Abstract
The activation of oxidative damage, neuroinflammation, and mitochondrial dysfunction has been implicated in secondary pathomechanisms following spinal cord injury (SCI). These pathophysiological processes lead to cell death and are tightly regulated by nuclear factor E2-related factor 2/antioxidant response element (Nrf2/ARE) signaling. Here, we investigated whether activation of Nrf2/ARE is neuroprotective following SCI. Female Fischer rats were subjected to mild thoracic SCI (T8) using the New York University injury device. As early as 30 min after SCI, levels of Nrf2 transcription factor were increased in both nuclear and cytoplasmic fractions of neurons and astrocytes at the lesion site and remained elevated for 3 days. Treatment of injured rats with sulforaphane, an activator of Nrf2/ARE signaling, significantly increased levels of Nrf2 and glutamate-cysteine ligase (GCL), a rate-limiting enzyme for synthesis of glutathione, and decreased levels of inflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) thus leading to a reduction in contusion volume and improvement in coordination. These results show that activation of the Nrf2/ARE pathway following SCI is neuroprotective and that sulforaphane is a viable compound for neurotherapeutic intervention in blocking pathomechanisms following SCI.
Key words: antioxidant response element, inflammation, neuroprotection, nuclear factor E2-related factor 2, oxidative stress, SCI
Introduction
Spinal cord injury (SCI) induces a multitude of deleterious processes, including oxidative stress, neuroinflammation, and mitochondrial dysfunction that contribute to neuronal damage and death. The pharmacological treatment of SCI has not advanced and there are no Federal Drug Administration-approved therapies to improve the long-term prognosis of SCI patients. Therefore, it is critical to develop therapeutic strategies that act on multiple pathways to effectively block the death of neurons.
The nuclear factor E2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway presents a viable target for neurotherapeutic intervention in neurodegeneration following SCI. The transcription factor Nrf2 was identified based on its role in the coordinated induction of a battery of cytoprotective genes, including those that encode antioxidant and anti-inflammatory proteins (Lee and Johnson, 2004). Nrf2 belongs to the basic leucine zipper transcription factor family characterized by a cap ‘n’ collar structure (Li and Kong, 2009). Under normal conditions, Nrf2 is sequestered in the cytoplasm via an interaction with the actin-binding protein Kelch-like ECH-associated protein 1 (Keap1) (Zhao et al., 2007a). The induction of neuroprotective genes depends upon the nuclear translocation of Nrf2 and binding to ARE, which is a cis-acting regulatory element or enhancer sequence that is present in promoter regions of genes encoding phase II detoxification enzymes and antioxidant proteins. It has been suggested that oxidation of a critical cysteine in Keap 1 releases Nrf2 to translocate into the nucleus. Nrf2 regulates >200 genes, which synergistically increase the efficiency of cellular defense systems (Lee and Johnson, 2004; Vargas et al., 2008).
Because the Nrf2/ARE signaling pathway acts as a master regulator of many protective genes, it may serve as a therapeutic target for neurodegenerative conditions involving oxidative stress, including SCI. Here, we demonstrate that SCI induces activation of the Nrf2/ARE signaling cascade. Administration of sulforaphane, a natural stimulus of Nrf2 derived from cruciferous vegetables (e.g., broccoli or cauliflower) to injured rats had a neuroprotective effect, and enhanced Nrf2 expression and phase II detoxification enzyme production, while attenuating inflammatory cytokine production leading to tissue sparing and improvement in coordination.
Methods
SCI
Adult female Fischer rats (180–200g) were used in these studies. The rat model of moderate contusive SCI used, was as described (Keane et al., 2001) with minor modifications. Briefly, animals were anesthetized with isoflurane (2%), and then a laminectomy was performed at vertebral level T8 and the cord was exposed without disrupting the dura. A moderate injury was induced using the New York University weight drop device (10 g, 12.5 mm). After injury, muscles were closed in layers, and the incision was closed with wound clips. Rats were returned to their cages and placed on computer-controlled warmed blankets, with access to water and food ad libitum. Gentamycin (5 mg/kg i.m.) was given once a day for a week following surgery to control infection, whereas buprenorphine (0.01 mg/kg s.c.) was given twice a day for 3 days after injury to relieve pain. The rats' bladders were manually voided twice a day until they were able to regain normal bladder function. Rats were killed at different times after SCI. Sham rats were used as control. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami.
