Abstract
Individually, motor training, pharmacological interventions, and housing animals in an enriched environment (EE) following spinal cord injury (SCI) result in limited functional improvement but, when combined, may enhance motor function. Here, we tested amphetamine (AMPH)-enhanced skilled motor training following a unilateral C3–C4 contusion injury on the qualitative components of reaching and on skilled forelimb function, as assessed using single-pellet and staircase reaching tasks. Kinematic analysis evaluated the quality of the reach, and unskilled locomotor function was also tested. Animals receiving AMPH and skilled forelimb training performed better than operated control animals on qualitative reaching, but not on skilled reaching. Those that received the combination treatment and were housed in EE cages showed significantly less improvement in qualitative reaching and grasping. Kinematic analysis revealed a decrease in digit abduction during skilled reaching among all groups, with no differences among groups. Kinematics provided no evidence that improved function was related to improved quality of reach. There was no evidence of neuroprotection in the cervical spinal cord. The absence of evidence for kinematic improvement or neuroprotection suggested that AMPH-enhanced motor training is due primarily to supraspinal effects, an enhancement of attention during skilled motor training, or plasticity in supraspinal circuitry involved with motor control.
Key words: amphetamine, cervical spinal cord injury, enriched environmental housing, motor training, skilled reaching
Introduction
In the United States, 55% of reported spinal cord injuries (SCIs) involve the cervical spinal cord, with the most frequent neurologic category being incomplete tetraplegia (National Spinal Cord Injury Statistical Center, 2008). While cervical injuries can impair both upper and lower extremity function, it is the impaired function of the upper limb that significantly limits the ability to carry out daily tasks (Anderson et al., 2009). Thus, people living with cervical SCI report the deficits of arm and hand function to be their primary concern (Anderson, 2004). Developing therapies to improve upper extremity function following cervical SCI is important, because even a small improvement in hand function can significantly increase the quality of life for an individual (Girgis et al., 2007).
Pharmacotherapy is a non-invasive intervention that can improve function after CNS injury. Amphetamine is a nervous system stimulant that releases monoamines from presynaptic neurons and blocks reuptake. The release of norepinephrine (NE) and dopamine (DA) are more prominent, but release of serotonin (5-HT) also occurs to a lesser extent (Martinsson and Eksborg, 2004; Wall et al., 1995). Following a cortical ischemic lesion in rats, paw reach accuracy improved when amphetamine (AMPH) was administered every third day for 26 days (Gilmour et al., 2005). AMPH has been shown to promote improved behavioral function such as beam walking in rats (Feeney et al., 1981), and tactile placing in cats (Feeney and Hovda, 1983) following cortical injury. AMPH also enhances the expression of proteins involved in neurite growth (GAP-43) and synaptogenesis (synaptophysin; Stroemer et al., 1998). In a stroke model, AMPH accelerated cognitive rehabilitation in the T-maze test compared to non-treated controls. Thus, AMPH likely improves memory and attention deficits following an embolic stroke (Rasmussen et al., 2006).
Motor training is another non-invasive intervention that can improve function. Motor training has a remarkable ability to modify structure and function in the motor cortex and spinal cord (Adkins et al., 2006). Specifically, skilled forelimb reach training is associated with an increase in the complexity and density of the dendritic processes in the undamaged forelimb motor cortex following unilateral stroke lesions (Biernaskie and Corbett, 2001; Bury and Jones, 2002). Animals that were retrained on the staircase forelimb motor task shortly after embolization of the external carotid artery significantly improved their ability to retrieve food pellets after only four training sessions (Rasmussen et al, 2006). Within the spinal cord, motor neurons exhibit increased protein synthesis and gene expression following exercise training. Therefore, training is an effective method for restoring activity, even in the absence of normal supraspinal input (Thuret et al., 2006).
Combination treatments are a likely direction for improving functional outcome since there are multiple targets that can be experimentally addressed (e.g., reduction in scar formation, providing growth-enhancing substrates for axonal regeneration/sprouting, countering the inhibitory environment of the injured CNS [Schwab et al., 2006], and activity-dependent plasticity). Several researchers (Adkins and Jones, 2005; Feeney et al., 1982; Gilmour et al., 2005; Hurwitz et al., 1991; Ramic et al., 2006) found improvements in skilled reaching tasks when motor training was paired with AMPH in animal stroke models. In all cases it was the pairing of AMPH and motor training that produced significant results, while AMPH alone did not. In one of the earliest studies demonstrating this effect, Hovda and Feeney (1984) showed that beam-walking experience while under AMPH intoxication accelerated locomotor recovery, but returning the animal to its cage following AMPH administration blocked this recovery. This has been attributed to an increase in the efficacy of the motor training mediated by AMPH. AMPH enhances monoamine neurotransmission, and also promotes neurotrophic and neuroplastic responses in the cortex after brain injury (Moroz et al., 2004; Stroemer et al., 1998). Other mechanisms by which AMPH administration may enhance recovery include temporary activation of circuitry involved in descending motor drive, and/or enhancement of attention during a period necessary to solidify the effects of motor training (Feeney et al., 1982).
Motor activity can be increased by both specific training protocols, and also non-specifically by housing in an enriched environment (EE). Spontaneous motor activity increases when animals are housed in an environment in which several animals share a larger space containing items (e.g., tubes, ramps, ladders, and running wheels) that increase motor activity, provide sensory feedback, and encourage social interaction (Kim et al., 2008). EE housing has been reported to increase the levels of neurotrophic factors (Dahlqvist et al., 1999) following middle cerebral occlusion in rats, in addition to an increase in the rate of injury-induced synaptogensis or stabilization of newly-formed synapses in the motor cortex following cervical SCI (Kim et al., 2008), along with increasing the dendritic arbors in the undamaged motor cortex (Biernaskie and Corbett, 2001). Furthermore, in a stroke model, motor performance was improved by a combination of AMPH, focused motor activity, and EE housing (Papadopoulos et al., 2009).
Our aim was to extend treatments shown to be effective following cortical injury to determine whether forelimb function could be improved following a spinal cord lesion. We evaluated D-AMPH administration, skilled forelimb training, and EE housing in rats given unilateral cervical contusion injuries to determine if this combination of non-invasive treatments would improve the animal's ability to recover forelimb function as tested both behaviorally and kinematically.
Methods
Subjects
Adult Sprague-Dawley rats (275–325 g; Taconic, Germantown, NY) were used. Male rats were studied because AMPH is known to interact with estrogen, leading to prolonged stereotypic behaviors and increased locomotor activity in females (Becker and Cha, 1989; Becker et al., 2001; Adriani et al., 2003), which would confound the interpretation of this study. Animals were selected for right limb preference prior to surgery by observing the animals' reaching performance in the Whishaw single-pellet reaching task (Stackhouse et al., 2008). Fifty percent of animals demonstrated right limb preference (n=125). Animals not selected for behavioral testing were used as anatomical controls (n=17), or passed to other studies. Twenty-six animals died from surgical complications within 24 h post-impact, likely due to respiratory impairment, and 15 animals were excluded due to incorrect lesion placement. Thus a total of 101 animals provided data for this study. Animals were housed 2/cage in standard cages (24×36×15 cm), or 5/cage in EE housing (76.2 cm wide×45.72 cm deep×121.92 cm high) in the animal facility under a 12/12-hour light/dark cycle (lights on at 7:00 am), and in conditions of controlled temperature and humidity. Animals were given a food-restricted diet of 12–15 g/d of standard rat chow so that they maintained approximately 85–90% of free-feeding body weight. The animals were weighed daily and given free access to food for the 2 days before and after surgery. Water was provided ad libitum. All behavioral data were collected preoperatively to provide baseline measures, and post-operatively at weeks 1, 5, 9, 13, and 17. All surgical and behavioral procedures were approved by the Drexel University College of Medicine's Institutional Animal Care and Use Committee, and followed National Institutes of Health (NIH) Guidelines for working with animals.
