Abstract
Clonus can interfere with self-care and rehabilitation of people with spinal cord injury. Our aim was to characterize clonus and to evaluate factors that influence clonus duration in muscles paralyzed chronically by spinal cord injury. Electromyographic activity was recorded from soleus and 7 other limb muscles (5 ipsilateral, 2 contralateral) during clonus. In 14 subjects, clonus frequency in soleus averaged 5.4±0.9 Hz and was slower when the reflex path was longer. Contraction frequency slowed at the beginning and end of clonus (sometimes by 2 Hz). The magnitude of one cycle changed the timing and magnitude of the next cycle. These data suggest that afferent input influences the frequency and maintenance of clonus. Recording from many muscles revealed that clonus was prolonged (>40 sec) when only ipsilateral triceps surae or triceps surae and tibialis anterior were involved. Therefore, localized inputs to spinal circuits were important to sustain clonus. Clonus was intermediate (median: 21 sec) with activation of three or four ipsilateral muscles and these contractions were associated with greater activation of ipsilateral flexors. Clonus was short (<5 sec) when ipsilateral and contralateral muscles were activated (five or six muscles). Activation of extraneous afferent input, particularly contralateral muscles, may provide a way to shorten clonus after spinal cord injury.
Key words: muscle spasms, spasticity, stretch reflex
Introduction
Within a few weeks of spinal cord injury, paralyzed muscles begin to contract involuntarily. Sometimes the muscles contract rhythmically at 5–8 Hz in response to stretch or cutaneous inputs. This clonus is common in triceps surae muscles and can disrupt function (Adams and Hicks, 2005; Little et al., 1989; Sköld et al., 1999; Westgren and Levi, 1998). Because clonus can last for >1 min (Wallace et al., 2005), it would help if people with spinal cord injury had strategies to manage these involuntary contractions as they occur. Crucial to developing clonus management techniques is an understanding of the neuromuscular mechanisms that generate and sustain these repeated muscle contractions.
Some studies suggest that repeated stretch reflexes are the primary mechanism regulating clonus because peripheral inputs are important to generate the contractions. For example, electrical stimulation of the appropriate peripheral nerve can induce clonus, whereas nerve compression can block conduction along Ia afferents and stop the contractions (Rossi et al., 1990). Changes in joint angle, the mechanical load acting at a joint, and the time course of reflex muscle contractions, also alter clonus frequency (Hidler and Rymer, 2000; Kozhina et al., 1998; Lin et al., 1999; Rack et al., 1984; Rossi et al., 1990). However, other investigators have argued that central factors are critical to clonus because contraction frequency is not modulated by changes in work load (Dimitrijevic et al., 1980; Walsh, 1976) and similar electromyographic (EMG) activity is seen when clonus occurs during a wide range of kinematical conditions that involve different proprioceptive inputs (Beres-Jones et al., 2003). Data from our laboratory suggest that interactions between central and peripheral processes are important in clonus. The afferent input generated by each muscle stretch was sufficient to bring motoneurons to threshold and produce one action potential in any given motor unit in each clonus cycle (Wallace et al., 2005). A train of unitary potentials was uncommon, possibly because persistent inward currents in motoneurons generated by the stretch were deactivated by the afterhyperpolarizations (Bennett et al., 2001a, b; Li et al., 2004a; Wallace et al., 2005). If this is the case, repeated excitation would be necessary to sustain clonus.
The first aim of this study was to characterize clonus in people with chronic (>1 year) cervical spinal cord injury (complete or motor-complete). Surface EMG was recorded from six muscles in the leg in which clonus was induced, and two muscles in the contralateral leg to describe muscles activation patterns during clonus. The second aim was to test the hypothesis that clonus is prolonged when primarily triceps surae muscles are activated. If segmental inputs to triceps surae motoneurons are important to generate these contractions, clonus may be sustained when the repeated input is focused on the parent motor pools that receive Ia projections from each other (Mendell and Henneman, 1971).
Methods
Subjects
Data were obtained from 13 men and 1 woman (mean±SD age: 34±8 years) with chronic (11±6 years) spinal cord injury at C3 (n=1), C4 (n=2), C5 (n=5), C6 (n=5) or C7 (n=1), determined using the standards of the American Spinal Injury Association Impairment scale (AIS). Injuries were complete in 4 subjects (AIS A) and incomplete in 10 subjects (AIS B) and resulted from diving mishaps (n=8), motor vehicle accidents (n=3), gunshots (n=2) or a fall (n=1). All subjects were able to manually trigger clonus in their leg muscles but they had no voluntary control of leg muscles, evidenced by an inability to generate any voluntary EMG or palpable muscle shortening during repeated attempts to contract these muscles. Therefore, the clonus that each subject elicited involved involuntary muscle contractions. Subjects were taking no medication to dampen their muscle spasms. All subjects gave informed, written consent before participating in the experiment. All procedures were approved by the University of Miami Institutional Review Board.
