (%) | 11 | 19 | 5 | 9 | 11 | 2 | 3 | 4 | 1 |
| Diarrhea (%) | 14 | 13 | 9 | 4 | 18 | 10 | 7 | 9 | 4 |
| Injection site reactions (%) | 30 | 15 | 13 | 10 | 10 | 7 (PBO) | 13 | 2 | |
| Minor hypoglycemia (%) | 5 | 6 | 4 | 3 | 1 | 3 | 1 | 8 | 26 |
The only adverse events not included here that occurred with an incidence ≥10% in any of these DURATION trials were as follows: with exenatide once weekly (ExQW), nasopharyngitis (13% DURATION-3), headache (10% DURATION-3), urinary tract infection (10% DURATION-1), and constipation (10% DURATION-1); with exenatide twice daily (ExBID), upper respiratory tract infection (17% DURATION-1); with pioglitazone, upper respiratory tract infection (10% DURATION-2); and with insulin glargine, nasopharyngitis (17% DURATION-3).
PBO, placebo microsphere injection (combined for sitagliptin and pioglitazone groups).
In all DURATION studies, there was a single incident of hypoglycemia in a patient treated with ExQW that necessitated the assistance of another person but did not require medical intervention or involve loss or severe impairment of consciousness.14,16–18 Overall, the incidence of minor hypoglycemia with ExQW, ExBID, sitagliptin, and pioglitazone was low throughout the DURATION studies (Table 3).14,16–18 When administered concomitantly with a sulfonylurea, minor hypoglycemia was experienced with both ExQW and ExBID at the rate of 15% (DURATION-1) and 12% (DURATION-5), compared with <1% (DURATION-1) and 0% (DURATION-5), respectively, for patients not receiving sulfonylurea (incidence rate was similar between ExBID and ExQW).16,17 In DURATION-3, the incidence of minor hypoglycemia was lowest with ExQW plus metformin (3%) and highest with insulin glargine plus metformin and sulfonylurea (42%) (adapted from Diamant et al.18).
Withdrawals due to adverse events were low and were consistent between studies. In DURATION-1 the withdrawal rate was low: 6% of ExQW patients (one-third were gastrointestinal) and 5% of ExBID patients (mostly gastrointestinal) withdrew because of adverse events.16 In DURATION-5, the discontinuation rate because of adverse events was 5% in both the ExQW and ExBID groups (mostly gastrointestinal).17 In DURATION-2, 7% of ExQW, 3% of sitagliptin, and 4% of pioglitazone patients withdrew because of adverse events.14 This compared with 5% of ExQW and 1% of insulin glargine patients withdrawing because of adverse events in DURATION-3.18
Throughout the DURATION program, two cases of pancreatitis were observed in patients treated with ExQW: specifically, single cases were reported in DURATION-3 (edematous pancreatitis) and DURATION-5.17,18 These cases resolved within 3 days, during which time ExQW would be circulating at therapeutic concentrations. In addition, two cases of pancreatitis were reported in patients treated with pioglitazone in DURATION-2 (necrotizing and non-necrotizing pancreatitis).14
The potential for thyroid neoplasms came to light from observations in rodents exposed to liraglutide,23 with unknown implications for the incretin-based therapies. There were no reports of thyroid neoplasms in patients treated with ExQW, ExBID, or insulin glargine in DURATION-1, DURATION-3, or DURATION-5.16–18 A single case of papillary thyroid cancer was reported in a patient treated with sitagliptin in DURATION-2.14
Conclusions
To date, in the DURATION clinical development program (DURATION-1, −2, −3, and −5), ExQW has demonstrated significantly greater improvements in HbA1c than ExBID, titrated insulin glargine, and maximum daily doses of sitagliptin or pioglitazone.14,16–18 Overall, 91% of patients were treated with background metformin therapy, alone or in combination with other oral antidiabetes agents, and 68% of patients were treated with background metformin monotherapy.14,16–18 Compared with ExBID, ExQW exhibited more robust FG improvement but less pronounced PPG improvement; these results are consistent with continuous exposure to exenatide with once-weekly dosing compared with mealtime exposure to exenatide with twice-daily dosing.16,17 ExQW elicited greater improvements in both PPG and FG than did the DPP-4 inhibitor sitagliptin, whereas the greater reduction in HbA1c achieved with ExQW versus pioglitazone was not accompanied with statistically significant differences in PPG or FG improvement.14 Insulin glargine treatment reduced FG to a greater extent than did ExQW; however, the greater PPG control exerted by ExQW likely led to the greater overall reduction in HbA1c compared with insulin glargine.18 Weight loss with ExQW was significantly greater than with sitagliptin, and ExQW treatment resulted in a significant 4–5 kg difference in weight change compared with pioglitazone or insulin glargine after 26 weeks of treatment.14,18 Weight loss with ExQW was similar to ExBID in both DURATION-1 and DURATION-5 trials.16,17