Experimental groups
All animals received a C3–C4 right side contusion. Injury to the C3–C4 spinal segments of the spinal cord affects muscles (acromiotrapezius, levitor claviculae, spinodeltoideus, biceps, and extensor carpi radialis brevis) used to perform skilled reaching and grasping (McKenna et al., 2000). Surgical lesions at this spinal level produce reproducible deficits in reaching and grasping performance, and transient deficits in forelimb locomotor function (Stackhouse et al., 2008), with no bladder impairment. The first group of animals (n=25) was used in a dose-response study, and received 0.5 (n=9), 1.0 (n=7), or 2.0 (n=9) mg/kg of AMPH to determine the optimal dose. The remaining animals were assigned to five experimental groups: injured controls (Ctl, n=13); training only (T, n=12); drug only (D, n=10); AMPH and training (D+T, n=14); and AMPH and training and EE housing (D+T+EE, n=10). Animals were tested in two different batches. The first batch contained half of the of animals in the Ctl, T, D, and D+T groups, while the second batch of animals (started 6 months later) contained the remaining animals from these groups and the D+T+EE animals. A one-way analysis of variance (ANOVA) comparing the same group between batches at all time points showed no differences between batches, thus data from both batches were combined for the overall analysis.
Spinal cord injury
Experimental animals were deeply anesthetized with a ketamine (60 mg/kg) plus xylazine (96 mg/kg) cocktail, and received a right unilateral C3–C4 dorsolateral contusion (modified from Sandrow et al., 2008). A partial laminectomy at C3 and C4 exposed the right side of the spinal cord. The vertebral column was stabilized with clamps positioned on the C2 and C6 spinous processes. The tip of the impactor (1.6 mm; Infinite Horizon, Lexington, KY) was positioned halfway between the dorsal vein and the right lateral edge of the spinal cord, and set to produce an impact of 175 kilodynes, which creates a 1500–1700 μm dorsal-ventral displacement. The impactor tip was positioned 3–4 mm above the spinal cord, and the field was filled with sterile saline to the top of the impactor tip. Following impact the contused area was covered with BioBrane (Bertek Pharmaceuticals, Morgantown, WV), the musculature was sutured shut with absorbable suture, and the skin was closed with wound clips (Biomedical Research Instruments, Silver Spring, MD). Three days post-injury, the animals were given a paralysis ranking based on a scale in which 1=digits and paw partially paralyzed, but the rat was able to support his weight, 2=digits and paw completely paralyzed, and 3=digit and paw are at least partially paralyzed and elbow was affected (Girgis et al,. 2007). About 80% of the animals survived and were assigned to either one of the dose-response groups (n=25), or one of the five experimental groups (n=59), so that each group contained an equal number of animals at each paralysis ranking.
Amphetamine preparation
D-amphetamine sulfate powder (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.9% sterile saline at 0.5, 1.0, or 2.0 mg/kg, and administered by IP injection beginning on day 13 following SCI. Animals received their designated dose every third day (Adkins and Jones, 2005; Gilmour et al., 2005) until the end of week 12 training, excluding testing weeks. AMPH was not administered during testing weeks, since the animals became hyperactive, and in addition, we did not want to test state-dependent learning (Ahmed et al., 1996). Control and training-only animals received 0.9% sterile saline on injection days. Injections were given between 7:00 and 8:00 am 45 min prior to the first training session.
Dose-response curve
The first experiment determined the optimal concentration of AMPH, based on both movement in a locomotor activity box (Vasilev et al., 2003), and performance in the single-pellet reaching task (Stackhouse et al., 2008). A dose of 1.0 mg/kg has been shown to increase skilled forelimb reaching success without interfering with the animal's ability to perform the task in a stroke model (Adkins and Jones, 2005). Thus we selected the doses of 0.5, 1.0, and 2.0 mg/kg to test in our lesion model. A SmartFrame open-field activity box (Kinder Scientific, Poway, CA) containing 32 infrared photobeams in both the x and y planes was used to assess the animal's locomotor activity level for 5 min starting at 30 min post-injection. This system recorded each time any part of the animal's body crossed a photobeam, and allowed us to determine the activity level of each animal at the three drug doses. Fifteen minutes after the trial in the activity box, the animals were placed in the single-pellet training box, and their motor performance was observed during their training session.
Enriched environmental housing
A group of animals (n=10) that received the optimal dose of AMPH and motor training were housed in an EE instead of conventional cages, to stimulate motor activity and promote forelimb use (Fig. 1). These animals were housed in groups of 5 in multilevel ferret cages (93.9 cm×61.60 cm×142.24 cm; Marshall Pet, Wolcott, NY) beginning 5 days following the lesion, and continuing for the duration of the study (Biernaskie and Corbett, 2001). This environment allowed the animals to climb to multiple levels, traverse through tunnels, cross ropes, run in a wheel, and forage for food. Food was placed in different corners of the cage, forcing the animals to climb and use the injured forelimb. Items such as tubes, ladders, and ropes were changed weekly to increase the chance of activity due to the novelty of the items. In addition the trough training device was placed in the EE cages 6 h per day 5 days per week to encourage skilled forelimb training.
FIG. 1.
Enriched environment (EE) housing cage. Multilevel cages allowed animals to transverse ladders, climb through tubes, run in a wheel, practice skilled forelimb reaching in a reaching trough, and forage for food. Food was also placed in different areas of the cage, encouraging the animals to climb.
Preoperative training
Behavior training/testing for forelimb function
Single-pellet test training
All animals were trained preoperatively on a reach-to-grasp task (Stackhouse et al., 2008) 5 sessions/week for 6 weeks, between 07:00 and 11:00 am, in an acrylic glass reaching chamber (45 cm deep×40 cm tall×12.5 cm wide). The chamber contained a 1-cm-wide slit on the front wall through which the animals reached to retrieve a 45-mg chocolate food pellet (Bio-Serv, Frenchtown, NJ) from a food well positioned on an acrylic glass tray 1.5 cm from the slit and at a height of 4 cm. The animals were operantly trained to reach with the right forelimb though the slit to retrieve and eat the food pellet. Training was done through successive stages. The animals were taught to shuttle back and forth in the single-pellet reaching box between reaches; this creates a natural separation between reaches, and allows the animal to re-align its body position before the next reach (Whishaw et al., 1992). The animal was considered successfully trained when it consistently reached with the right forelimb, grasped the food pellet with greater than 40% success (8/20 pellet retrievals), and shuttled to the back of the chamber before the next attempt.
Staircase test training
The animals were trained on the staircase-reaching test for 2 weeks preoperatively (two 15-min sessions/day). The apparatus is a small chamber with an opening at one end that leads to a plinth on top of a platform with a staircase on each side (Montoya et al., 1991). The staircase contains seven wells equally spaced along the side of the platform in a step-like fashion. Each well contains three 45-mg food pellets (Bio-Serv). The plinth overhangs the platform to prevent the animals from raking the pellet up against the side of the platform. Baseline scores were obtained during the second week.
Post-operative skilled motor training
Task-specific forelimb motor training
Training began on day 13 following injury and continued 7 days a week for 12 weeks excluding testing weeks. To encourage use of the injured forepaw during the first week of training (post-operative week 2), the animals were trained for 20 min/day to climb a 3’×1’ grid surface (1”×1”grid) inclined 50–60° to receive cereal rewards. Motor training during weeks 3–12 consisted of two 15-minute training sessions in the reaching tasks, with a 30-min interval between the two sessions. Which training task was performed first was randomly selected daily. One training session was carried out in a trough-reaching task, which is similar to the staircase pellet retrieval task, while the other session was performed in a tray-reaching task, similar to the single-pellet reaching task (Whishaw and Pellis, 1990). The trough task apparatus is a small chamber (35×6 cm) with an opening on one end, and a chamber with a platform onto which the animal can climb at the other end. The trough on the right side of the platform was filled with flavored food pellets (Bio-Serv). The platform overhangs its base, preventing the animal from raking the pellets, thus testing the animal's grasping ability. The tray task is an acrylic glass box (35×13×30 cm) fitted with a face consisting of 2-mm bars spaced 10 mm edge to edge. An acrylic glass tray (11 cm×4 cm×1.5 cm) was filled with chocolate food pellets (Bio-Serve) and mounted on the front of the box. To obtain a food pellet, the rat reached through the bars, grasped, and retracted the pellet. Following surgery, the rats tended to reach for the food reward with the uninjured forepaw; in this event, acrylic glass inserts (25×10 cm) were placed inside the reaching box to create a channel to the front of the box and force the use of the injured forepaw. The food tray can be adjusted in 1-cm increments from 1–4 cm above the ground, and can also be moved forward in 0.5-cm increments. Adjusting the shelf height allows for a gradual increase to 4 cm, which accommodates the animals' more impaired reaching abilities for the first few weeks following injury; the tray can also be moved away from the box, creating a space between the tray and box, forcing the animals to grasp the food pellet for retrieval.