Sulforaphane delivery
Sulforaphane was purchased from LKT Laboratories Inc (St. Paul, MN), and dissolved in dimethyl sulfoxide (DMSO; 1 mg/mL). A dose of 5 mg/kg sulforaphane was injected intraperitoneally 15 min after injury, once a day for 3 days following injury for histological analysis, and once at 1 day for biochemical, immunoblotting, and immunohistochemical analysis. DMSO alone was used as vehicle control.
Immunoblotting procedures
T7–T9 spinal cords were harvested at different time points after injury. Rats were deeply anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg). T7–T9 spinal cords were cut quickly, removed, and stored in liquid nitrogen until use. Spinal cords were cut sagitally into two halves at the epicenter of T8. One half of each cord was homogenized in lysis buffer (20 mM Tris–HCl, pH: 7.5, 150mM NaCl, 1% Triton X-100, 1mM ethylenediaminetetraacetic acid, 1mM ethylene glycol tetraacetic acid, 2.5mM pyrophosphate, 1mM β-glycerophosphate) with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The other half of the cord was used for isolation of nuclear and cytoplasmic fractions using an extraction kit (Thermos Scientific, Rockford, lL) according to the manufacturer's instructions. Purity of nuclear and cytoplasmic extractions was confirmed by immunoblotting using primary antibodies to Lamin B1 (Abcam, Cambridge, MA). Equal amounts of protein (25 μg) were loaded onto 10–20% Tris-HCl Criterion pre-casted gels (Bio-Rad, Richmond, CA), transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were incubated for 1 h with primary antibodies diluted in blocking buffer, 1:1250 rabbit anti-Nrf2 (Abcam, Cambridge, MA), 1:1250 rabbit anti-heme oxygenase-1 (HO1) (Abcam), 1:500 rabbit anti-catalytic subunit of glutamate-cysteine ligase (GCLC) (Abcam), 1:1250 mouse anti-NAD(P)H:quinone oxidoreductase-1 (NQO1) (Abcam), 1:1000 rabbit anti-cleaved interleukin-1β (IL-1β) (Cell Signaling, Beverly, MA), 1:1000 rabbit anti-tumor necrosis factor-α (TNF-α) (Abcam), 1:1000 rabbit anti-IkBα (Cell Signaling) and 1:1000 rabbit anti-phospho-IkBα (Cell Signaling) followed by appropriate secondary horseradish peroxidase (HRP)-linked antibodies (Cell Signaling). Visualization of signal was by enhanced chemiluminescence using a phototope-HRP detection kit (Cell Signaling). Quantification of band density was performed using the UN-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT), and data were normalized to β-actin and presented as relative density units.
Immunohistochemistry
Immunohistochemistry of spinal cord sections was done as previously described (de Rivero Vaccari et al., 2008). Briefly, after paraformaldehyde perfusion fixation, spinal cords were harvested and fixed in 4% paraformaldehyde overnight then stored in phosphate-buffered saline (PBS) containing 20% sucrose at 4°C until use. Fifty-five micrometer sections were cut using Leica SM 2000R Sliding Microtome. Sections were blocked by treatment overnight with normal goat serum (Vector Laboratories, Burlingame, CA). Tissue sections were rinsed with 0.1 M PBS, pH 7.4, and incubated overnight at 4°C with primary antibody rabbit anti-Nrf2 (Anaspec, San Jose, CA), and double stained with mouse anti-neuronal nuclei (NeuN, Millipore, Billerica, MA) (a neuron-specific marker) or mouse anti-glial fibrillary acidic protein (GFAP, Millipore, Billerica, MA). Alexa Fluor 594 goat anti-rabbit and 488 goat anti-mouse antibodies (Invitrogen, Carlsbad, CA) were used as secondary antibodies. Sections were cover-slipped with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA) and analyzed with an Olympus Fluorview 1000 confocal microscope.
