Abstract
In this work we assess the usefulness of the Randall-Selitto test as a method to detect and quantify neuropathic pain responses in rats subjected to different spinal cord injuries. The mechanical nociceptive thresholds were significantly reduced during follow-up after spinal cord contusion or transection. Our results demonstrate that the Randall-Selitto test allows the detection of neuropathic pain both in forepaws and hindpaws, as well as in dorsal and plantar surfaces. Moreover, it does not require weight support capacity, so it can be used at early time points after the injury. This is the first time that this method has been used to describe the changes in nociceptive thresholds that take place after spinal cord injuries of different severities over time.
Key words: hyperalgesia, neuropathic pain, Randall-Selitto test, spinal cord injury, withdrawal threshold
Introduction
Spinal cord injury (SCI) causes loss of motor, sensory, and autonomic functions below the lesion level, in addition to plastic changes in neural circuits below and above the injury site that may trigger positive symptoms such as spasticity and neuropathic pain. Neuropathic pain is a major cause of disability, and interferes with functional recovery and patient's quality of life (Soler et al., 2007; Widerstrom-Noga et al., 2001). Based on the body areas where symptoms appear in relation to the site of the injury, neuropathic pain is classified as above-level, below-level, or at-level pain (Siddall et al., 1997). Animal models, such as the spinal cord contusion that parallels the injury characteristics described in most traumatic human SCI, have been demonstrated to induce mechanical and thermal allodynia in forelimbs (above-level), “girdling” (at-level), and in hindlimbs (below-level) (Hulsebosch et al., 2000).
Different techniques have been used to quantify and assess the development of neuropathic pain after SCI in animal models (Christensen et al., 1996; Christensen and Hulsebosch, 1997). Some of the most commonly used tests, in particular the Von Frey filaments (Detloff et al., 2010; Hogan et al., 2004; Le Bars et al., 2001) and the electronic Von Frey aesthesiometer (Liu et al., 2008), are designed for the detection of cutaneous mechanical hyperalgesia by applying mechanical stimuli to the plantar surfaces of the hindpaws. The Randall-Selitto test (Randall and Selitto, 1957), intended to serve as a tool to assess the effect of analgesic agents on the response thresholds to mechanical pressure stimulation, has been used by a number of investigators to evaluate inflammatory painful responses (Anseloni et al., 2003; Bujalska and Gumulka, 2001; Khasar et al., 1998; Lee et al., 2001). An electronic device based on the Randall-Selitto principle, which allows testing pain in a quantitative manner by pressuring different areas of the animal's body, has been developed. This test can be applied in both plantar and dorsal surfaces of forelimbs and hindlimbs of awake animals independent of their weight support ability, thus allowing the evaluation at early time points after injury. Moreover, this test can be considered a complement to cutaneous mechanical hyperalgesic tests since the Randall-Selitto test also evaluates nociceptive responses to deep mechanical stimuli.
The purpose of this study is to evaluate the potential of customary algesimetry tests, specifically the Randall-Selitto algesimetry test, to detect and quantify above- and below-level neuropathic pain in animals subjected to SCI of different severities. Since an important problem regarding pain assessment techniques is the high variability obtained in intra-individual measurements (Hogan et al., 2004), we analyze the repeatability of the technique with the aim of validating the obtained data. The results show that the Randall-Selitto technique is a reliable and repeatable method to detect and quantify below-level and above-level neuropathic pain in a rat model of SCI. Moreover, it can be used to distinguish different kinds of SCIs, such as spinal cord contusion from complete section.
Methods
Laboratory animals
Adult female Sprague-Dawley rats (250–300 g body weight) were used. The animals were kept in standard laboratory conditions with 12 h light/dark periods at a temperature of 22±2°C and supplied with dry rat food and drinking water ad libitum. A total of 43 animals were used to perform all experiments: 33 for the algesimetry assay (25 injured and eight control intact animals, see below section on surgical procedure) and 10 for the repeatability analysis (see section on Randall-Selitto test). All experimental protocols were approved by the Ethics Committee of our institution, and followed the European Communities Council Directive 86/609/EEC.