Experimental arrangement
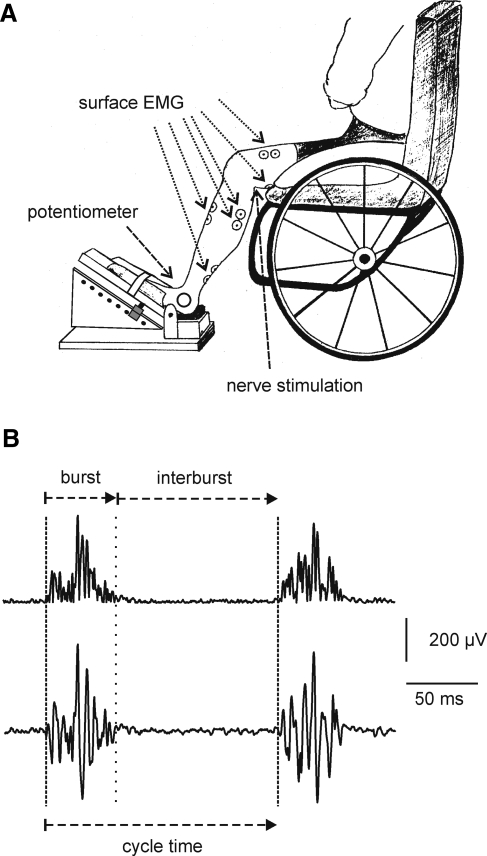
Each subject sat in his or her wheelchair with the foot of the leg in which clonus could be consistently triggered secured on a sloped platform such that the hip, knee, and ankle joints were at ∼90, 120, and 90 degrees respectively (Fig. 1A). This leg was termed ipsilateral with respect to EMG recordings. Surface EMG was recorded with pairs of self-adhesive gel electrodes (Medtronic Andover Medical Inc., Haverhill, MA) attached to the skin over six muscles of the ipsilateral leg (vastus lateralis, hamstrings, tibialis anterior, medial gastrocnemius, lateral gastrocnemius, soleus). Electrodes were also placed over the soleus and tibialis anterior muscles of the contralateral leg (12 subjects). The skin beneath each electrode site was shaved and cleaned with 70% alcohol. One electrode was positioned on the middle of each muscle belly while the indifferent electrode was positioned 5 cm distal. Ankle joint angle was monitored with a potentiometer strapped to the lateral malleolus (10 subjects). Surface EMG and ankle joint position were amplified, filtered (30–1000 Hz, DC-100 Hz, respectively), and sampled online (3200 Hz, 400 Hz, respectively) using a SC/Zoom system (Physiology Section, Umeå University, Sweden).
FIG. 1.
Experimental arrangement. (A) Surface EMG electrodes were attached to six muscles of the ipsilateral leg (indicated by arrows) and two contralateral leg muscles. A potentiometer strapped to the lateral malleolus of the ipsilateral leg registered ankle joint angle. (B) Rectified and unprocessed (lower) soleus surface EMG to show two consecutive bursts of EMG during clonus. The period of relative EMG silence between them was defined as the interburst. Dashed lines and a dotted line mark the beginning of the EMG burst and interburst, respectively. A clonus cycle was composed of one EMG burst and the subsequent interburst.
Protocol
Each subject triggered clonus up to six times by manually lifting the test knee, then gently releasing the leg and letting the heel of the foot strike the platform. Subjects were asked to stop the clonus if it continued for >60 sec. Subjects had a minimum of 5 min rest between each spasm involving clonus.
Clonus timing analysis
Surface EMG from soleus in the ipsilateral leg was rectified and the integral measured at the onset and offset of each burst of EMG (Fig. 1B, dashed and dotted lines respectively). The period of relative silence following the burst of EMG in soleus was termed the interburst. Together, one burst of surface EMG and the following interburst comprised one clonus cycle. The durations of the bursts, interbursts, and clonus cycles were calculated from the soleus burst onset and offset times. Mean clonus frequency was calculated for each spasm and then across all spasms for each subject. In six spasms, tonic extensor muscle activity preceded clonus. In these spasms, measurements were taken from the point at which clonus started in the ipsilateral soleus. The distance from the ipsilateral anterior superior iliac spine to the proximal soleus surface electrode was doubled to approximate reflex path length (n=5 subjects, Campbell et al., 1991).
When spasms involved at least 25 cycles of clonus, the data were grouped according to relative clonus duration because spasm duration varied from spasm to spasm. The total duration of each clonus represented 100%. The average (±SD) duration of the bursts, interbursts, and clonus cycles was calculated for every 2% of clonus time for the first and last 10% of a spasm. The same parameters were calculated over 5% intervals for the remainder of the spasm.
Average EMG and EMG ratios
The EMG integral measured for each burst (and interburst; defined by EMG onset and offset in the ipsilateral soleus) was divided by the duration of the integration time to provide the average EMG for that burst (and interburst). The average burst EMG (and interburst EMG) for each muscle was normalized to the respective ipsilateral soleus data. Burst (interburst) ratios > 1 meant that the test muscle was more active than the ipsilateral soleus.
Interrelationships between clonus parameters
When spasms involved at least 25 cycles of clonus, the average soleus burst EMG for the current cycle was compared to the timing (the duration of the next interburst, burst, and cycle) and average EMG of the next cycle. This analysis was used to assess whether the amount of EMG in one clonus cycle was related to the magnitude or timing parameters of the subsequent cycle.
Prevalence and patterns of muscle activity
Prevalence of activity in each muscle was determined at the start, middle, and end of each spasm. A muscle was deemed active at the start of clonus if EMG was present during the third cycle of soleus EMG; in the middle of the clonus if there was EMG for at least 10 % of clonus duration (from 45 to 55% clonus duration for each spasm); at the end of clonus if EMG was present during the third last cycle of soleus EMG. These cycles were chosen because many changes occurred within the first and last five cycles (Fig. 2). As the ipsilateral triceps surae muscles (soleus, medial gastrocnemius, lateral gastrocnemius) were activated synergistically, they were counted as one muscle group. Clonus data from seven spasms that lasted >60 sec were excluded from this analysis because the subject terminated these spasms. The relationship between clonus duration and the number of muscles activated during clonus was evaluated in the middle of the clonus for the 12 subjects with contralateral EMG recordings. Because certain combinations of muscles were coactive across different spasms, these data defined the muscle activation patterns.