Histopathological assessment
At 1 week after SCI, rats were deeply anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg) and perfused with 500 mL of physiological saline followed by 500 mL of 4% paraformaldehyde. A 10-mm segment of spinal cord encompassing the injury site was obtained, transverse sectioned at 15 μm, and then stained with hematoxylin and eosin (H&E) plus luxol fast blue for gray and white mater visualization. Sections spaced at every 1000 μm were used for lesion volume analysis by using computer-assisted microscopy, and Stereo Investigator software. In each section, the total area was first determined, and then damaged gray and white matter areas were determined, characterized by the presence of infiltrating immune cells, myelin breakdown, shrunken eosinophilic neurons, and hemorrhage as described (de Rivero Vaccari et al., 2008, 2009). The area of each section was calculated by Neurolucida software and then summated for the volumes of each spinal cord (de Rivero Vaccari et al., 2008, 2009).
Behavioral testing
Hindlimb locomotor score
Hindlimb locomotor function was evaluated by using the Basso, Beattie and Bresnahan (BBB) locomotor score (Basso et al., 1995). Each rat was evaluated before surgery and once a week for 6 weeks after surgery by two experienced examiners who were blinded to the experimental groups.
CatWalk-assisted gait analysis
At 6 weeks after SCI, gait analysis was performed by using the CatWalk 7.0 system (Hamers et al., 2006). One week prior to the analysis, rats were trained and habituated to cross the walkway by being rewarded with food pellets after each successful walking. A minimum of three correct crossings per animal without any interruption or hitch was obtained and then analyzed for stride length, base of support, and regularity index.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. One-way analysis of variance (ANOVA) followed by Tukey post-hoc comparison tests was used to compare the levels of different experimental groups. P values of significance used were: #p<0.001, *p<0.05, ##p<0.01 and **p<0.1.
Results
SCI induces activation of the Nrf2/ARE pathway in the rat spinal cord
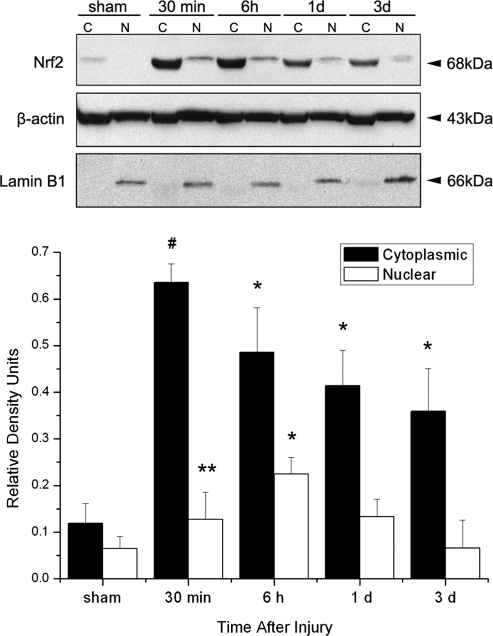
In order to determine whether SCI induced activation of the Nrf2/ARE signaling pathway, we analyzed nuclear and cytoplasmic fractions of spinal cord lysates from injured and sham-operated animals for Nrf2 expression (Fig. 1). As early as 30 min after SCI, there was a significant accumulation of the Nrf2 protein in the cytoplasmic and nuclear fractions of injured cords. The protein levels of Nrf2 in the nuclear fraction peaked at 6 h following SCI. Sham-operated animals showed no statistically significant difference in protein levels in either fraction at different time points after SCI. Therefore, SCI induces increased Nrf2 expression in the cytoplasm followed by translocation into the nucleus.
FIG. 1.
Spinal cord injury (SCI) induces activation of nuclear factor E2-related factor 2 (Nrf2) in cytoplasmic (C) and nuclear (N) fractions. Representative immunoblots showing Nrf2 levels were elevated as early as 30 min after SCI in cytoplasmic fractions and remained elevated for 3 days, whereas levels in nuclear fractions peaked at 6 h following SCI. Lamin B1 was used to assess the purity of the nuclear fraction. β-actin was used as a loading control. Data are presented as relative density units normalized to β-actin and expressed as mean±SEM. #p<0.001, *p<0.05, **p<0.1 compared to sham; n=5 per group.