The safety and efficacy data for ExQW were consistent across the DURATION trials (Fig. 1 and Tables 2 and 3). The most common adverse event with ExQW treatment was mild to moderate nausea; injection site reactions were also reported with ExQW treatment in all trials and were more common with ExQW than with ExBID or insulin glargine.14,16–18 The incidence of mild hypoglycemia with ExQW was low in all trials and was greater with concomitant sulfonylurea treatment.14,16–18 There was a single incident of hypoglycemia in a patient treated with ExQW who required assistance but did not require medical intervention and did not result in loss or severe impairment of consciousness.14,16–18 Withdrawals due to adverse events with ExQW were low.14,16–18 Four cases of pancreatitis were reported in the DURATION trials: two with ExQW and two with pioglitazone, resulting in three study withdrawals.14,17,18 A single case of papillary thyroid cancer was reported in a patient taking sitagliptin, an issue of importance due to reports of thyroid neoplasms in rodents during liraglutide development; however, the implications for the wider class of incretin-based therapies are unclear.14,23
The results of the studies summarized herein suggest that ExQW has greater efficacy than does ExBID or sitagliptin. These effects were further demonstrated in the open-label extensions of the DURATION-1 and DURATION-2 trials, in which patients switched from ExBID or sitagliptin treatment to ExQW treatment.24,25 Patients who were originally treated with ExBID experienced improvements in FG after switching to ExQW, whereas patients who were originally treated with sitagliptin experienced improvements in HbA1c, FG, and weight after switching to ExQW.25 Of note is that patients switching from pioglitazone to ExQW experienced sustained glycemic control, a reversal of pioglitazone-associated weight gain, and mixed effects on cholesterol and triglycerides.25 The improved or sustained effects on efficacy with ExQW were accompanied with low levels of nausea and hypoglycemia.24,25
The DURATION clinical development program to date has demonstrated that ExQW significantly improved glycemic control and weight, with a low risk of hypoglycemia. ExQW also has the potential to improve β-cell function and markers of cardiovascular risk and warrants further investigation into these measures.14,16–18 Of note is that ExQW in combination with oral agents is currently under consideration by the European Commission for Europe-wide marketing authorization, following the positive opinion rendered by the Committee for Medicinal Products for Human Use of the European Medicines Agency.26
Acknowledgments
The development of this article was funded by Amylin Pharmaceuticals, Inc.
Author Disclosure Statement
A.S. is a former employee of Amylin Pharmaceuticals, Inc. B.W. is an employee and stock holder of Amylin Pharmaceuticals, Inc. R.C. serves as a principal investigator or co-investigator in clinical trial research sponsored by the following companies: Amylin Pharmaceuticals, Inc., Abbott Diagnostics Division, Bayer Diabetes Care, Daiichi Sankyo, Inc., DexCom, Inc., Edwards Lifesciences, LLC, Intarcia Therapeutics, Inc., Eli Lilly and Company, Johnson & Johnson/LifeScan, MannKind Corporation, Medtronic, Inc., Merck & Co., Inc., Novo Nordisk A/S, Quotient Diagnostics Ltd., ResMed Inc., Roche Laboratories, Inc., Sanofi-Aventis Group, and the Takeda Pharmaceutical Company Ltd. In addition, R.C. is an advisory board member for Abbott Diagnostics Division, Bayer Diabetes Care, CeQur Ltd., Eli Lilly and Company, Novo Nordisk A/S, and Roche Laboratories, Inc. All honoraria, speaking fees, consulting fees, and research and educational support are paid directly to the non-profit International Diabetes Center, of which R.C. is a salaried employee. R.C. receives no personal payments for any of these activities.