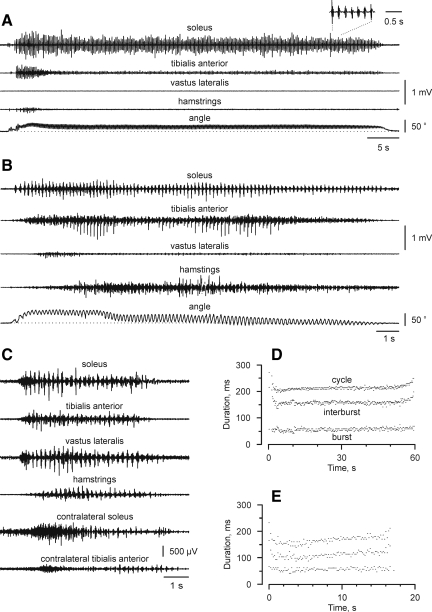
FIG. 2.
Surface EMG from ipsilateral and contralateral muscles. Surface EMG from soleus, tibialis anterior, vastus lateralis, hamstrings, and ankle joint position (top to bottom) recorded during typical clonus of long (A) and intermediate (B) duration. (C) Short-duration clonus included activation of contralateral muscles. Data are from three different subjects. Each spasm stopped naturally. The inset in A magnifies the signals from seven clonus cycles. (D, E) Symbols show the duration of each cycle, interburst, and burst for the spasms shown in A and B, respectively.
Joint angle analysis
The right angle between the dorsum of the foot and the line of the fibula was assigned as the reference position of the ipsilateral ankle joint (90 degrees). Plantar flexion excursions were taken as values >90 degrees and dorsiflexion was assigned values <90 degrees. Ankle joint angle was measured at the onset of every clonus cycle. Peak ankle joint angle in every clonus cycle was also measured. The difference between baseline and peak measurements yielded the excursion of the ankle joint for each cycle. Mean ankle joint excursion for all clonus cycles of each spasm was calculated.
Statistical analysis
Mean (±SD) values are given except where noted. Linear-regression analyses were used to compare clonus duration with reflex path length, and the number of muscles active midway during clonus. Analysis of variance on ranks was used to compare the median duration of clonus involving different numbers of muscles. Data were grouped according to whether clonus involved activation of one or two ipsilateral muscles (triceps surae, tibialis anterior), three or four ipsilateral muscles (triceps surae, tibialis anterior, vastus lateralis, hamstrings), or five or six muscles (three or four ipsilateral muscles and one or two contralateral muscles). Statistical significance was set at p<0.05.
Results
Clonus duration
A total of 60 spasms were recorded, with subjects triggering clonus an average of 4±1 times (range: 3–6). The duration of clonus varied across different subjects. One subject consistently had clonus of long duration (>60 sec) that he stopped by extraneous input (by changing the position of the leg in which clonus was triggered). Another four subjects generated clonus of intermediate length (15–60 sec), whereas four other subjects had short-duration clonus (<5 sec). The remaining five subjects had clonus of less predictable duration. Typical examples of long, intermediate, and short duration clonus are shown in Figure 2A–C.
Muscles active during clonus change
Within a few cycles of clonus onset, there was often a transition period in which muscles that were highly active decreased in activity (ipsilateral tibialis anterior) or become silent (hamstrings, vastus lateralis, Fig. 2A). Other muscles (triceps surae) continued working in concert. In some subjects, the inverse situation sometimes occurred. Muscles that had been relatively inactive at the start of clonus (hamstrings; Fig. 2B) were activated more in the middle of clonus or toward its end.
Number of muscles coactive with the burst of soleus EMG
Triceps surae and ipsilateral tibialis anterior were almost always active throughout every clonus (Fig. 3A). Hamstrings were the only muscle group whose prevalence increased from the start to the middle of clonus, but it waned at clonus end. Fewer vastus lateralis muscles were active relative to hamstrings and their prevalence decreased with time. Contralateral soleus and tibialis anterior were always activated less often than were ipsilateral muscles. Overall, most muscles stopped contracting near the end of clonus, leaving primarily ipsilateral triceps surae and tibialis anterior active.
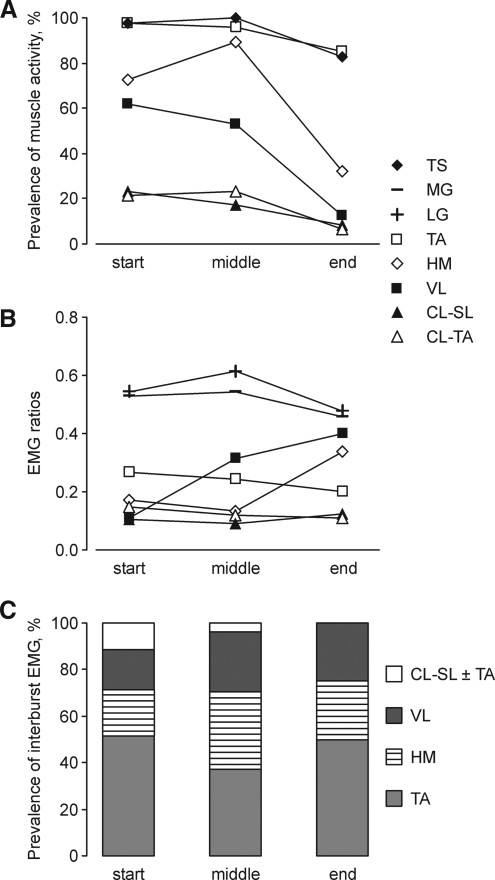
FIG. 3.