SCI induces Nrf2 expression in motor neurons and astrocytes
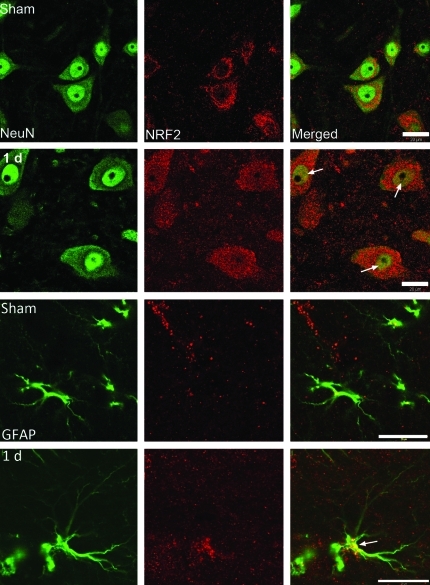
Figure 2 shows confocal images of the cell type expression and regional distribution of Nrf2 in motor neurons and GFAP-containing astrocytes in the ventral horn of sham and injured spinal cords at 1 day after injury. Sections were stained for Nrf2 (red), the neuronal marker NeuN (green) and the astrocyte marker GFAP (green). Nrf2 immunoreactivity was seen in the cytoplasm and cell processes in neurons in sham animals. Moderate SCI resulted in increased immunoreactivity of Nrf2 in the nucleus of motor neurons (arrows). Additionally, increased Nrf2 immunoreactivity was observed in astrocytes (arrows) at 1 day after injury, suggesting activation of the Nrf2 pathway after SCI and further demonstrating translocation of the transcription factor from the cytoplasm to the nuclear compartment after trauma.
FIG. 2.
Nrf2 immunoreactivity increases after spinal cord injury (SCI) in motor neurons and astrocytes of the spinal cord. Confocal images of frozen sections of the ventral horn stained with anti-nuclear factor E2-related factor 2 (Nrf2), the neuronal marker anti-NeuN and the astrocyte marker glial fibrillary acidic protein (GFAP). In sham sections of the spinal cord, Nrf2 is present mainly in the cytoplasm. One day after SCI, increased Nrf2 staining was observed both in the cytoplasm and nucleus (arrows) of motor neurons and astrocytes. Bar=20 μm.
SCI induces expression of Nrf2/ARE regulated phase II detoxifying enzymes
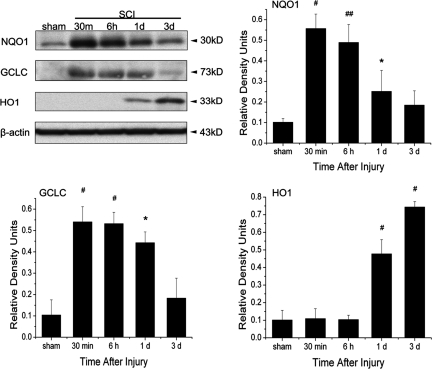
Next, we measured the expression of downstream proteins in the antioxidative and anti-inflammatory pathways regulated by Nrf2/ARE (Fig. 3). These include proteins with an antioxidative function, NQO1, a glutathione generating enzyme such as GCLC, a rate-limiting enzyme for synthesis of glutathione (GSH), and an anti-inflammatory function as HO1. By 30 min after SCI, significant increases in the levels of NQO1 and GCLC were present in spinal cord lysates from injured animals compared to sham. By 1 day after injury, there was a significant increase in the levels of HO1 and the level continued to increase by 3 days post-trauma. Therefore, SCI induces expression of phase II detoxifying enzymes via Nrf2.
FIG. 3.
Spinal cord injury (SCI) induces increased protein levels of the Nrf2/ARE downstream signaling proteins NQO1, GCLC, and HO1. Representative immunoblots indicate that levels of NQO1 and GCLC were elevated 30 min after SCI and remained elevated for 3 days, whereas protein levels of HO1 increased at 1 and 3 days following SCI. β-actin was used as a loading control and data are presented as relative density units normalized to β-actin. Data are expressed as mean±SEM. #p<0.001, *p<0.05, ##p<0.01 compared to sham; n=5 per group.