Surgical procedure
Operations were performed under deep pentobarbital anesthesia (50 mg/kg i.p., Sigma, St. Louis, MO) and after subcutaneous injection of buprenorfine (0.05 mg/kg, Buprex, Schering-Plough, Kenilworth, NJ) near the incision site. The dorsum of the animal was shaved and disinfected with povidone iodine. A longitudinal midline incision was made through the skin and fascia, and paravertebral muscle insertions were gently removed along T8–T10 vertebral bodies. A T8 selective laminectomy was then practiced to expose the spinal cord, which was subjected to different injuries. In two groups of rats, the spinal cord was contused using the Infinite Horizon Impactor device (Precision Scientific Instruments, Lexington, UK), applying a fixed force of 100 kilodynes (group 100 kdyn, n=8) or 200 kdyn (group 200 kdyn, n=8). The actual force used to produce the injury was recorded, as well as the resultant displacement of the spinal cord. In a third group, the spinal cord was completely transected by means of a sharp scalpel at T8 vertebral level (complete section group, n=9). To ensure that the injury transected the whole spinal cord, both stumps were gently lifted away and repositioned back into the vertebral channel. In all surgeries, the wound was sutured with 5/0 silk thread in the muscular plane and small surgical clips in the skin plane to disinfect. Animals were kept in a warm environment until full recovery from anesthesia. Bladders were expressed twice a day until reflex voiding of the bladder was re-established.
Functional assessment
Locomotor hind paw function after injuries was assessed in an open field using the Basso, Beattie, and Bresnahan (BBB) rating scale (Basso et al., 1995). Briefly, the BBB testing scale scores locomotor ability from 0 points (no discernable hind paw movement) to 21 points (coordinated gait with parallel hind paw placement and consistent trunk stability). Scores from 0 to 8 indicate isolated movements in the three joints (hip, knee, and ankle). Scores 9 to 13 indicate the return of paw placement and coordinated movements with the forelimbs, whereas scores 14 to 21 indicate the return of consistent coordination, toe clearance during stepping, predominant parallel paw position, trunk stability, and elevation of the tail. Locomotor recovery was evaluated one animal at a time in an open enclosure of 90 cm diameter×24 cm wall height, allowing individuals to move freely for 5 min. Hind paw movements were observed by two examiners and the average score was used as the recorded value. The locomotor test was performed weekly up to 42 days post-operation.
Randall-Selitto test
Description
The nociceptive withdrawal threshold was assessed by using the Randall-Selitto electronic algesimeter (IITC 2500 Digital Paw Pressure Meter, IITC Life Science, Woodland Hills, CA). Before the test, each animal received 5 min of handling to get used to manipulation; then it was placed into a soft cotton cloth and carefully immobilized with the same hand used to hold the tested paw. The test consisted of the application of an increasing mechanical force, in which the tip of the device was applied onto the medial portion of the plantar or the dorsal surfaces of both fore and hind paws until a withdrawal response resulted (Fig. 1). The point of application was marked with ink in order to maintain the location over repeated trials. The maximum force applied was limited to 250 g to avoid skin damage. Measurements in the skin of the dorsal and lateral parts of the trunk were also performed to assess at-level neuropathic pain after SCI, with a maximum force of 350 g (400 g is the maximum reliable measurement suggested by the manufacturer). Because no response from the animals was observed in the trunk sites, the device used did not seem sensitive enough to detect pain response at this level; therefore, no further reference will be made regarding at-level pain testing.
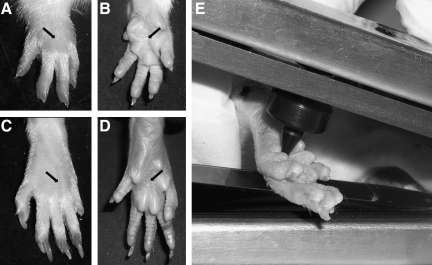
FIG. 1.
Dorsal and plantar surfaces of fore (A, B) and hind paws (C, D), with black arrows indicating the point of application of the mechanical stimuli in the Randall-Selitto test. (E) Application of the tip of the Randall-Selitto probe during a test.
Repeatability of technique
Repeatability quantifies the proportion of the total variance in multiple measurements of a trait that is due to differences among individuals. It is traditionally estimated using the intra-class correlation coefficient (Sokal and Rohlf, 1995), which is derived from one-way analysis of variance (ANOVA). It is understood that if repeated measurements of a given individual are very similar relative to the differences among individuals, then repeatability is high, and there may be little practical reason to obtain multiple measurements. If measurements within individuals are highly variable, then repeatability is low and a gain in precision is achieved by taking multiple measurements of individuals.