On days when AMPH was paired with motor training, animals participated for 15 min in the first training task (randomly selected) beginning 45 min following injection. The animals were then returned to their home cage for 30 min before participating for 15 min in the second reaching task. The total testing time from AMPH injection to the end of the second training session was 1 h 45 min. The half-life of AMPH is approximately 5–6 h (Feeney et al., 2004), and at a dose of 1 mg/kg, male rats are active for approximately 140 min (Milesi-Halle et al., 2007). This dosing/training schedule was selected to ensure that the animals were experiencing AMPH effects while participating in motor training (Table 1).
Table 1.
Behavioral Timeline
| Weeks | 1–8 | 9 | 10 | 11 | 12–13 | 14 | 15–17 | 18 | 19–21 | 22 | 23–25 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reach training, baseline testing | Injury, paralysis rating | Week 1 testing | Week 2 maze training | Weeks 3–4 of motor training | Week 5 testing | Week 6–8 of motor training | Week 9 testing | Weeks 10–12 of motor training | Week 13 testing | Retention period | Week 17 testing |
Post-operative behavioral testing
Behavioral testing and scoring was carried out by two trained individuals, who demonstrated 95% inter-rater reliability on behavioral scoring. The lead scorer was blinded to treatment group.
Skilled (trained) tests
Single pellet
Qualitative components of the single-pellet reach-to-grasp task were scored using a movement rating scale (McKenna and Whishaw, 1999; Whishaw et al., 1993), which assesses 10 different components of a reach. These components include limb lift, digits closed, aim, advance, digits open, pronation, grasp, supination I, supination II, and release. A component was rated 2 if the movement appeared normal, 1 if the movement was abnormal but recognizable, or 0 if the movement was absent or if the animal compensated for its deficit by moving other parts of the body (uninjured forelimb, tongue, and torso; McKenna and Whishaw, 1999). The ratings from each component of the reach were summed to produce a deficit score from each trial (range: 20=no deficit to 0=maximum deficit). Six trials for each animal were scored from video replay by two raters and averaged to obtain one score per animal.
Quantitative assessment of the reach-to-grasp task was recorded using a Sony digital video camera. The number of pellets successfully retrieved out of approximately 20 reaches was counted. A success was defined as a reach during which the rat grasps the food pellet with active digit flexion. Unsuccessful attempts were counted when the animal raked the pellet from the well without actively grasping it, or dropped the pellet once it had retrieved it from the platform. The percentage of successful reaches was calculated as: (no. successful reaches/no. total reaches)×100.
Kinematics
To distinguish between recovery and compensation, kinematic analysis of selected components of the single-pellet qualitative reach (Whishaw et al., 1993) was completed. Each animal's digit tips were inked prior to testing, and an average of 3–5 reaches during single-pellet reaching were analyzed per animal. To capture reaching movements, a high-speed (500 frames/sec) camera recording system was positioned to record a frontal view of the animal. Because movements of the distal forelimb (digits open, grasp, supination I, and supination II, and release) were more impaired than movements depending on the integrity of shoulder function (limb lift, aim, and advance), following a cervical dorsolateral lesion (Stackhouse et al., 2008), we assessed two components, the degree of paw pronation excursion that occurred during the pronation phase, using the angle of a plane made between the second and fifth digits and the horizontal at the start and end of the pronation phase when pellet contact has occurred, and the extent of digit spread (abduction) between the second and fifth digits during the digits open and pronation phases. WINanalyze (Mikromak, Erlangen, Germany) tracking software was used to digitize the reach frame-by-frame. Custom-written software (Microsoft Excel) was used to take the Cartesian coordinates from digitization, and calculate the pronation excursion and digit abduction for each animal.
All three single-pellet reaching tasks (qualitative and quantitative reaching and kinematic analysis) were recorded at the same time for each animal to limit the amount of behavioral testing the animals underwent, and were analyzed separately at a later date.
Staircase-reaching test
The staircase-reaching test was used to examine reaching performance in a task that minimizes the need for shoulder function against gravity (Montoya et al., 1991; Stackhouse et al., 2008), and focuses on grasping. Thus, this test requires that the animal make a coordinated grasp and lift up to 21 pellets from 7 wells (3 pellets per well) at different levels within the chamber. The number of pellets retrieved, averaged from the three best trials out of 10, was used as a quantitative measure of forelimb-reaching ability.
Spontaneous (untrained) locomotor tests
Grid walking is a locomotor test of sensorimotor integration in which animals walk on an elevated grid (36 cm L×38 cm W×30 cm H, with 3×2-cm openings) for 2 min (Grill et al., 1997, Sandrow et al., 2008). The percentage of correct placements by the affected forepaw was used as the outcome measure (the total number of correct placements of steps on the grid rungs by the affected forepaw over the number of total steps, steps, and missteps, made by the affected forepaw, and expressed as the percentage of correct steps).
Forelimb Locomotor Score (FLS)
Open field locomotor testing and scoring was conducted as previously described (Cao et al., 2008; Sandrow et al., 2008). Briefly, the animals were placed in an enclosure and videotaped in an open-field apparatus for 4 min. The animals were scored on a scale of 0 (no observable forelimb movement) to 17 (normal locomotion), based on the amount of forelimb movement. Only preoperative and end-point (week 13) scores were analyzed.
Histology
The animals were deeply anesthetized with Euthasol® (Virbac Corp., Can Antonio, TX) at the end of the behavioral studies, and transcardially perfused with 150 mL of physiological saline, followed by 500 mL of ice-cold 4% paraformaldehyde in 0.1 phosphate buffer (PB) solution with a pH of 7.4. Spinal cords were dissected and the lesion block and a segment caudal to the lesion block were placed in 0.1 M PB containing 30% sucrose for 72 h. The lesion block was approximately 6 mm long for the lesion region, and the caudal block was about 2–3 mm long. The blocks were frozen in M-1 embedding matrix (Thermo Electron Corp., Pittsburgh, PA), and serially sectioned at 20 μm on a cryostat in the transverse plane.
Spared tissue
A Nissl/myelin stain was used to assess tissue sparing. White and gray matter areas were outlined and quantified on the intact (left) and lesioned (right) sides of the spinal cord using ImageJ software. The percentage of spared tissue (total spared gray and white matter) was calculated by summing every section that included the lesion cavity (Sandrow-Feinberg et al., 2009), and dividing the area of the lesioned side by that of the intact side and multiplying by 100 through the lesion. The percentage of spared tissue was used for regression analysis with qualitative and staircase-reaching performance.
Statistical analysis
A sphericity test was used to check for normal distributions of the data for each outcome measure. For parametric behavioral data, a two-way repeated-measure ANOVA between treatment groups over time, with time taken as a repeated measure, was used. If significant, a one-way ANOVA at each time point was performed, followed by the Bonferroni post-hoc test. A one-way ANOVA followed by Dunnett's t-test was used to identify the proper drug dose. For non-parametric behavioral data a Kruskal-Wallis test was performed, and if significant a Mann-Whitney U test comparing each treatment pair was used to determine significance between treatment groups. Reaching attempts at week 1 and week 13 were binned into groups of 0–10 and 11–20+ and analyzed by chi-square (Fisher's 2×2 exact test). Anatomical measures were found to be parametric and were analyzed by one-way ANOVA between groups, followed by the Bonferroni post-hoc test when appropriate. Significance levels were set to 0.05 for all comparisons.