Sulforaphane treatment increases the expression of Nrf2 and decreases neuroinflammation after SCI
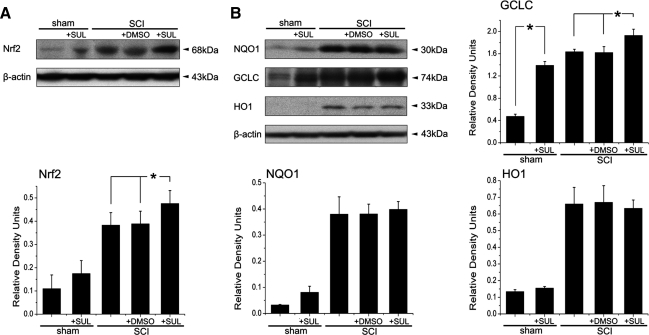
The activation of antioxidant genes and suppression of neuroinflammatory genes via the Nrf2/ARE signaling pathway can potentially have a major effect in blocking or slowing neurodegenerative processes induced by SCI. Sulforaphane is a small molecule inducer of Nrf2. To test whether sulforaphane alters the expression of Nrf2 in the spinal cord after SCI, a dose of 5 mg/kg sulforaphane was injected intraperitoneally immediately after SCI. One day after SCI, cords containing the epicenter were harvested and immunoblotted for expression of GCLC, NQO1 and HO1 (Fig. 4). Sulforaphane treatment significantly increased the expression of Nrf2 in spinal cord lysates 1 day after SCI (Fig. 4A). Moreover, sulforaphane treatment also resulted in significant increases in the levels of GCLC at 1 day after SCI, but no significant increases were measured in the levels of NQO1 and HO1 (Fig. 4B).
FIG. 4.
Sulforaphane (SUL) increases protein expression of Nrf2 and downstream signaling intermediates 1 day after spinal cord injury (SCI). A dose of 5 mg/kg SUL was injected intraperitoneally 15 min after injury and cords were harvested 1 day later. Representative immunoblots of injured cord lysates from animals subjected to SCI and treated with SUL at 1 day after SCI are shown. SUL increased expression of Nrf2 (A) and the downstream signaling component GCLC but not NQO1 and HO1 (B). Data are expressed as mean±SEM. *p<0.05. Dimethyl sulfoxide (DMSO) was used as a treatment control and β-actin was used as a loading control; n=5 per group.
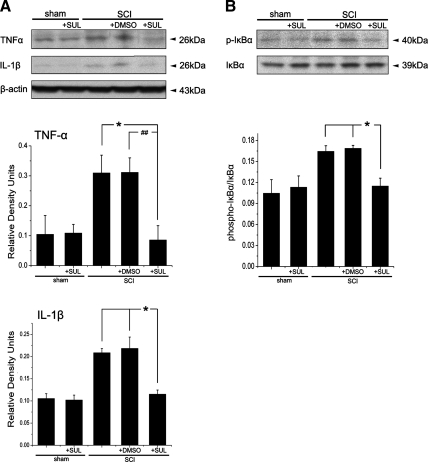
We next evaluated whether sulforaphane treatment altered expression of pro-inflammatory cytokines, TNF-α and IL-1β. As shown in Figure 5, administration of sulforaphane immediately after SCI completely reversed the increase in levels of TNF-α and IL-1β as compared to animals treated with vehicle (DMSO) alone. Because activation of Nrf2/ARE downregulates nuclear factor-κB (NF-κB), the transcription factor regulating TNF-α and IL-1β, we analyzed whether sulforaphane decreased NF-κB activation as measured by phosphorylation of IκBα (Fig. 5B). Decreased levels of IκBα phosphorylation were observed in spinal cord lysates from injured animals treated with sulforaphane compared to those receiving vehicle alone. Therefore, it appears that sulforaphane treatment not only increases expression of antioxidant proteins, but it also blocks expression of pro-inflammatory cytokines via influencing the activation of the NF-κB signaling pathway.
FIG. 5.