References
- 1.Nauck MA. Wollschläger D. Werner J. Holst JJ. Orskov C. Creutzfeldt W. Willms B. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7–36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ. Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. J Physiol Endocrinol Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 3.Nauck MA. Baller B. Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes. 2004;53(3 Suppl):S190–S196. doi: 10.2337/diabetes.53.suppl_3.s190. [DOI] [PubMed] [Google Scholar]
- 4.Vilsbøll T. Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47:357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 5.Toft-Nielsen MB. Damholt MB. Madsbad S. Hilsted LM. Hughes TE. Michelsen BK. Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer TJ. McIntosh CHS. Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 7.Hinnen D. Nielsen LL. Waninger A. Kushner P. Incretin mimetics and DPP-4 inhibitors: new paradigms for the treatment of type 2 diabetes. J Am Board Fam Med. 2006;19:612–620. doi: 10.3122/jabfm.19.6.612. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM. Buse JB. Davidson MB. Ferrannini E. Holman RR. Sherwin R. Zinman B. American Diabetes Association; European Association for Study of Diabetes: Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agersø H. Jensen LB. Elbrønd B. Rolan P. Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ. Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim D. MacConell L. Zhuang D. Kothare PA. Trautmann M. Fineman M. Taylor K. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA. Okerson T. Viswanathan P. Guan X. Holcombe JH. MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–2952. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 13.Pratley RE. Nauck M. Bailey T. Montanya E. Cuddihy R. Filetti S. Thomsen AB. Søndergaard RE. Davies M. 1860-LIRA-DPP-4 Study Group: Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 14.Bergenstal RM. Wysham C. MacConell L. Malloy J. Walsh B. Yan P. Wilhelm K. Malone J. Porter LE. DURATION-2 Study Group: Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 15.Tracy MA. Ward KL. Firouzabadian L. Wang Y. Dong N. Qian R. Zhang Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;20:1057–1062. doi: 10.1016/s0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 16.Drucker DJ. Buse JB. Taylor K. Kendall DM. Trautmann M. Zhuang D. Porter L. DURATION-1 Study Group: Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 17.Blevins T. Pullman J. Malloy J. Yan P. Taylor K. Schulteis C. Trautmann M. Porter L. DURATION-5: Exenatide once weekly resulted in greater improvements in glycemic control compared to exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–1310. doi: 10.1210/jc.2010-2081. [DOI] [PubMed] [Google Scholar]
- 18.Diamant M. Van Gaal L. Stranks S. Northrup J. Cao D. Taylor K. Trautmann M. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–2243. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 19.Amylin Pharmaceuticals, Inc. [press release] investors.amylin.com/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=1484584&highlight. [Nov 8;2010 ]. investors.amylin.com/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=1484584&highlight
- 20.van Wijk JP. de Koning EJ. Martens EP. Rabelink TJ. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2003;23:1744–1749. doi: 10.1161/01.ATV.0000090521.25968.4D. [DOI] [PubMed] [Google Scholar]
- 21.Bunck MC. Cornér A. Eliasson B. Heine RJ. Shaginian RM. Taskinen M-R. Smith U. Yki-Jarvinen H. Diamant M. Three-year exenatide therapy, followed by a 4-week off-drug period, had a sustainable effect on β-cell disposition index in metformin treated patients with type 2 diabetes [abstract] Diabetes. 2010;59(Suppl 1):728-P. [Google Scholar]
- 22.Kendall DM. Riddle MC. Rosenstock J. Zhuang D. Kim DD. Fineman MS. Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 23.Liraglutide prescribing information. www.victozapro.com/pdf/Victoza_ComboPI_5.24.pdf. [Nov 15;2010 ]. www.victozapro.com/pdf/Victoza_ComboPI_5.24.pdf
- 24.Buse JB. Drucker DJ. Taylor KL. Kim T. Walsh B. Hu H. Wilhelm K. Trautmann M. Shen LZ. Porter LE. DURATION-1 Study Group: DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33:1255–1261. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wysham C. Bergenstal RM. Malloy J. Walsh B. Yan P. Malone J. Taylor K. Boies L. DURATION-2: Effect of switching to once-weekly exenatide from maximum daily doses of sitagliptin or pioglitazone. Diabet Med. 2011;28:705–714. doi: 10.1111/j.1464-5491.2011.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amylin Pharmaceuticals, Inc. [press release] phx.corporate-ir.net/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=1550871&highlight= [May 12;2011 ]. phx.corporate-ir.net/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=1550871&highlight=