Results
Post-injury motor function
The unilateral C3–C4 contusion injury initially produced a moderate deficit in forelimb function. The affected (right) forelimb was unable to provide full weight support, dorsal stepping was common, the paw and digits were often paralyzed, and in some cases the elbow was affected, preventing full extension of the forelimb during stepping. These deficits showed partial to full recovery over the remainder of the study. All animals were always able to locomote and care (groom, eat, drink, and move around the cage) for themselves. At 3 days post-operatively, the paralysis ranking scale (Girgis et al., 2007) rated 26 animals as 1 (digits and paw partially paralyzed but the rat was able to support his weight), 23 animals as 2 (digits and paw completely paralyzed), and 9 animals as 3 (digits and paw are at least partially paralyzed and the elbow was affected).
Dose selection
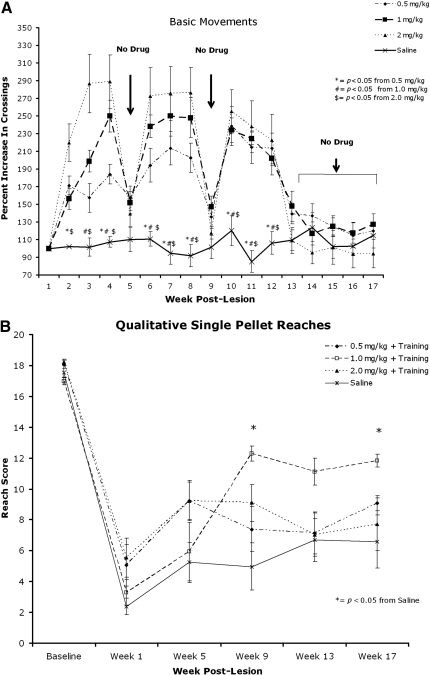
Administration of AMPH (0.5, 1.0, and 2.0 mg/kg) elicited significantly more locomotor movements compared to saline throughout the course of the 17-week experiment (Fig. 2A). The highest dose (2.0 mg/kg) produced the most locomotor activity, and observation of these animals also showed greater activity in the training apparatus during drug administration days. There was no significant difference in locomotor activity during testing weeks (5, 9, and 13), and during the retention period (weeks 14–17), indicating that none of these dosing regimens caused sensitization. Qualitative reaching scores showed that animals receiving the dose of 1.0 mg/kg performed significantly better during weeks 9 and 17 of testing (Fig. 2B), with less hyperactivity seen during training. Consequently, we used 1.0 mg/kg AMPH in the subsequent experiments.
FIG. 2.
Dose-response curves. (A) Locomotor activity is significantly increased in animals that received amphetamine (AMPH) compared to saline. Results are normalized to week 1. No difference was seen among treatment groups or control animals during weeks when no drug was administered (weeks 5 and 9, and 13–17). (B) Animals that received 1.0 mg/kg AMPH performed significantly better during qualitative reaching at weeks 9 and 17 compared to the saline group (p<0.05). Means and standard errors are shown (*p<0.05 for all figures).
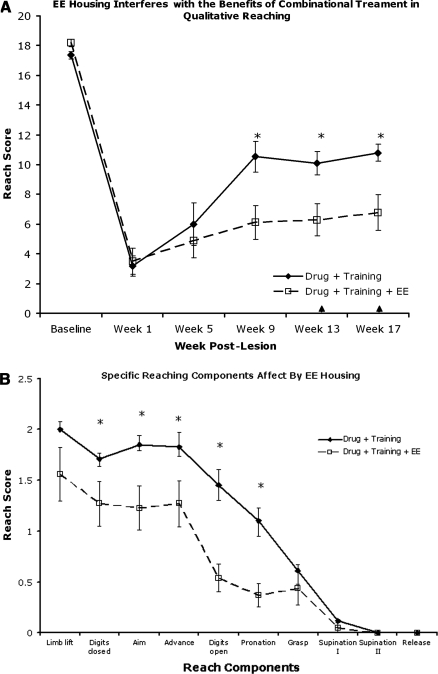
Combination treatment improved skilled forelimb reaching in rats
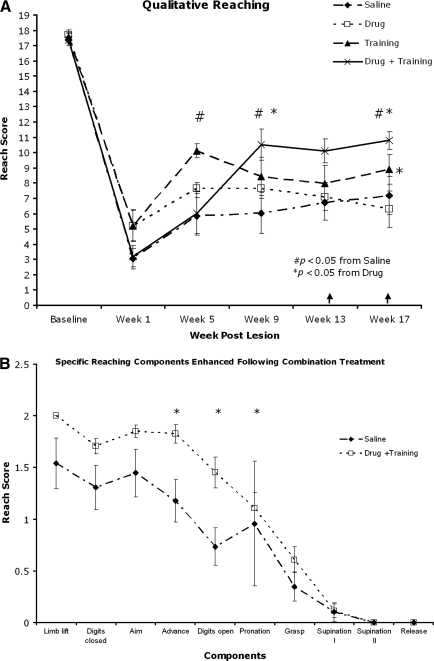
Qualitative forelimb reaching (Fig. 3A) assessed the animals' ability to perform the 10 components of a reach (Whishaw et al., 1998). Post-hoc analysis showed the qualitative reaching score to be higher (p<0.05) in animals that received both drug and training during weeks 9 and 17 post-lesion, and approached significance (p<0.08) at week 13 compared to control and drug-only animals. Specifically, at week 17 the drug and training (D+T) animals performed better on the advance, digits open, and pronation phases of reaching (Fig. 3B). At week 17, following a 4-week retention period with neither drug (D) nor training (T), the T group performed significantly better than the D group in qualitative reaching.
FIG. 3.
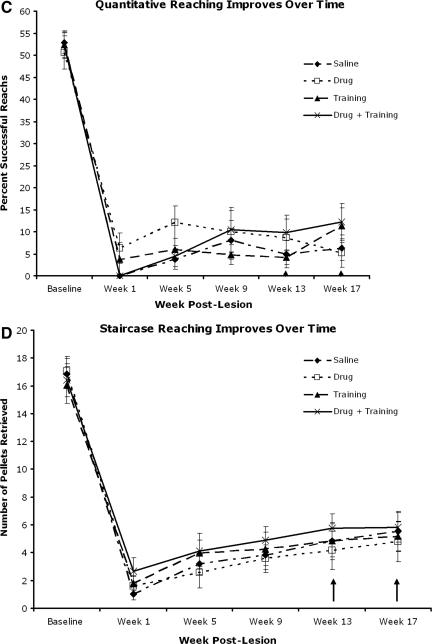
Skilled motor reaching assessment of the right forelimb. (A) Qualitative reaching score. Drug+training animals performed significantly better then saline and drug-only animals on weeks 9 and 17 post-lesion. Training alone was more effective than drug after 1 month with no drug (week 17). (B) The advance, digits open, and pronation phases were enhanced compared to the saline group during week 17. (C) Quantitative reaching was impaired in all groups following injury, but all groups showed an increase in pellet retrieval over time. (D) The staircase-reaching test showed a severe deficit in grasping ability in all groups, with improvement in any group over time (↑ indicates retention period).
In contrast, forelimb function assessed using both the Whishaw single-pellet (quantitative measure; Fig. 3C) and staircase (Fig. 3D) reaching tests showed deficits in reaching and grasping with no differences seen among groups during testing weeks. There was a main effect over time, indicating some recovery in grasping ability, but with no effect of treatment.