We estimated both intra- and inter-day repeatability from a sample of 10 control rats as follows. The Randall-Selitto test was performed once on each individual and, after an entire round on all individuals, it was repeated again on the same day for up to five times (the harmonic mean was 3.4). The observer was blind with respect to the results from previous measurements. The whole experimental protocol was repeated after one week. Intra-day repeatability was estimated as the intra-class correlation. Inter-day repeatability can also be estimated using the intra-class correlation coefficient from the averages of each rat on each day. However, this coefficient is very sensitive to changes in the average values of traits through time (Bulmer, 1985); for this reason, we first tested for average differences, which were very similar in magnitude for the different body parts (fore paw plantar surface, fore paw dorsal surface, hind paw plantar surface, and hind paw dorsal surface) in the one-week period.
Experimental assays
The potential use of the Randall-Selitto test to detect and quantify neuropathic pain in rats subjected to SCI was evaluated using the three groups of animals described in the surgical procedure section (100 kdyn contusion, 200 kdyn contusion, and complete section) plus a control group (n=8; these animals were an independent sample to that used in the repeatability test). The Randall-Selitto test was performed on days 14, 28, and 42 after injury, and the order of stimulated sites was randomly assigned on each day. Because the estimated repeatability of the test was relatively low (see section on Results), the average of the measurements was used as the estimated value for neuropathic pain detection in a repeated-measures design ANOVA (see below section on neuropathic pain detection).
Statistical analysis
Side differences
Possible differences between right and left paws were assessed using conventional two-way ANOVA with individuals as a random factor and side as a fixed effect. Control and injured groups were analyzed separately at each time interval, and non-significant side differences were detected after correcting for multiple testing by using a sequential Bonferroni test. We therefore pooled right and left paw measurements to simplify statistical analyses.
Neuropathic pain detection
Since preliminary tests detected a positive correlation between the averages and standard deviations of the different groups, analyses were done after log-transforming the data (taking natural logarithms of each one of the repeated measurements for each individual on each day). Data analysis was performed with a multifactorial repeated-measurement ANOVA, considering the injury group and the body part as independent variables. To control for type I errors, post-hoc Bonferroni paired comparisons were performed when statistical significance (p<0.05) was detected. The statistical software packages Statistica, v. 9 (StatSoft Inc., Tulsa, OH) and SPSS v. 15 (SPSS Inc., Chicago, IL) were used for statistical analyses.
Results
Spinal cord injury parameters and functional recovery
We estimated the accuracy of the actual force applied and the displacement suffered by the spinal cord in the two contusion injuries of 100 and 200 kdyn, since they are the main variables in the contusion technique (Cao et al., 2005; Scheff et al., 2003; Zhang et al., 2008). Animals from the 100 kdyn group received a mean impact force of 104.9±2.08 kdyn, which is not statistically different from 100 (p>0.05), whereas animals from the 200 kdyn group received a mean of 209.14±3.61 kdyn (p>0.05). The displacements suffered by the spinal cord averaged 807.63±47.57 μm and 1468.71±89.35 μm for the 100 kdyn and 200 kdyn groups, respectively. Therefore, the different forces applied lead to lesions of different severity.
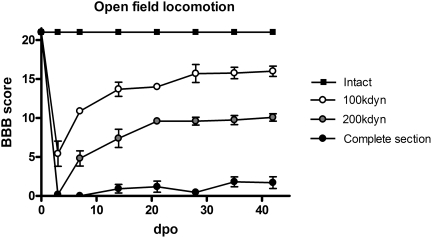
The severity of the SCI is directly related to the functional recovery (Zhang et al., 2008). Functional recovery, assessed by the evaluation of voluntary locomotion (BBB test), is plotted in Figure 2. In animals from the 100 kdyn group the BBB score increased from 10.89±0.31 one week after surgery to 16.0±0.65 at the end of the study, when the rats displayed plantar stepping and consistent coordination, frequent toe clearance and rotated position of the paws at lift off. In the 200 kdyn group the BBB score averaged 4.82±0.98 after one week and 10.07±0.47 at six weeks; animals displayed occasional weight support and plantar stepping but no coordination between fore and hindlimbs. The average BBB scores were clearly different between these two groups (Fig. 2), but their rate of recovery was similar (that is, an average increase of 5.11 points from day 7 to day 42 in the 100 kdyn group, and 5.25 points in the 200 kdyn group). Animals with complete section had a mean score of ∼1 point throughout the follow-up, indicating paralysis of the hind paws, with only some slight movements of one or two joints, most likely of reflex origin.