Muscles active at the start, middle, and end of clonus. (A) Prevalence of muscles active during the burst of soleus EMG. (B) EMG ratios for individual muscles coactive with the burst of soleus EMG. (C) Prevalence of interburst EMG for different muscles. Muscles include triceps surae (TS), ipsilateral medial gastrocnemius (MG), lateral gastrocnemius (LG), tibialis anterior (TA), hamstrings (HM), vastus lateralis (VL), contralateral soleus (CL-SL), and tibialis anterior (CL-TA).
Extent of muscle activation relative to the burst of soleus EMG
EMG burst ratios, a measure of the extent of muscle activation relative to ipsilateral soleus, also changed during clonus. In 84 % of cases EMG ratios were <1. In 12 % of cases medial gastrocnemius (MG) and lateral gastrocnemius (LG) EMG ratios were >1 indicating that these synergists were more active than soleus. In 4 % of cases, ipsilateral flexors were more active than soleus. EMG ratios for ipsilateral gastrocnemeii (MG, LG) were the highest at the start, middle, and end of clonus (Fig 3B). Tibialis anterior ratios decreased from start to end of clonus whereas vastus lateralis ratios increased. Hamstrings ratios increased at the end of clonus. Contralateral soleus and tibialis anterior ratios remained low throughout clonus.
Muscles active during the soleus interburst
Interburst EMG activity was less common with time. At clonus inception, almost half of the spasms contained interburst EMG activity (52 %). In the middle of clonus, interburst EMG was present in 32 % of spasms, but declined to 18% of spasms at the end of clonus. Ipsilateral tibialis anterior activity was most common in the interburst (range: 37–51% at start, middle, or end of clonus), followed by hamstrings (20–33%), vastus lateralis (17–26%), and then contralateral muscle activity (0–11%; Fig 3C).
Number of active muscles influences clonus duration
Clonus was of longer duration when fewer limb muscles were active midway through the spasm (Fig. 4). For example, the clonus shown in Figure 2A–C lasted for 60 sec, 17 sec, and 5 sec respectively, and involved two, four, and six muscles at the middle of these spasms. Clonus that lasted >60 sec (seven spasms) involved activation of only triceps surae or triceps surae and tibialis anterior muscles.
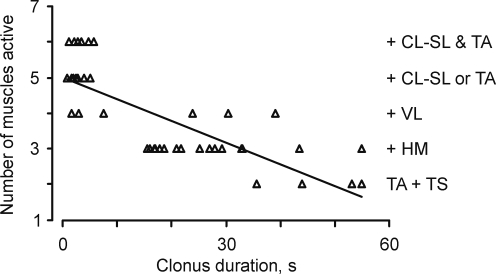
FIG. 4.
Active muscles and clonus duration. The number of muscles active during clonus as a function of clonus duration (r=0.76, p<0.01, n=47 spasms, 12 subjects). A muscle was deemed “active” if it produced EMG for at least 10% of clonus duration around the middle of the spasm (45–55% of individual spasm duration). Active muscles are listed (right). Two active muscles included triceps surae and ipsilateral tibialis anterior (TS+TA). Activation of a third muscle (hamstrings, + HM), a fourth muscle (vastus lateralis, + VL), and five or six muscles (one or two contralateral muscles, + CL-SL±TA) corresponded to three, four, five, or six muscles, respectively.
Overall, clonus that involved only ipsilateral triceps surae or triceps surae and tibialis anterior stopped spontaneously after a median of 48 sec (range: 36–60 sec). Clonus that involved activation of three or four ipsilateral muscles lasted for a median of 21 sec (range: 2–55 sec), whereas most of the spasms with contralateral EMG activity lasted only a few seconds (median: 4 sec, range: 1–22 sec 5 or 6 active muscles). The differences between these median durations were statistically significant.
Muscle activation patterns during clonus
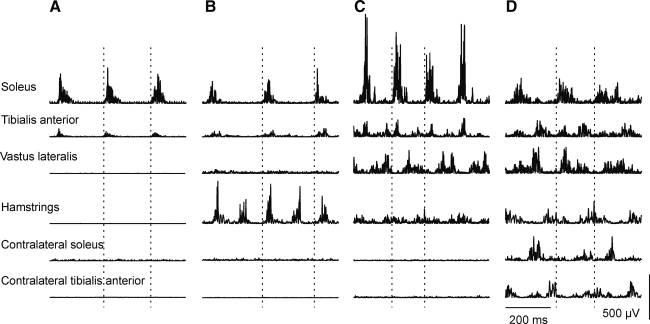
Four predominant muscle activation patterns were seen during clonus (Fig. 5): 1) activation of ipsilateral triceps surae and tibialis anterior (two muscles, Fig. 5A); 2) activation of ipsilateral triceps surae, tibialis anterior, and hamstrings (three muscles, Fig. 5B); 3) activation of ipsilateral triceps surae, tibialis anterior, hamstrings, and vastus lateralis (four muscles; Fig. 5C); and 4) activation of ipsilateral triceps surae, tibialis anterior, hamstrings, vastus lateralis, and contralateral muscle soleus or tibialis anterior (five or six muscles; Fig. 5D).
FIG. 5.
Patterns of muscle activity during clonus. Surface EMG from ipsilateral soleus, tibialis anterior, vastus lateralis, hamstrings, contralateral soleus, and tibialis anterior (top to bottom) recorded at the middle of clonus when two (A), three (B), four (C), or six (D) muscles were active. Data are from four different subjects. Dashed lines mark one clonus cycle.