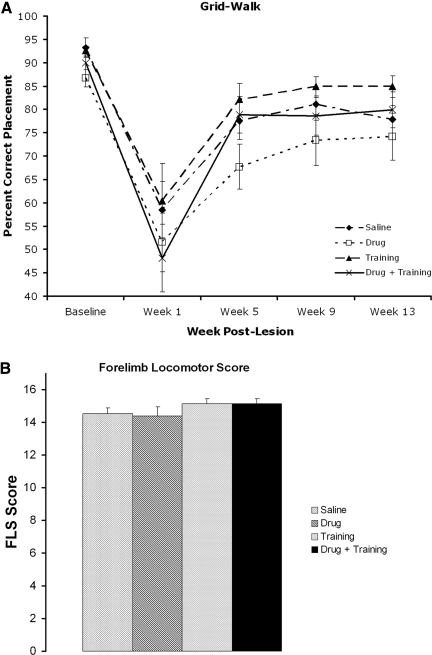
Locomotor function improved spontaneously
Sensorimotor function was assessed using the grid test. All animals showed a marked deficit in the right forelimb at week 1, with a partial improvement by week 5 (Fig. 4A), and a main effect over time, but with no differences among groups. Forelimb locomotor function was assessed at the final treatment testing week (week 13) using the FLS test. Most animals displayed few locomotor deficits at this time, with continuous plantar stepping and occasional toe clearance (FLS=14), but this test also showed no significant difference among groups (Fig. 4B). All animals displayed normal forelimb locomotor patterns prior to surgery (FLS=17). Thus, untrained locomotor function showed good recovery with no additional benefits of AMPH-enhanced training.
FIG. 4.
Locomotor measures of the right forelimb. (A) Grid walking (sensorimotor) showed a deficit with a partial recovery by week 5. (B) Forelimb Locomotor Score (FLS) at week 13 showed few deficits (e.g., incomplete toe clearance), with no difference among groups. All animals had normal locomotion function (FLS=17) prior to surgery.
Enriched environmental housing interferes with the benefits of drug-enhanced motor training
Surprisingly, animals that received drug and training and were housed in an EE performed significantly worse on skilled tasks compared to animals that received drug and training but were housed in standard cages (Fig. 5A, B, and C; p<0.05). Both groups showed marked deficits at week 1, with partial improvement subsequently, but the extent of improvement by rats in the EE-housed group was less than that of rats housed in standard cages at weeks 9, 13, and 17. These two treatment groups did not differ on tests that showed no recovery (quantitative skilled reaching; Fig. 5D), or those that revealed few deficits (grid and FLS; data not shown).
FIG. 5.
Enhanced training with enriched environment (EE). Animals that received drug and training and were housed in EE cages performed significantly worse (p<0.05) than animals receiving drug and training and housed in standard cages, in both qualitative (A and B) and staircase (C) reaching. These differences were significant at week 9, and persisted for the remainder of the study. Quantitative reaching displayed no difference among groups, but improved over time (D).
Assessment of motivation
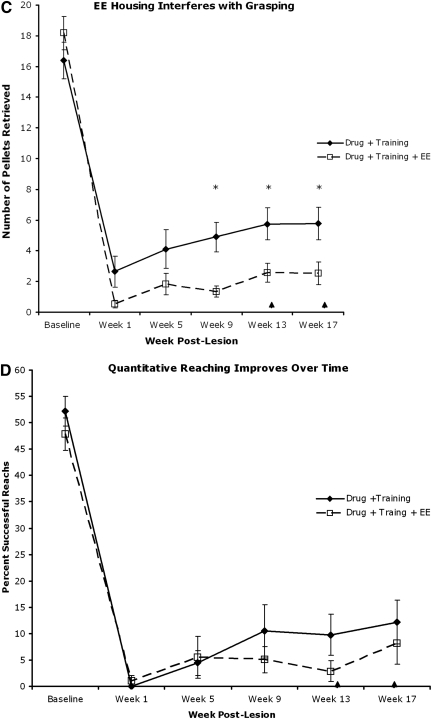
The reaching and grasping tasks were difficult for intact animals and more so for injured animals. In order to examine if the experimental animals' failure to grasp and retrieve pellets in the single-pellet reaching test was a result of their willingness to engage in the task, we counted the number of reach attempts each animal made during week 1 and week 13. A reach attempt was scored when the wrist of the animal's extended forelimb successfully broke the plane of the shelf in the Whishaw single-pellet reaching box, regardless of success. The number of reach attempts 1 week following injury compared to the number at week 13 indicated more attempts at later stages (Fig. 6), indicating continued engagement in the task. Thus the animals' inability to regain partial to full reaching and grasping function following cervical SCI likely reflects the degree of difficulty of the test rather than their lack of motivation to perform the test.
FIG. 6.
The number of reach attempts made by the animals at weeks 1 (A) and 13 (B). More attempts were made at week 13 than at week 1 (Saline, control; D, drug only; T, training only; D+T, drug and training; D+T+EE, drug and training in enriched environmental housing; *p<0.05 by Fisher's 2×2 exact test).
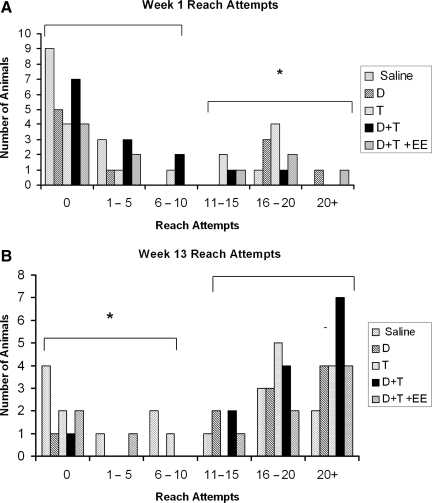
Kinematic analysis
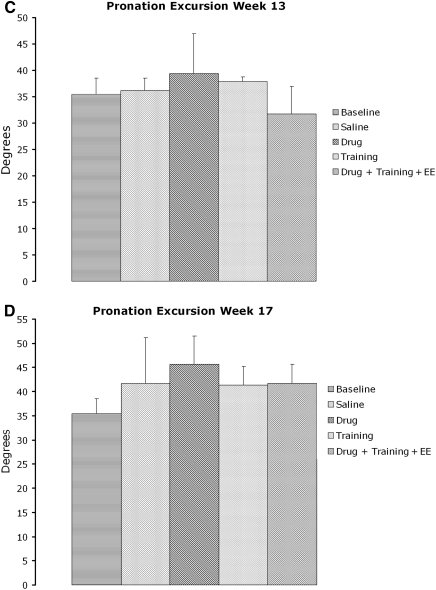
High-speed video analysis of digit abduction and pronation excursion components of the reach-to-grasp single-pellet task were analyzed from a subset (n=34) of animals from each group. Animals were chosen based on their ability to extend the limb through the slot during week 13 of qualitative reaching testing. The results revealed a significant deficit in digit abduction in all groups compared to baseline. These differences were present in all but the training group at week 13 (p<0.05), and in all groups at week 17 (p<0.008) post-injury (Fig. 7A and B). Only 3 training-only animals fit the inclusion criteria at week 13, and it is likely that this number was insufficient. Pronation excursion also revealed no difference among groups (Fig. 7C and D). Thus, kinematic measures did not reveal a beneficial effect of treatment that was predicted by the single-pellet reaching task.
FIG. 7.
Kinematic analysis of the digit abduction and pronation excursion components of the Whishaw qualitative reach-to-grasp test. (A) Digit abduction was significantly decreased at week 13 in saline controls, drug, drug plus treatment, and drug plus treatment plus enriched environment (EE) animals compared to preoperative measures. (B) All groups showed a significant deficit in digit abduction during week 17. Pronation excursion revealed no difference among groups at weeks 13 and 17 (C and D).