FIG. 2.
Open field locomotion assessed by the BBB scale in the four groups of rats tested. All groups are significantly different (p<0.05) from day 7 to the end of the follow-up.
Repeatability
Individual differences were significantly repeatable (intra-class correlation coefficients) based on the individual ANOVAs. The repeatability tended to be highest for plantar fore and hind paw testing (Table 1). As an average, individuals accounted for 41.4% and 21.0% of the intra-day variance and 38.2% and 28.9% of the inter-day variance for plantar and dorsal surfaces, respectively.
Table 1.
Correlation Matrix of Repeatability (Intra-Class Correlation) for Nociceptive Withdrawal Threshold Using the Randall-Selitto Test
| |
Plantar fore paw |
Dorsal fore paw |
Plantar hind paw |
Dorsal hind paw |
||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | Day 1 | Day 2 | Day 1 | Day 2 | |
| Day 1 | 0.471 (0.002) | 0.509 (0.048) | 0.307 (0.035) | 0.578 (0.026) | 0.417 (0.005) | 0.254 (0.215) | 0.047 (0.346) | 0 (0.910)* |
| Day 2 | 0.405 (0.009) | 0.255 (0.058) | 0.363 (0.009) | 0.230 (0.078) | ||||
For each body part, the diagonal values are the intra-day repeatability and the upper diagonal values are the inter-day repeatability (numbers in parentheses represent significance level from the one-way ANOVA). Repeatability underlined is significant (p<0.05) after correcting for multiple testing using Bonferroni test.
The between-rats estimated variance from the ANOVA method was negative.
Neuropathic pain responses
When analyzing results from intact animals we found that thresholds found with the Randall-Selitto test were quite similar between fore- and hindlimbs. Paw dorsal surfaces were more sensitive to mechanical stimuli than plantar surfaces, so the mean threshold values were about 60% lower in the former (Table 2).
Table 2.
Supported Pressure (in Grams) in Fore- and Hindlimbs after SCI in Rats Submitted to Contusion or Complete Section at 14, 28, and 42 Days Post-Surgery
| |
|
|
100 kdyn |
200 kdyn |
Complete section |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Control | 14 | 28 | 42 | 14 | 28 | 42 | 14 | 28 | 42 | |
| Forelimb | Plantar | 170.3±12.5 | 117.3±9.65 | 116.8±12.7 | 128.2±12.0 | 105.9±16.1 | 124.8±13.0 | 117.12±16.2 | 116.3±14.3 | 162.2±14.5 | 176.7±7.5 |
| Dorsal | 113.8±7.1 | 76.7±3.5 | 83.1±5.8 | 71.7±4.8 | 73.4±4.7 | 87.4±7.2 | 77.1±3.7 | 69.9±6.9 | 98.9±4.2 | 89.8±10.9 | |
| Hindlimb | Plantar | 172.9±17.7 | 89.9±5.3 | 90.8±5.7 | 88.2±3.9 | 69.3±7.1 | 79.0±6.2 | 63.6±6.4 | 57.5±10.2 | 65.6±3.0 | 53.3±3.1 |
| Dorsal | 97.6±8.6 | 73.9±5.6 | 81.2±5.3 | 73.3±2.4 | 57.6±5.5 | 57.3±5.5 | 51.2±5.5 | 50.0±6.6 | 53.5±2.5 | 46.3±4.0 | |
Values are shown as mean±SEM. Values for control animals are shown as the mean of the three testing times. n=8–9 per group.