Sulforaphane (SUL) decreases pro-inflammatory cytokine expression of tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β) (A) and decreases phosphorylation of IκBα (B) 1 day after spinal cord injury (SCI). Data are expressed as mean±SEM. *p<0.05, ##p<0.01. Dimethyl sulfoxide (DMSO) was used as a treatment control and β-actin was used as a loading control; n=5 per group.
Sulforaphane treatment decreases spinal cord lesion volume
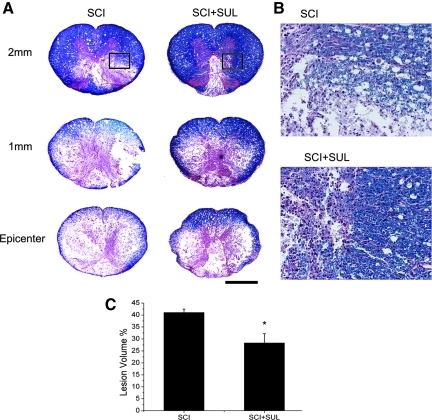
To determine whether sulforaphane treatment resulted in tissue sparing after SCI, we measured the lesion volume of sulforaphane-treated and untreated animals at 1 week after injury. Figure 6A shows representative spinal cord sections of the lesion epicenter and areas rostral to the impact site at 1 week after trauma. Spinal cords from animals treated with sulforaphane demonstrated smaller areas containing shrunken neurons in gray matter and reduced white matter degeneration (Figure 6B). Importantly, administration of sulforaphane significantly reduced the lesion volume by ∼15% as determined by diminished white matter degeneration and preservation of motor neuron morphology (SCI, 41.07±1.40%; SCI+SUL, 28.34±3.86%, p=0.011, Fig. 6C).
FIG. 6.
Sulforaphane (SUL) improves histopathological outcomes and decreases spinal cord lesion volume. (A) Representative cross sections of spinal cords stained with hematoxylin-eosin and luxol fast blue of sulforaphane-treated and untreated animals at 1 week after SCI. Sections correspond to the injury epicenter and sites 1 and 2 mm rostral to the epicenter. (B) Enlarged area corresponding to inset in (A) demonstrates that animals treated with SUL had smaller areas of shrunken neurons in gray matter and reduced white matter degeneration. (C) Areas of degeneration were quantified for lesion volume by Neurolucida software. Lesion volume analysis indicates that SUL-treated animals have lower lesion volume when compared to control groups. Data are expressed as mean±SEM; *p<0 .05, n=6 per group. Bar=50μm.
Sulforaphane treatment significantly improved coordination after SCI
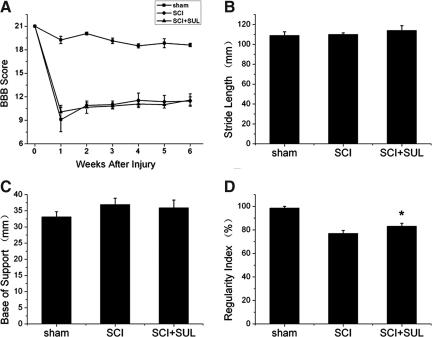
To investigate the long-term consequences of sulforaphane treatment, we conducted tests that evaluated locomotor performance using the BBB test, and gait analysis using the CatWalk-assisted gait test (Fig. 7). Although sulforaphane treatment did not significantly improve performance in BBB, stride length, or base of support, this treatment strategy did result in a significant improvement in the regularity index (sham, 98.43±1.53; SCI, 76.97±2.55; SCI+SUL, 83.10±2.47, *p=0.028 SCI vs. SCI+SUL means *p<0.05), which evaluates the degree of coordination in quadruped animals.
FIG. 7.
Sulforaphane (SUL) improves coordination (regularity index) at 7 weeks following SCI. A person blinded to the treatment protocols assessed behavioral outcomes following SUL-treatment with the following tests: Basso, Beattie and Bresnahan (BBB) locomotor score (A), stride length (B), base of support (C), and regularity index (coordination, D). SUL-treated animals have a higher regularity index than SCI animals that did not receive SUL. Data are expressed as mean±SEM. *p<0.05, compared to sham, n=10 per group.