Histological evaluation
Tissue sparing
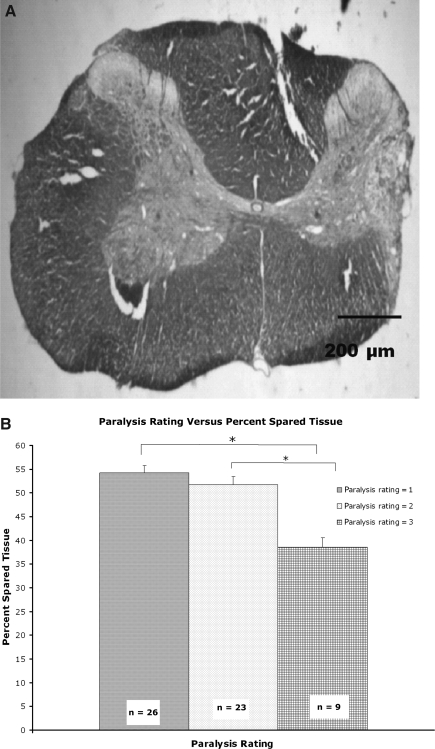
The amount of spared tissue (gray and white matter; Fig. 8A) was measured. Animals were excluded if the lesion did not include the entire dorsolateral funiculus, or if the lesion crossed the midline. Fifteen animals were eliminated because the lesion was too large, and two animals were sacrificed early due to autophagia of their left hindlimb, presumably due to damage to the spinothalamic tract. These animals were not included in the study, but this had no effect on overall power analysis per group. There was no difference between groups in the amount of tissue spared, and no correlation between qualitative and staircase-reaching success with the amount of tissue spared (data not shown). Animals that were given a paralysis rating of 3, however, had significantly less spared tissue compared to animals that received a paralysis rating of 1 or 2 (Fig. 8B). The paralysis rating thus appears to be a predictor of lesion size.
FIG. 8.
(A) Representative cross-section of a cervical dorsolateral contusion. Injury ablates dorsolateral white matter, often produces partial damage to lateral gray matter, and may extend into the ventral-lateral white matter. (B) Paralysis rating conducted 3 days post-lesion showed that animals with a paralysis rating of 3 (highest degree of impairment) had larger lesions than animals rated 1 or 2.
Discussion
We used a right-sided C3–C4 contusion injury, which induces detectable and reproducible deficits in the right forelimb, to evaluate the effects of AMPH, skilled motor training, and locomotion on forelimb function. Some aspects of forelimb function improved spontaneously, but others remained impaired. We also found a greater improvement in some tasks following a combination treatment of skilled forelimb motor training paired with AMPH. Lastly, we found that EE housing combined with AMPH and skilled motor training diminished the extent of forelimb recovery.
Behavioral outcomes following chronic treatments
Skilled functional recovery
We compared forelimb recovery from a cervical SCI using a novel combination of non-invasive treatments and a battery of behavioral tests. While the early components (limb lift, digits closed, and aim) of the qualitative single-pellet reaching task showed some recovery independent of treatment, AMPH combined with daily motor training promoted recovery of the middle phases (advance, digit open, and pronation), but not the later phases (grasp, supination I, supination II, and release), of the reach. These improvements persisted for 4 weeks when tested for retention (no drug administration or motor training during this time) indicating a long-term benefit of this combination treatment. The C3–C4 injury affects the motor neuron pools of the shoulder and upper forearm muscles (McKenna et al., 2000), while the dorsal lateral contusion disrupts the rubrospinal tracts (Liu et al., 1999), lateral cortiospinal fibers (Weidner et al., 2001), and parts of the reticulospinal tract (Houle and Jin, 2001). Ablation of the red nucleus resulted in functional changes in the aim, advance, pronation, grasp, supination I and II, and release phases of the reach (Whishaw et al., 1992,1998). Anatomical data indicate that some rubrospinal fibers do make direct projections onto the motoneurons of the extensor and flexor digitorum and the flexor and abductor digit, and electrophysiological data show that the red nucleus is active during reaching. Microstimulation of the red nucleus resulted in shorter electromyographic latencies in the distal forepaw musculature than in the proximal forelimb/shoulder musculature (reviewed by Stackhouse et al., 2008). Therefore, lesion location may permit the combination treatment to elicit the reorganization/sprouting of fiber tracts involved in proximal muscle movements, rather than the more distal movements.
Two mechanisms by which AMPH administration following a SCI may promote improved motor function, including temporary activation of the circuitry involved in the descending drive for locomotion, a motor mechanism, and/or enhancement of attention during a period necessary to solidify the effects of motor training, a cognitive mechanism (Feeney et al., 1982). AMPH may exert its beneficial effects by increasing monoamine function in the cortex, or by restoring monoaminergic control to the spinal cord, or both. It has been suggested that AMPH activates sites distant from the injury (diaschisis) that were transiently depressed following a CNS injury (Feeney and Hovda, 1983; Feeney et al., 1982; Sutton et al., 1989). This combination has also been shown to be promising in a stroke animal model. Adkins and Jones (2005) also described improvement that persisted for 4 weeks following the last testing session, although performance deteriorated at 8 and 12 weeks following the cessation of training. Periodic motor training is likely required to maintain the functional recovery.
Ramic and associates (2006) proposed that the combination treatment of AMPH and motor training following a stroke lesion may lead to an enhancement of expression of growth proteins, permitting the remodeling of cortico-efferent pathways that are important for motor recovery. Another study in cats with bilateral cortical lesions suggested that AMPH may also act on subcortical motor regions, in addition to cortical motor regions, to enhance recovery (Sutton et al., 1989).
Two quantitative tasks, the staircase-reaching and the single-pellet tasks, were used for several reasons (Stackhouse et al., 2008). The staircase-reaching test assesses reaching function independent of shoulder control, which may be impaired following a dorsolateral C3–C4 SCI if the motoneuron pool supplying the shoulder musculature is disrupted. The staircase test requires the animal to make a coordinated grasp and to lift the pellet to its mouth with no possibility of raking the pellet. The location of the shelf of the single-pellet reaching apparatus, 4 cm above the floor, requires shoulder muscle control to aim the forelimb in order to contact the pellet. The single-pellet test, however, allows the possibility for the rat to rake the pellet through the slot rather than grasping it. Thus grasping, which is one of the components of reaching that remains challenging following injury, showed no benefits of the therapies. In an embolic stroke rat model there also was no improvement in performance when other tests (45 min daily exposure to staircase and T-maze testing) were paired with AMPH administration in the staircase test or in a test of cognition, the T-maze test (Rasmussen et al., 2006).
Unskilled (untrained) behavioral recovery
There was an initial deficit in unskilled locomotor function, followed by a considerable improvement, as indicated by the grid-walking and FLS tests. The lesion spares the dorsal columns and ventral pathways (dorsal and ventral corticospinal tract), which are important for locomotion. This type of motor recovery was also found in a similar lesion model using the cylinder paw-placement test (Stackhouse et al., 2008). Our results demonstrate that the pathways located in the dorsolateral spinal cord are important for skilled forelimb movements such as reaching and grasping, but are less important in movements associated with locomotion.
Housing in enriched environments impaired functional improvement
A number of studies have shown that EE housing in combination with other treatments improves motor function following CNS injury (Biernaskie and Corbett, 2001; Dai et al., 2009; Eaton et al., 2008; Knieling et al., 2009). In a stroke model, animals that were housed in an EE and receiving focused motor activity sessions paired with AMPH, recovered to preoperative performance, and this was associated with a measurable increase in axonal growth in the cortex (Papadopoulos et al., 2009). Our results showed no benefits of treatment in the spinal cords of animals that received the combination therapy and were housed in an EE, compared to those who received drug and training but were housed in standard cages. In fact, EE diminished the extent of recovery. The duration of AMPH administration may be an important variable in the success of this combination treatment, since Papadopoulos and associates (2009) used a more restricted AMPH regimen. Indeed, for clinical application it would be important to limit the number of AMPH-enhanced sessions due to the potential side effects of AMPH (reflex bradycardia, behavioral arousal, and hypermotility; Goldstein, 2000). Others (Feeney et al., 1982; Ramic et al., 2006) have shown improvement in reaching in EE-housed animals when motor training is provided under AMPH intoxication. We chose not to test function until AMPH had been cleared, since we did not want state-dependent learning to confound the interpretation of the results. Recently, a cervical SCI model that used EE as a form of rehabilitation treatment in combination with chondroitinase ABC, also showed a decrease in the recovery of the single-pellet and staircase-reaching tests compared to injured controls (Garcia-Alias et al., 2009). In that study, animals were only exposed to an EE 1 h a day for 4 weeks, indicating that the “interference” that occurred happened rapidly. As there are no standard protocols for EE housing, differences in details of the housing and the experimental interventions used are likely to contribute to differences in outcomes. Thus EE seems to have selective effects depending on the parameters of the experiment. It has been shown that training in one task might come at the expense of another. For example, transected cats that are trained to stand cannot step, and those trained to step cannot stand (de Leon et al., 1998). Garcia-Alias and colleagues (2009) hypothesize that interference may occur when training for one task, but testing for another. This interference may be due to limited plasticity, so that one behavior, general skills acquired in EE housing, is acquired at the expense of another, skilled-forelimb reaching. There are specific time windows following SCI when therapeutic interventions appear to be more effective (Clarke et al., 2009). In our study animals were placed in EE housing 5 days post-operatively, and AMPH-enhanced skilled training begun 13 days post-operatively. The EE might have been more successful if animals were housed in EE cages following the commencement of training, to let forelimb function recover first, thus allowing EE housing to enhance this plasticity in subsequent weeks.