Prevalence of muscle activation patterns
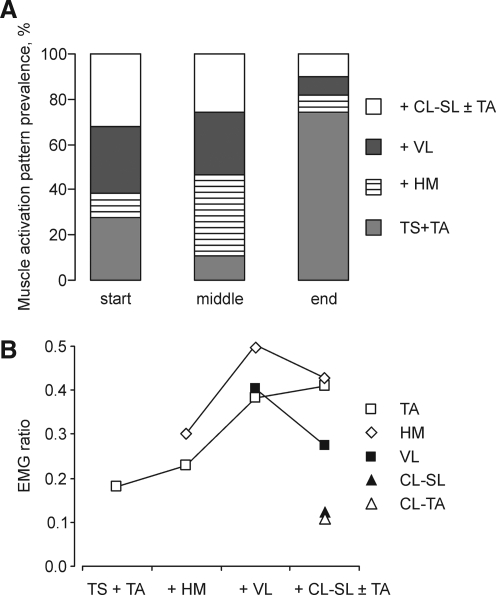
At the beginning of clonus, activation of two, four or five to six muscles was equally prevalent (Fig. 6A). Activation of three muscles was the least common pattern at the start of clonus but became the most common pattern in the middle of clonus. Clonus cessation was dominated by ipsilateral triceps surae and tibialis anterior activity, as other muscles were inactivated.
FIG. 6.
Evolution of muscle activation patterns. (A) Prevalence of muscle activation patterns at the start, middle, and end of clonus. (B) EMG ratios for individual muscles at clonus end plotted against muscle activation patterns. Muscles include ipsilateral tibialis anterior (TA), hamstrings (HM), vastus lateralis (VL), contralateral soleus (CL-SL), and tibialis anterior (CL-TA). Two active muscles included ipsilateral triceps surae and tibialis anterior (TS+TA). Activation of a third muscle (hamstrings, + HM), a fourth muscle (vastus lateralis, + VL), and five or six muscles (one or two contralateral muscles, + CL-SL±TA) corresponded to three, four, five, or six muscles, respectively.
EMG ratios at the end of clonus in relation to muscle activation patterns
At clonus end, EMG ratios increased for ipsilateral flexor muscles, particularly when more muscles were activated (Fig. 6B). For example, with activation of ipsilateral hamstrings (three muscles), the amount of ipsilateral tibialis anterior EMG increased relative to soleus EMG. Excitation of vastus lateralis as well (four muscles) was associated with relative increases in both hamstrings and tibialis anterior EMG.
Muscle activation patterns may include interburst EMG
At the start of clonus, the burst of EMG in ipsilateral tibialis anterior was primarily in phase with the EMG in ipsilateral triceps surae (59 % spasms), in both phases (30 %), or out of phase (11 %). Hamstrings EMG in both phases was prevalent at the middle of clonus (20 % of spasms; Fig. 5B). Figure 5C and D show other examples of interburst EMG recorded in the middle of clonus.
Cycle timing
Figures 2D and E show the durations of each burst, interburst, and cycle measured from the clonus shown in Figures 2A and B, respectively. Regardless of clonus duration, there was more variability in cycle duration in the first and last few seconds of every spasm. For example, at the start of the clonus in Figure 2E, the longest cycle was 234 ms. After only 2 sec, the cycle had reduced to 155 ms, representing an increase in clonus frequency from 4.3 Hz to 6.5 Hz (a 51% increase). Thereafter, a contraction rhythm was established such that cycle duration slowly increased up until near the termination of the clonus. The duration of the burst of EMG was relatively consistent throughout clonus (Fig. 2E). Variability in cycle duration therefore related largely to changes in the duration of the interburst. These phenomena were not necessarily observed when clonus was of short duration, because of its brief nature.
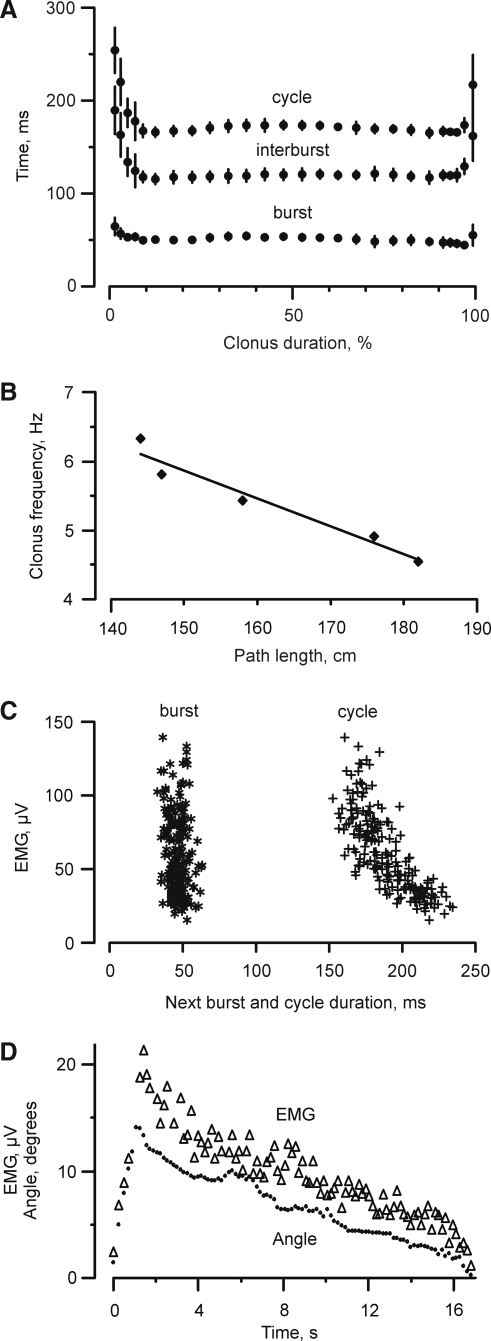
Figure 7A shows the cycle timing during clonus for one subject. The five spasms ranged in length from 14 to 22 sec. Each was normalized by the time of the respective clonus. Irrespective of the absolute length of the clonus, fluctuations in cycle duration were greatest during the first and last 10% of each spasm, largely because of variations in the duration of the interburst. These data emphasize that clonus frequency was not fixed.