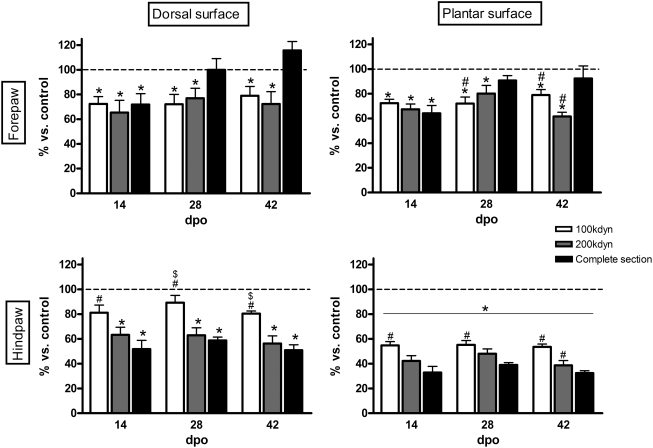
Table 2 shows the absolute values of the pain withdrawal threshold resulting from the application of the Randall-Selitto probe for each group of SCI rats on day of follow-up. Evidences of neuropathic pain were detected in all injury groups on nearly all the follow-up days compared to the control group. For the hindlimb, the decrease in the withdrawal threshold was comparatively greater when testing the plantar than the dorsal skin. The two groups with contusion injury (100 and 200 kdyn) showed roughly constant reduction of nociceptive threshold throughout the study, which was more marked, although not significantly, in the 200 kdyn group (Fig. 3). The complete section group displayed the highest pain response 2 weeks after surgery; its mechanical nociceptive threshold was statistically lower compared to the 100 kdyn group, but it tended to increase 4 weeks after surgery. Interestingly, Randall-Selitto tests performed on the forelimb also revealed a significant reduction in nociceptive thresholds, indicative of mechanical hyperalgesia following SCI. In this case, the reduction was similar and stable for 100 and 200 kdyn contusion groups during the study, whereas for the complete section group the thresholds were reduced 1 week after lesion but tended to reverse to normal values 4 weeks thereafter (Fig. 3).
FIG. 3.
Randall-Selitto measurements to assess neuropathic pain responses. Results are expressed as percentage of control values. Note that animals with spinal cord contusions show a slightly different pattern of hyperalgesic response than animals with complete transection. Dashed line represents the control values in each graph. Statistical significance: *p<0.05 vs. control group; #, p<0.05 vs. complete section; $, p<0.05 vs. 200 kdyn.
Regarding the manifestation of neuropathic pain in different body areas with respect to the SCI site, values for the plantar surfaces showed that 100 and 200 kdyn groups presented significantly reduced mechanical nociceptive thresholds at both above- and below-level injury, while the complete section group presented more marked below- but not above-level pain.
Discussion
One of the main problems when studying neuropathic pain in animal models is the low availability of versatile techniques; hence most of them focus on the detection of pain in the hind paws. Hyperalgesic manifestations have already been described after traumatic lesions of the spinal cord (Christensen and Hulsebosch, 1997; Christensen et al., 1996). The Randall-Selitto test appears to be a useful method for studying neuropathic pain conditions affecting both the fore- and hindlimbs, and thus responses corresponding to above and below the segmental level of the lesion. The test does not require the weight support ability of the animal, can be performed at early time points after the injury, and introduces the possibility of studying the dorsal surface area of the paws. In this study, we demonstrate the usefulness of the Randall-Selitto test to detect and quantify the appearance of neuropathic pain after SCI of different severities.
When focusing on the hindlimb responses, measurements obtained from rats after contusion injuries (100 and 200 kdyn) presented similarly reduced nociceptive thresholds compared to those of intact animals. No significant differences were detected between lesion groups, indicating that the severity of the SCI does not necessarily correspond to the degree of neuropathic pain signs (Redondo Castro et al., 2011; Zhang et al., 2008). Nonetheless, in the hindlimb measurements there is a tendency toward greater reduction in pain withdrawal threshold as the severity of the lesion increases, although without statistical significance. Other studies reported that a more severe lesion induced more pain (Knerlich-Lukoschus et al., 2008).
Regarding the side of the paw, the application of the pointed pressure on the dorsal surface caused less reduction in withdrawal threshold than when testing the plantar surface, as previously reported (Kauppila et al., 1998). The differences between plantar and dorsal surfaces may be due to intrinsic differences, since plantar thresholds were higher than dorsal thresholds in intact animals, probably because plantar stepping confers more resistance to the plantar skin. The hairy skin of the dorsum of the paw is thinner and presents a higher density of mechanoreceptors than the glabrous plantar skin (except in the plantar pads) (Verdú and Navarro, 1997). It is well-known that in conditions causing central sensitization (as in the case of SCI), not only nociceptors but also low threshold mechanoreceptors need a lower intensity of stimulation to elicit sensory responses (Cervero and Laird, 1996).