The combination of AMPH and physical therapy has been studied for many years in stroke patients with results that are so far inconclusive. There have been both positive (Crisostomo et al., 1988; Walker-Batson et al., 1995) and negative (Sonde et al., 2001; Treig et al., 2003) findings that can be attributed to differences in time of intervention following injury, drug dose, types of rehabilitation, time between drug administration and type of physical therapy, heterogeneity in stroke type, lesion size, lesion location, and comorbidities, among the studies. Standard protocols will need to be developed for the clinical evaluation of this treatment.
Kinematic analysis revealed long-term deficits in digit abduction
In agreement with our previous work (Stackhouse et al., 2008), we found a persisting although milder deficit in digit abduction among all groups compared to normal animals. It has been suggested that digit abduction is a component of single-pellet reaching that is under the control of the rubrospinal tract (Stackhouse et al., 2008), which is directly affected by our lesion. We found no differences among treatment groups, operated controls, and normal controls (baseline animals), during the pronation phase of qualitative reaching in the subset of animals selected for kinematic analysis. Observations of the videos indicated that animals often reached across the pellet rather than pronating over it, as a compensatory response to their motor impairment. We examined the kinematics of the first supination component of skilled reaching, but this component never improved. Similar results were also found following a dorsal column lesion, and pronation was greatly reduced and supination did not occur (McKenna and Whishaw, 1999). Strain differences may play a role in some of the differences seen in the deficits. Sprague-Dawley rats have slower movements that appear abbreviated when extending and flexing the arm and digits, and when pronating and supinating the paw, relative to Long-Evans rats (Whishaw et al., 2003). In Sprague-Dawley rats, we found the average baseline qualitative reaching score to be 18/20 because of incomplete supination, rather than the 20/20 that the Whishaw group reported for Long-Evans rats.
Anatomical outcomes
Exercise increases the release of several neurotrophic factors (Dupont-Versteegden et al., 2004; Houle et al., 1999) in spinal cord and muscle that can be neuroprotective, and AMPH has been shown to increase neuronal sprouting in the neocortex (Stroemer et al., 1998). We found no differences in the amount of tissue that was spared at the injury site among treatment groups, suggesting that neither AMPH nor training offered measurable neuroprotective effects. We also found no correlations between the amount of spared tissue and behavioral performance at week 17 in the qualitative and staircase-reaching tasks or with kinematic analysis. We therefore saw neither anatomical nor kinematic indications of benefits from the treatments.
Conclusions
Noninvasive combination treatments following CNS injuries have provided an effective approach to recovery (Adkins and Jones, 2005; Gilmour et al., 2005; Sandrow-Feinberg et al., 2010) that can be translated to the clinic (Gladstone et al., 2006; Walker-Batson et al., 1995). This study demonstrated that the pairing of AMPH and skilled forelimb motor training following a cervical SCI improved qualitative reaching, and that this combination is an additive effect since neither drug nor training alone resulted in significant improvement over saline treatment. AMPH may increase the focus/attention during motor training, and/or activate the motor mechanisms associated with the formation of new motor pathways, and the skilled training targets areas needed to enhance motor function (Schallert et al., 2000). Interestingly, EE housing decreased forelimb recovery in both qualitative and staircase reaching. It is possible that the limited plasticity in the spinal cord becomes dedicated to a general task improved by housing in an EE at the expense of what is needed to increase skilled reaching (Garcia-Alias et al., 2009).
Without further anatomical or kinematic evidence, we postulate that AMPH enhances attention during a period necessary to solidify the effects of motor training, and/or it causes functional activation of pathways able to remodel in response to motor training.
Acknowledgments
The authors would like to thank Theresa Connors, Robert Kushner, and Barry T. Himes, Ph.D., for their histological assistance. This research was supported by Paralyzed Veterans of America, the Craig H. Neilsen Foundation (J.S.S.), and NIH grant PO1 NS055976.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Adkins D.L. Boychuk J. Remple M.S. Kleim J.A. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- Adkins D.L. Jones T.A. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci. Lett. 2005;380:214–218. doi: 10.1016/j.neulet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Adriani W. Seta D.D. Dessi-Fulgheri F. Farabollini F. Laviola G. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ. Health Perspect. 2003;111:395–401. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.H. Oberling P. Di Scala G. Sandner G. Amphetamine-induced conditioned activity does not result from a failure of rats to habituate to novelty. Psychopharmacology (Berl.) 1996;123:325–332. doi: 10.1007/BF02246642. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Sharp K.G. Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp. Neurol. 2009;220:9–22. doi: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Becker J.B. Cha J.H. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav. Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker J.B. Molenda H. Hummer D.L. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann. NY Acad. Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Biernaskie J. Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J. Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury S.D. Jones T.A. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J. Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Shumsky J.S. Sabol M.A. Kushner R.A. Strittmatter S. Hamers F.P. Lee D.H. Rabacchi S.A. Murray M. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil. Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. Mala H. Windle V. Chernenko G. Corbett D. The effects of repeated rehabilitation “tune-ups” on functional recovery after focal ischemia in rats. Neurorehabil. Neural Repair. 2009;23:886–894. doi: 10.1177/1545968309341067. [DOI] [PubMed] [Google Scholar]
- Crisostomo E.A. Duncan P.W. Propst M. Dawson D.V. Davis J.N. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann. Neurol. 1988;23:94–97. doi: 10.1002/ana.410230117. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P. Zhao L. Johansson I.M. Mattsson B. Johansson B.B. Seckl J.R. Olsson T. Environmental enrichment alters nerve growth factor-induced gene A and glucocorticoid receptor messenger RNA expression after middle cerebral artery occlusion in rats. Neuroscience. 1999;93:527–535. doi: 10.1016/s0306-4522(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Dai H. MacArthur L. McAtee M. Hockenbury N. Tidwell J.L. McHugh B. Mansfield K. Finn T. Hamers F.P. Bregman B.S. Activity-based therapies to promote forelimb use after a cervical spinal cord injury. J. Neurotrauma. 2009;26:1719–1732. doi: 10.1089/neu.2008-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon R.D. Hodgson J.A. Roy R.R. Edgerton V.R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden E.E. Houle J.D. Dennis R.A. Zhang J. Knox M. Wagoner G. Peterson C.A. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Eaton M.J. Pearse D.D. McBroom J.S. Berrocal Y.A. The combination of human neuronal serotonergic cell implants and environmental enrichment after contusive SCI improves motor recovery over each individual strategy. Behav. Brain Res. 2008;194:236–241. doi: 10.1016/j.bbr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Feeney D.M. De Smet A.M. Rai S. Noradrenergic modulation of hemiplegia: facilitation and maintenance of recovery. Restor. Neurol. Neurosci. 2004;22:175–190. [PubMed] [Google Scholar]
- Feeney D.M. Gonzalez A. Law W.A. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Feeney D.M. Gonzales A. Law W.A. Amphetamine restores locomotor function after motor cortex injury in the rat. Proc. West Pharmacol. Soc. 1981;24:15–17. [PubMed] [Google Scholar]