FIG. 7.
Clonus timing and interrelationships. (A) Mean (±SD) cycle, interburst and burst durations for five spasms recorded from one subject. Data for each spasm were normalized to individual clonus duration (% of total length). (B) Mean clonus frequency as a function of reflex path length (n=5 subjects, r=0.97, p<0.005). (C) The magnitude of the EMG recorded in the current clonus cycle plotted as a function of the duration of the next burst and cycle. Each symbol represents data from one cycle (n=204). (D) Integrated soleus surface EMG (Δ) and ankle excursion (•) during clonus that lasted for 17 sec. Each symbol represents data from one cycle of clonus.
Mean burst, interburst, and cycle durations for all subjects were 58±10 ms (range 43–76 ms), 128±22 ms (range: 87–155 ms) and 186±26 ms (range: 150–221 ms), respectively. The burst of EMG lasted 31±6% of the cycle, on average (range: 21–52%).
Interrelationships between parameters
Mean clonus frequency for all subjects was 5.4±0.9 Hz in soleus (range: 4.5–7.0 Hz). There was a significant, inverse relationship between soleus clonus frequency and reflex path length (r=0.97, p=0.005, Fig. 7B). Therefore, clonus frequency was slower for subjects with longer legs.
The magnitude of the EMG produced in the clonus cycle influenced the timing and the intensity of the next cycle. Figure 7C shows data from one spasm that contained 204 cycles of clonus. Cycles with smaller bursts of EMG, those typically occurring near the start and end of clonus, were followed by cycles with longer durations because of alterations in the length of the interbursts. These same relationships were found in 21 of the 23 spasms (91%) that lasted for > 25 cycles. In contrast, the durations of the bursts of EMG were relatively consistent throughout the clonus.
The initial ankle angle at the onset of the burst of EMG in soleus averaged 89±7 degrees (n=10 subjects). Peak ankle joint excursion usually occurred after a few seconds of clonus, then decreased slowly in parallel with the decline in soleus EMG (Fig. 7D). The maximal and mean ankle joint excursions during clonus were 16±5 degrees (range 7–26 degrees) and 10±3 degrees (range: 5–15 degrees), respectively.
Discussion
Our data show that clonus was of shorter duration when more muscles were activated (Fig. 4). In contrast, clonus was persistent when EMG activity was largely confined to the synergistic triceps surae muscles (Fig. 2A). Clonus was shortened when knee extensors or flexors in the same leg became involved (Fig. 2B). Activation of contralateral muscles resulted in earlier termination of clonus (Fig. 2C). Stronger contractions of ipsilateral flexors (tibialis anterior, hamstrings) occurred, relative to soleus contractions, when more muscles were activated (Fig. 6B). Overall, these data suggest that ankle clonus in these spinal cord injured individuals is sustained when local spinal circuits are primarily involved.
Triceps surae excitation dominates prolonged ankle clonus
Triceps surae motoneurons receive widespread Ia projections from their parent muscle and synergists (Mendell and Henneman, 1971); therefore, repetitive stretch of the triceps muscles during clonus must provide afferent input to these same motor pools. This repeated peripheral input may prolong clonus. Consistent with this suggestion, soleus EMG almost always exceeded that recorded from all other muscles during clonus. The highest EMG ratios were in medial and lateral gastrocnemius (Fig. 3B). Even though ipsilateral tibialis anterior was often coactivated with triceps surae, the tibialis anterior EMG ratio was low. Ankle extensors are also stronger than flexors (Fukunaga et al., 1996). Together, these results likely explain the predominance of extensor movements during prolonged clonus (Fig. 2A and B).
A relative lack of inhibition after spinal cord injury may prolong clonus. Spinal cord injury reduces inhibition at rest (Ia presynaptic, reciprocal Ia, post-activation depression, and possibly Ib inhibition; Ashby et al., 1974; Calancie et al., 1993; Delwaide and Oliver, 1988; Hultborn et al., 1987; Little et al., 1999; Morita et al. 2001; Okuma et al. 2002; Rudomin, 1990; Schindler-Ivens and Shields, 2000). There is also less inhibition during prolonged triceps surae clonus. Both short-lasting Ia presynaptic inhibition of motoneurons and long-lasting post-activation depression is reduced because periodic depression and recovery of the H-reflex occurs in each clonus cycle (Kozhina et al., 1998). This behavior persists throughout the duration of the spasm. It is of note that the ipsilateral tibialis anterior EMG ratio was low during prolonged clonus (0.18, Fig. 6B). This coactivation of extensors and flexors during clonus suggests there may be a dynamic, in-phase balance between excitation and inhibition during clonus that influences its duration. This suggestion is consistent with in-phase synaptic excitation and inhibition in limb motoneurons during rhythmic scratch-like behavior in the turtle (Berg et al., 2007).
Other studies have reported soleus and tibialis anterior activity during the muscle shortening phase of clonus (Cook, 1967; Hidler and Rymer, 2000), as well as coactivation of other muscles (Beres-Jones et al., 2003; Latash et al., 1989), which indicates that clonus can be more complex than a stretch reflex. The EMG in tibialis anterior may reflect reductions in reciprocal Ia inhibition after spinal cord injury (Okuma et al., 2002), sprouting within the spinal cord that results in co-contraction of agonist–antagonist pairs (Ashby and Wiens, 1989) or both possibilities. Contamination of tibialis anterior EMG by cross talk from the much larger triceps surae muscle group seems unlikely, as many of the signals we recorded in one channel were absent in the other.