Discussion
In this study, we show a neuroprotective role for sulforaphane, a natural compound derived from cruciferous vegetables, in determining outcomes after moderate thoracic SCI in the rat. Our data show that SCI initiates the activation of the Nrf2/ARE signaling pathway that regulates genes involved in detoxification and antioxidant defenses, collectively termed the “phase II detoxifying enzymes”. Sulforaphane treatment after SCI further enhanced expression of the Nrf2/ARE pathway resulting in increased expression of the glutathione-generating enzyme GCLC, and the reduced expression of inflammatory cytokines TNF-α and IL-1β, leading to significant improvement in tissue sparing and beneficial effects in coordination. Thus, the Nrf2/ARE signaling pathway presents a viable target for enhancement of neuroprotective mechanisms following SCI.
The induction of Nrf2/ARE protective genes is dependent upon the nuclear translocation of Nrf2 and binding to ARE, a regulatory sequence that is present in the promoter region of these genes (Liu et al., 2008). Our results are in agreement with this idea, and show that SCI induces increased expression of Nrf2 in the cytoplasm of neurons and astrocytes, as early as 30 min following injury, and by 6 h there was a significant increase in the expression of Nrf2 in the nuclear compartment. In recent years, small molecular inducers of Nrf2, including sulforaphane, which coordinately upregulate proteins in several lines of cellular defense: 1) oxidative scavenging of ROS, 2) neutralization of electrophiles and xenobiotics by glucuronidation and glutathione conjugation, and 3) nicotinamide adenine dinucleotide phosphate (NADPH)-generating metabolic pathways, have been well characterized (Alam et al., 1999; Ishii et al., 2000). Moreover, studies using Nrf2 gene knockout mice and cells show that sulforaphane does not provide protection against these diverse cellular defenses (Innamorato et al., 2008; Lin et al., 2008), thus demonstrating that sulforaphane is selective for the Nrf2 pathway. However, our results do not discern which substances in the injured spinal cord act as transcriptional stimulators of the Nrf2/ARE signaling pathway, but suggest cellular defense mechanisms involved in resistance to oxidative stress.
The function of Nrf2 and its downstream target genes is important for protection against oxidative stress or chemical-induced cellular damage. Numerous studies using Nrf2 knockout mice have shown that decreased levels of phase II detoxification enzymes and antioxidant proteins render Nrf2 knockout mice highly susceptible to cytotoxic compounds compared to wild-type mice (Aoki et al., 2001; Chan and Kan, 1999; Enomoto et al., 2001; Mao et al., 2010; Ramos-Gomez et al., 2001). Nrf2 has neuroprotective functions against oxidative stress in neurons (Khodagholi et al., 2010; Soane et al., 2010), astrocytes (Reddy et al., 2010; Vargas et al., 2005) and microglia (Brandenburg et al., 2010). Moreover, astrocytes express higher levels of Nrf2 and downstream signaling intermediates than neurons. Primary astrocytes and neurons derived from Nrf2 knockout mice are more susceptible to oxidative stress induced by H2O2 or mitochondrial inhibitors (Shih et al., 2003). Moreover, overexpression of Nrf2 protects cells from Fas-induced apoptosis suggesting an important role of Nrf2 in anti-apoptotic pathways (Kotlo et al., 2003). Activation of the Nrf2/ARE pathway has been shown to be neuroprotective following several types of acute insults to the central nervous system (CNS), such as intracerebral hemorrhage (Wang et al., 2007; Zhao et al., 2007b, 2009), focal cerebral ischemia (Zhao et al., 2006) and traumatic brain injury (TBI) (Jin et al., 2009; Yan et al., 2008; Zhao et al., 2007a). Collectively, these observations suggest that Nrf2/ARE signaling plays an important role in cellular survival by modulating both cellular antioxidant potential and apoptosis pathway. Because SCI induces oxidative stress that contributes to progression of secondary injury pathomechanisms (Liu et al., 2002), activation of the Nrf2/ARE pathway may reduce oxidative stress leading to neuroprotection.