- Feeney D.M. Hovda D.A. Amphetamine and apomorphine restore tactile placing after motor cortex injury in the cat. Psychopharmacology (Berl.) 1983;79:67–71. doi: 10.1007/BF00433018. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G. Barkhuysen S. Buckle M. Fawcett J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Gilmour G. Iversen S.D. O'Neill MF. O'Neill M.J. Ward M.A. Bannerman D.M. Amphetamine promotes task-dependent recovery following focal cortical ischaemic lesions in the rat. Behav. Brain Res. 2005;165:98–109. doi: 10.1016/j.bbr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Girgis J. Merrett D. Kirkland S. Metz G.A. Verge V. Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Gladstone D.J. Danells C.J. Armesto A. McIlroy W.E. Staines W.R. Graham S.J. Herrmann N. Szalai J.P. Black S.E. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37:179–185. doi: 10.1161/01.STR.0000195169.42447.78. [DOI] [PubMed] [Google Scholar]
- Goldstein L.B. Effects of amphetamines and small related molecules on recovery after stroke in animals and man. Neuropharmacology. 2000;39:852–859. doi: 10.1016/s0028-3908(99)00249-x. [DOI] [PubMed] [Google Scholar]
- Grill R. Murai K. Blesch A. Gage F.H. Tuszynski M.H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle J.D. Jin Y. Chronically injured supraspinal neurons exhibit only modest axonal dieback in response to a cervical hemisection lesion. Exp. Neurol. 2001;169:208–217. doi: 10.1006/exnr.2001.7645. [DOI] [PubMed] [Google Scholar]
- Houle J.D. Morris K. Skinner R.D. Garcia-Rill E. Peterson C.A. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hovda D.A. Fenney D.M. Amphetamine with experience promotes recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res. 1984;298:358–361. doi: 10.1016/0006-8993(84)91437-9. [DOI] [PubMed] [Google Scholar]
- Hurwitz B.E. Dietrich W.D. McCabe P.M. Alonso O. Watson B.D. Ginsberg M.D. Schneiderman N. Amphetamine promotes recovery from sensory-motor integration deficit after thrombotic infarction of the primary somatosensory rat cortex. Stroke. 1991;22:648–654. doi: 10.1161/01.str.22.5.648. [DOI] [PubMed] [Google Scholar]
- Kim B.G. Dai H.N. McAtee M. Bregman B.S. Modulation of dendritic spine remodeling in the motor cortex following spinal cord injury: effects of environmental enrichment and combinatorial treatment with transplants and neurotrophin-3. J. Comp. Neurol. 2008;508:473–486. doi: 10.1002/cne.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knieling M. Metz G.A. Antonow-Schlorke I. Witte O.W. Enriched environment promotes efficiency of compensatory movements after cerebral ischemia in rats. Neuroscience. 2009;163:759–769. doi: 10.1016/j.neuroscience.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Liu Y. Kim D. Himes B.T. Chow S.Y. Schallert T. Murray M. Tessler A. Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J. Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson L. Eksborg S. Drugs for stroke recovery: the example of amphetamines. Drugs Aging. 2004;21:67–79. doi: 10.2165/00002512-200421020-00001. [DOI] [PubMed] [Google Scholar]
- McKenna J.E. Prusky G.T. Whishaw I.Q. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J. Comp. Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McKenna J.E. Whishaw I.Q. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J. Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Halle A. McMillan D.E. Laurenzana E.M. Byrnes-Blake K.A. Owens S.M. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya C.P. Campbell-Hope L.J. Pemberton K.D. Dunnett S.B. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Moroz I.A. Pecina S. Schallert T. Stewart J. Sparing of behavior and basal extracellular dopamine after 6-hydroxydopamine lesions of the nigrostriatal pathway in rats exposed to a prelesion sensitizing regimen of amphetamine. Exp. Neurol. 2004;189:78–93. doi: 10.1016/j.expneurol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. University of Alabama; Birmingham: 2008. [Google Scholar]
- Papadopoulos C.M. Tsai S.Y. Guillen V. Ortega J. Kartje G.L. Wolf W.A. Motor recovery and axonal plasticity with short-term amphetamine after stroke. Stroke. 2009;40:294–302. doi: 10.1161/STROKEAHA.108.519769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramic M. Emerick A.J. Bollnow M.R. O'Brien T.E. Tsai S.Y. Kartje G.L. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Rasmussen R.S. Overgaard K. Hildebrandt-Eriksen E.S. Boysen G. D-amphetamine improves cognitive deficits and physical therapy promotes fine motor rehabilitation in a rat embolic stroke model. Acta Neurol. Scand. 2006;113:189–198. doi: 10.1111/j.1600-0404.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- Sandrow-Feinberg H.R. Izzi J. Shumsky J.S. Zhukareva V. Houle J.D. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma. 2009;26:721–731. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrow-Feinberg H.R. Zhukareva V. Santi L. Miller K. Shumsky J.S. Baker D.P. Houle J.D. PEGylated interferon-beta modulates the acute inflammatory response and recovery when combined with forced exercise following cervical spinal contusion injury. Exp Neurol. 2010;223:439–451. doi: 10.1016/j.expneurol.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrow H.R. Shumsky J.S. Amin A. Houle J.D. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp. Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T. Leasure J.L. Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J. Cereb. Blood Flow Metab. 2000;20:1513–1528. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Schwab J.M. Brechtel K. Mueller C.A. Failli V. Kaps H.P. Tuli S.K. Schluesener H.J. Experimental strategies to promote spinal cord regeneration—an integrative perspective. Prog. Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Sonde L. Nordstrom M. Nilsson C.G. Lokk J. Viitanen M. A double-blind placebo-controlled study of the effects of amphetamine and physiotherapy after stroke. Cerebrovasc. Dis. 2001;12:253–257. doi: 10.1159/000047712. [DOI] [PubMed] [Google Scholar]
- Stackhouse S.K. Murray M. Shumsky J.S. Effect of cervical dorsolateral funiculotomy on reach-to-grasp function in the rat. J. Neurotrauma. 2008;25:1039–1047. doi: 10.1089/neu.2007.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer R.P. Kent T.A. Hulsebosch C.E. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–2393. doi: 10.1161/01.str.29.11.2381. discussion 2393–2385. [DOI] [PubMed] [Google Scholar]
- Sutton R.L. Hovda D.A. Feeney D.M. Amphetamine accelerates recovery of locomotor function following bilateral frontal cortex ablation in cats. Behav. Neurosci. 1989;103:837–841. doi: 10.1037//0735-7044.103.4.837. [DOI] [PubMed] [Google Scholar]
- Thuret S. Moon L.D. Gage F.H. Therapeutic interventions after spinal cord injury. Nature Rev. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Treig T. Werner C. Sachse M. Hesse S. No benefit from D-amphetamine when added to physiotherapy after stroke: a randomized, placebo-controlled study. Clin. Rehabil. 2003;17:590–599. doi: 10.1191/0269215503cr653oa. [DOI] [PubMed] [Google Scholar]
- Vasilev V. Veskov R. Janac B. Rakic L. Stojiljkovic M. Age-related differences in MK-801- and amphetamine-induced locomotor and stereotypic activities of rats. Neurobiol. Aging. 2003;24:715–723. doi: 10.1016/s0197-4580(02)00232-4. [DOI] [PubMed] [Google Scholar]
- Walker-Batson D. Smith P. Curtis S. Unwin H. Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Further evidence. Stroke. 1995;26:2254–2259. doi: 10.1161/01.str.26.12.2254. [DOI] [PubMed] [Google Scholar]
- Wall S.C. Gu H. Rudnick G. Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol. Pharmacol. 1995;47:544–550. [PubMed] [Google Scholar]
- Weidner N. Ner A. Salimi N. Tuszynski M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw I.Q. Gorny B. Foroud A. Kleim J.A. Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav. Brain Res. 2003;145:221–232. doi: 10.1016/s0166-4328(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Gorny B. Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav. Brain Res. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Pellis S.M. Gorny B. Kolb B. Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 1993;56:59–76. doi: 10.1016/0166-4328(93)90022-i. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Pellis S.M. Pellis V.C. A behavioral study of the contributions of cells and fibers of passage in the red nucleus of the rat to postural righting, skilled movements, and learning. Behav. Brain Res. 1992;52:29–44. doi: 10.1016/s0166-4328(05)80322-5. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Pellis S.M. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav. Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]