In examining the forelimb measurements in the contused animals, there were also reduced mechanical nociceptive thresholds compared to intact animals. These differences were maintained during the entire follow-up, thus indicating the appearance of above-level neuropathic pain after SCI (Carlton et al., 2009; Hulsebosch et al., 2009). On the other hand, animals with complete spinal cord section showed hyper-reactive responses at 2 weeks after injury but not at later times, indicating normal nociception in the forelimbs. The difference in responses points to differences in plastic changes taking place at cord segments cranial to the lesion between incomplete and complete SCI. The maintenance over time of forelimb mechanical hyperalgesia in the contusion rats in contrast to the normalization in the complete section group may suggest that hyperexcitability in nociceptive spinal system cranial to the lesion may be sustained chronically by the persistent ascending spinothalamic pathways, which are abnormally active after SCI and have been suggested to function as a pain generator (Wasner et al., 2008). These findings may be of practical use for differentiating complete from incomplete spinal cord lesions based on the performance in the Randall-Selitto test when applied to the forelimbs.
In the case of the animals with complete section, all painful manifestations in hindlimbs should be attributed to reflex responses, since the absence of motor and sensory evoked potentials proved that the section was complete and that no regeneration of ascending or descending pathways took place (data not shown) (Valero-Cabré et al., 2004). Thus, differences detected between contusion and section groups should be attributed to the residual ascending and descending tracts, and also the supraspinal modulation of nociceptive information (apart from the contribution of the reflex pathways), present in all cases. The distinction between abnormal pain and hyper-reflexia is rarely available in studies on the detection of neuropathic pain after SCI (Detloff et al., 2010).
When analyzing the data from the same day, the nociceptive withdrawal threshold using the Randall-Selitto test was significantly repeatable for plantar but not for dorsal surfaces (see Table 1). However, when repeatability was calculated between non-adjacent data, the associated probabilities were only sometimes significant. This marginal significance might reflect a low statistical power because only 10 individuals were used in the repeatability analysis. A recent meta-analysis using 759 estimates of repeatability suggests that 35% of the variation among individuals in behavior could be attributed to individual differences (Bell et al., 2009). The values of 41% for the intra-day and 38% for the inter-day variance for nociceptive withdrawal threshold of plantar surfaces are within the expected repeatability, which explains why pain assessment techniques are highly variable. Therefore, increasing the number of observations per individual will decrease the error around the withdrawal thresholds in this type of experiment. Moreover, the finding that higher repeatability was generally found for the plantar surfaces leads us to propose that Randall-Selitto test using only plantar surfaces is enough to provide reliable values to detect above- and below-level pain.
In conclusion, the results of this study highlight the use of the Randall-Selitto test as an adequate and sensitive method to evaluate neuropathic pain in both fore and hind paws, even at early stages, after SCI in experimental models, because it does not require animals to support their weight. Moreover, it permits the distinction of complete and incomplete spinal cord lesions. This supposes a new range of possibilities for the study of pain itself, as well as for the study of new therapies and treatments to modulate pain appearance.
Acknowledgments
This work was supported by grants from the Fundació MaratóTV3 (grant 070210), the Ministerio de Ciencia y Innovación (grant SAF2009-12495), and funds from Red de Terapia Celular (TERCEL, Instituto de Salud Carlos III) of Spain, and from FEDER. We thank Mauro Santos for his help with the statistical analysis, and Marta Morell, Abel Torres, and Samantha Dawley for their technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Anseloni V.C. Ennis M. Lidow M.S. Optimization of the mechanical nociceptive threshold testing with the Randall-Selitto assay. J. Neurosci. Methods. 2003;131:93–97. doi: 10.1016/s0165-0270(03)00241-3. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bell A.M. Hankison S.J. Laskowski K.L. The repeatability of behaviour: a meta-analysis. Anim. Behav. 2009;77:771–783. doi: 10.1016/j.anbehav.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalska M. Gumulka W.S. Effect of cyclooxygenase and NO synthase inhibitors on antinociceptive action of acetaminophen. Pol. J. Pharmacol. 2001;53:341–350. [PubMed] [Google Scholar]