By measuring the entire spasm, we also show that EMG in the ipsilateral tibialis anterior can be out of phase with the burst of EMG in triceps surae (52 % of spasms at the start of clonus; 10 subjects), as found in people with thoracic spinal cord injury (Beres-Jones et al. 2003). In the present study, the out-of-phase tibialis anterior EMG stopped after a few seconds in eight subjects, but clonus continued. Therefore, coactivation of other muscles with soleus is a more important determinant of clonus duration than is activation of muscles during the soleus interburst.
Coactivation of many muscles shortens ankle clonus
Activation of more muscles successively reduced clonus duration (Fig. 4). Changes in ipsilateral ankle position were not obvious with excitation of more muscles when only ankle extension and flexion were measured. However, contractions in ipsilateral flexors were stronger relative to those in ipsilateral soleus when more muscles were activated (Fig. 6B). One interpretation of these results is that stronger muscle activity extraneous to the triceps surae somehow inhibits the soleus motoneurons involved in clonus. Inhibition from ipsilateral and interlimb sources presumably involves many interneurons that act directly to inhibit the soleus motoneurons or to increase inhibition of afferents acting on the triceps surae (Butler et al., 2006; Li et al., 2004b; Pierrot-Deseilligny and Burke, 2005; Zehr and Duysens, 2004; Zijdewind and Thomas, 2001). Consistent with this idea, vibration of the triceps surae tendon depressed clonus in multiple muscles (Butler et al., 2006). Furthermore, local commissural interneurons can act on 1a afferents presynaptically, and on motoneurons directly to inhibit extensor motoneurons (Kiehn, 2006), a possible mechanism to explain short clonus when contralateral muscles are activated. Other factors that may result in earlier termination of clonus are changes in the activation thresholds for muscle spindles or golgi tendon organs or declines in excitation. Soleus EMG and ankle angle declined slowly during clonus (Fig. 7D). The magnitude of EMG in each clonus cycle also influenced the magnitude of the next cycle (Fig. 7C).
Afferent inputs influence clonus frequency
Our data also provide new evidence for the importance of afferent inputs and their interaction with central processes to generate and maintain clonus. First, the magnitude of one cycle changed the timing and magnitude of the next cycle (Fig. 7C), supporting those studies that propose a reflex component to clonus (Hidler and Rymer, 2000; Lin et al., 1999; Rack et al., 1984; Rossi et al., 1990; Wallace et al., 2005). Second, cycle frequency varied considerably within clonus. Analysis of the entire spasm showed dramatic increases, and decreases in clonus frequency occurred at the beginning and end of clonus (Figs. 2 and 7). Only the duration of the burst of soleus EMG was relatively consistent throughout clonus (Fig. 7C) suggesting that afferent drive is seen by the motoneurons for a similar time during each clonus cycle. Consistent with these suggestions, microneurographical recordings have shown that muscle spindle afferents only discharge late in the muscle relaxation phase of sustained clonus (Hagbarth et al., 1975; Szumski et al., 1974). The time that afferent input arrives at the spinal cord therefore alters clonus frequency by changing the duration of the interburst (Fig. 7C). Clonus frequency also depends upon reflex path length (Fig. 7B). Longer reflex pathways caused by differences in leg length resulted in slower clonus frequencies (Iansek, 1984).
Contractions of flexors and contralateral muscles shorten clonus
In summary, our data show that repeated contraction of only triceps surae or synchronous triceps surae and tibialis anterior activity prolongs the duration of clonus. Specific activation of local circuitry with little opposition from antagonists is important to maintain clonus. Clonus was shorter when three or four ipsilateral muscles were activated. In these spasms, contractions of flexor muscles became stronger relative to those in soleus. Contralateral muscle activation shortened clonus further. Therefore, instead of taking medication, people with spinal cord injury may be able to activate numerous limb muscles to terminate clonus.
Acknowledgments
The authors thank Dr. Galina Kozhina for help during experiments and Ms. Adriana Martinez for reference work. This work was supported by United States Public Health Service (grant NS-30226); The Miami Project to Cure Paralysis; and the Faculty of Education, The University of Auckland.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams M.M. Hicks A.L. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- Ashby P. Verrier M. Lightfoot E. Segmental reflex pathways in spinal shock and spinal spasticity in man. J. Neurol. Neurosurg. Psychiatr. 1974;37:1352–1360. doi: 10.1136/jnnp.37.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P. Wiens M. Reciprocal inhibition following lesions of the spinal cord in man. J. Physiol. 1989;414:145–157. doi: 10.1113/jphysiol.1989.sp017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D.J. Li Y. Harvey P.J. Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J. Neurophysiol. 2001a;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bennett D.J. Li Y. Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J. Neurophysiol. 2001b;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Beres-Jones J.A. Johnson T.D. Harkema S.J. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp. Brain Res. 2003;149:222–236. doi: 10.1007/s00221-002-1349-5. [DOI] [PubMed] [Google Scholar]
- Berg R.W. Alaburda A. Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Butler J.E. Godfrey S. Thomas C.K. Depression of involuntary activity in muscles paralyzed by spinal cord injury. Muscle Nerve. 2006;33:637–644. doi: 10.1002/mus.20500. [DOI] [PubMed] [Google Scholar]