- Bulmer M.G. The Mathematical Theory of Quantitative Genetics. Clarendon Press; Oxford: 1985. [Google Scholar]
- Cao Q. Zhang Y.P. Iannotti C. DeVries W.H. Xu X.M. Shields C.B. Whittemore S.R. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp. Neurol. 2005;191(Suppl 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Carlton S.M. Du J. Tan H.Y. Nesic O. Hargett G.L. Bopp A.C. Yamani A. Lin Q. Willis W.D. Hulsebosch C.E. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F. Laird J.M. Mechanisms of touch-evoked pain (allodynia): a new model. Pain. 1996;68:13–23. doi: 10.1016/S0304-3959(96)03165-X. [DOI] [PubMed] [Google Scholar]
- Christensen M.D. Hulsebosch C.E. Chronic central pain after spinal cord injury. J. Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Christensen M.D. Everhart A.W. Pickelman J.T. Hulsebosch C.E. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Detloff M.R. Clark L.M. Hutchinson K.J. Kloos A.D. Fisher L.C. Basso D.M. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp. Neurol. 2010;225:366–376. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan Q. Sapunar D. Modric-Jednacak K. McCallum J.B. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hulsebosch C.E. Hains B.C. Crown E.D. Carlton S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C.E. Xu G.Y. Perez-Polo J.R. Westlund K.N. Taylor C.P. McAdoo D.J. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Kauppila T. Kontinen V.K. Pertovaara A. Weight bearing of the limb as a confounding factor in assessment of mechanical allodynia in rat. Pain. 1998;74:55–59. doi: 10.1016/S0304-3959(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Khasar S.G. Miao F.J. Janig W. Levine J.D. Vagotomy-induced enhancement of mechanical hyperalgesia in the rat is sympathoadrenal-mediated. J. Neurosci. 1998;18:3043–3049. doi: 10.1523/JNEUROSCI.18-08-03043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F. Juraschek M. Blomer U. Lucius R. Mehdorn H.M. Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J. Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- Le Bars D. Gozariu M. Cadden S.W. Animal models of nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Lee E.B. Li D.W. Hyun J.E. Kim I.H. Whang W.K. Anti-inflammatory activity of methanol extract of Kalopanax pictus bark and its fractions. J. Ethnopharmacol. 2001;77:197–201. doi: 10.1016/s0378-8741(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Liu Y.M. Zhu S.M. Wang K.R. Feng Z.Y. Chen Q.L. Effect of tramadol on immune responses and nociceptive thresholds in a rat model of incisional pain. J. Zhejiang Univ. Sci. 2008;9:895–902. doi: 10.1631/jzus.B0820039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L.O. Selitto J.J. A method for measurement of analgesic activity on inflammed tissue. Arch. Int. Pharmacodyn. Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Redondo Castro E. Udina E. Verdú E. Navarro X. Longitudinal study of wind-up responses after graded spinal cord injuries in the adult rat. Restor. Neurol. Neurosci. 2011 doi: 10.3233/RNN-2011–0585. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. Taylor D.A. Cousins M.J. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- Sokal R.R. Rohlf F.J. Biometry. 3rd. Freeman; New York: 1995. [Google Scholar]
- Soler M.D. Sauri-Ruiz J. Curcoll-Gallemi M.L. Benito-Penalva J. Opisso-Salleras E. Chamarro-Lusar A. Vidal-Samso J. Characteristics of chronic neuropathic pain and their relationship with psychological well-being in spinal cord injury patients. Rev. Neurol. 2007;44:3–9. [PubMed] [Google Scholar]
- Valero-Cabré A. Forés J. Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J. Neurophysiol. 2004;91:2838–2848. doi: 10.1152/jn.01177.2003. [DOI] [PubMed] [Google Scholar]
- Verdú E. Navarro X. Comparison of immunohistochemical and functional reinnervation of skin and muscle after peripheral nerve injury. Exp. Neurol. 1997;146:187–198. doi: 10.1006/exnr.1997.6517. [DOI] [PubMed] [Google Scholar]
- Wasner G. Lee B.B. Engel S. McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–2400. doi: 10.1093/brain/awn169. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga E.G. Felipe-Cuervo E. Yezierski R.P. Chronic pain after spinal injury: interference with sleep and daily activities. Arch. Phys. Med. Rehabil. 2001;82:1571–1577. doi: 10.1053/apmr.2001.26068. [DOI] [PubMed] [Google Scholar]
- Zhang Y.P. Burke D.A. Shields L.B. Chekmenev S.Y. Dincman T. Zhang Y. Zheng Y. Smith R.R. Benton R.L. DeVries W.H. Hu X. Magnuson D.S. Whittemore S.R. Shields C.B. Spinal cord contusion based on precise vertebral stabilization and tissue displacement measured by combined assessment to discriminate small functional differences. J. Neurotrauma. 2008;25:1227–1240. doi: 10.1089/neu.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]