- Calancie B. Broton J.G. Klose K.J. Traad M. Difini J. Ayyar D.R. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr. Clin. Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- Campbell J.W. Herbison G.J. Chen Y.T. Jaweed M.M. Gussner C.G. Spontaneous electromyographic potentials in chronic spinal cord injured patients: relation to spasticity and length of nerve. Arch. Phys. Med. Rehabil. 1991;72:23–27. [PubMed] [Google Scholar]
- Cook W.A. Antagonistic muscles in the production of clonus in man. Neurology. 1967;17:779–796. doi: 10.1212/wnl.17.8.779. [DOI] [PubMed] [Google Scholar]
- Delwaide P.J. Oliver E. Short-latency autogenic inhibition (IB inhibition) in human spasticity. J. Neurol. Neurosurg. Psychiatr. 1988;51:1546–1550. doi: 10.1136/jnnp.51.12.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic M.R. Nathan P.W. Sherwood A.M. Clonus: the role of central mechanisms. J. Neurol. Neurosurg. Psychiatr. 1980;43:321–332. doi: 10.1136/jnnp.43.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T. Roy R.R. Shellock F.G. Hodgson J.A. Edgerton V.R. Specific tension of human plantar flexors and dorsiflexors. J. Appl. Physiol. 1996;80:158–165. doi: 10.1152/jappl.1996.80.1.158. [DOI] [PubMed] [Google Scholar]
- Hagbarth K.E. Wallin G. Löfstedt L. Aquilonius S.M. Muscle spindle activity in alternating tremor of Parkinsonism and in clonus. J. Neurol. Neurosurg. Psychiatr. 1975;38:636–641. doi: 10.1136/jnnp.38.7.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidler J.M. Rymer W. Z. Limit cycle behavior in spasticity: analysis and evaluation. IEEE Trans. Biomed. Eng. 2000;47:1565–1575. doi: 10.1109/10.887937. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Meunier S. Morin C. Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J. Physiol. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansek R. The effects of reflex path length on clonus frequency in spastic muscles. J. Neurol. Neurosurg. Psychiatry. 1984;47:1122–1124. doi: 10.1136/jnnp.47.10.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kozhina G. Ross B.H. Wallace D. Thomas C.K. Clonus in leg muscles after human spinal cord injury. Neural Control of Movement Meeting; Key West, Florida: 1998. [Google Scholar]
- Latash M.L. Penn R.D. Corcos D.M. Gottlieb G.L. Short-term effects of intrathecal baclofen in spasticity. Exp. Neurol. 1989;103:165–172. doi: 10.1016/0014-4886(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Li Y. Gorassini M.A. Bennett D.J. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J. Neurophysiol. 2004a;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y. Harvey P.J. Li X. Bennett D.J. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J. Neurophysiol. 2004b;91:2236–2246. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Lin J.P. Brown J.K. Walsh E.G. Continuum of reflex excitability in hemiplegia: influence of muscle length and muscular transformation after heel-cord lengthening and immobilization on the pathophysiology of spasticity and clonus. Dev. Med. Child Neurol. 1999;41:534–548. doi: 10.1017/s0012162299001152. [DOI] [PubMed] [Google Scholar]
- Little J.W. Ditunno J.F. Stiens S.A. Harris R.M. Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Arch. Phys. Med. Rehabil. 1999;80:587–599. doi: 10.1016/s0003-9993(99)90204-6. [DOI] [PubMed] [Google Scholar]
- Little J.W. Micklesen P. Umlauf R. Britell C. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am. J. Phys. Med. Rehabil. 1989;68:32–36. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- Mendell L.M. Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J. Neurophysiol. 1971;34:171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Morita H. Crone C. Christenhuis D. Petersen N.T. Nielsen J.B. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain. 2001;124:826–837. doi: 10.1093/brain/124.4.826. [DOI] [PubMed] [Google Scholar]
- Okuma Y. Mizuno Y. Lee R.G. Reciprocal Ia inhibition in patients with asymmetric spinal spasticity. Clin. Neurophysiol. 2002;113:292–297. doi: 10.1016/s1388-2457(02)00004-4. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- Rack P.M. Ross H.F. Thilmann A.F. The ankle stretch reflexes in normal and spastic subjects. The response to sinusoidal movement. Brain. 1984;107:637–654. doi: 10.1093/brain/107.2.637. [DOI] [PubMed] [Google Scholar]
- Rossi A. Mazzocchio R. Scarpini C. Clonus in man: a rhythmic oscillation maintained by a reflex mechanism. Electroencephalogr. Clin. Neurophysiol. 1990;75:56–63. doi: 10.1016/0013-4694(90)90152-a. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S. Shields R.K. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp. Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld C. Levi R. Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch. Phys. Med. Rehabil. 1999;80:1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- Szumski A. J. Burg D. Struppler A. Velho F. Activity of muscle spindles during muscle twitch and clonus in normal and spastic human subjects. Electroencephalogr. Clin. Neurophysiol. 1974;37:589–597. doi: 10.1016/0013-4694(74)90072-8. [DOI] [PubMed] [Google Scholar]
- Wallace D.M. Ross B.H. Thomas C.K. Motor unit behavior during clonus. J. Appl. Physiol. 2005;99:2166–2172. doi: 10.1152/japplphysiol.00649.2005. [DOI] [PubMed] [Google Scholar]
- Walsh E.G. Clonus: beats provoked by the application of a rhythmic force. J. Neurol. Neurosurg.Psychiatr. 1976;39:266–274. doi: 10.1136/jnnp.39.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgren N. Levi R. Quality of life and traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 1998;79:1433–1439. doi: 10.1016/s0003-9993(98)90240-4. [DOI] [PubMed] [Google Scholar]
- Zehr E.P. Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10:347–361. doi: 10.1177/1073858404264680. [DOI] [PubMed] [Google Scholar]
- Zijdewind I. Thomas C.K. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve. 2001;24:952–962. doi: 10.1002/mus.1094. [DOI] [PubMed] [Google Scholar]