The ability of Nrf2 to attenuate inflammatory cytokines such as IL-1β and TNF-α has been suggested to be regulated by inactivation of NF-κB (Li et al., 2008; Peng et al., 2010). However, recent experiments suggest that intracellular levels of GSH influence NF-κB activation (Lian et al., 2010). Cellular GSH homeostasis is maintained by the rate of GSH synthesis in which GCL (also known as γ-glutamylcysteine synthetase) works as a first step and time-limiting enzyme in GSH synthesis (Lu, 2009). Thus, the intracellular redox status maintained by GSH may influence the activity of the redox-sensitive NF-κB transcription factor (Heiss et al., 2001). Moreover, the relative levels of GCL subunits are a major determinant of cellular GCL activity and are highly regulated at the transcriptional and post-transcriptional level in response to oxidative stress. The GCL subunits are often coordinately induced in response to oxidative stress, but distinct transcriptional and post-transcriptional mechanisms mediate their differential rates and levels of induction (Franklin et al., 2009). In addition, NQO1 expression is also regulated at the post-transcriptional level (Tsvetkov et al., 2011). Our findings show that sulforaphane treatment enhanced the expression of Nrf2 and GCLC in the spinal cord at 1 day after SCI, which may inhibit IκBα phosphorylation leading to decreased production of NFκB-dependent inflammatory cytokine IL-1β and TNF-α production. In previous work, we also found that Nrf2 depresses inflammatory cytokines production, including TNF-α, IL-1β, interleukin-6 (IL-6), and intercellular adhesion molecule 1 (ICAM-1) via depressing activation of NF-κB in a TBI model in mice (Jin et al., 2008a,b). Moreover, significant levels of IL-1β regulated by inflammasomes in CNS cells contribute to inflammatory pathomechanisms after SCI (de Rivero Vaccari et al., 2008). Therefore, further studies are needed to evaluate the interactions among the Nrf2/ARE, NF-κB, and inflammasome signaling pathways and to determine the post-transcriptional modifications that regulate downstream signal proteins after SCI.
Increased sparing of white and gray matter paralleled the improvement in coordination after sulforaphane treatment. However, we did not observe significant improvements in the BBB score, base of support, or stride length. Because sulforaphane was administered once a day for 3 days following SCI and its half-life is ∼2 h (Pledgie-Tracy et al., 2007), it is possible that alterations in dosage or timing of drug administration would result in a more robust improvement in functional outcomes. We propose that sulforaphane enhances expression of phase II detoxifying enzymes and decreases pro-inflammatory cytokine production, thus inhibiting cell death. Substances that coordinately upregulate enzymes involved in several important lines of cellular defense may have the potential to mitigate neuronal death caused by oxidative stress following SCI, stroke, or even neurodegenerative disease.
Acknowledgments
These studies were supported by National Institutes of Health grant NS059836 and Craig Neilsen Foundation 124671 to R.W.K., grant from Jinling Hospital of China (2009M014), and grant from China Scholarship Council (2009619088). We thank Frank Brand III, Geoffrey Yurcisin, Denise Koivisto, and Ileana Oropesa for technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Alam J. Stewart D. Touchard C. Boinapally S. Choi A.M. Cook J.L. Nrf2, a Cap‘n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26,071–26,078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Aoki Y. Sato H. Nishimura N. Takahashi S. Itoh K. Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Brandenburg L.O. Kipp M. Lucius R. Pufe T. Wruck C.J. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res. 2010;59:443–450. doi: 10.1007/s00011-009-0116-5. [DOI] [PubMed] [Google Scholar]
- Chan K. Kan Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12,731–12,736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari J.P. Lotocki G. Marcillo A.E. Dietrich W.D. Keane R.W. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari J.P. Marcillo A. Nonner D. Dietrich W.D. Keane R.W. Neuroprotective effects of bone morphogenetic protein 7 (BMP7) treatment after spinal cord injury. Neurosci. Lett. 2009;465:226–229. doi: 10.1016/j.neulet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Enomoto A. Itoh K. Nagayoshi E. Haruta J. Kimura T. O'Connor T. Harada T. Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Franklin C.C. Backos D.S. Mohar I. White C.C. Forman H.J. Kavanagh T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers F.P. Koopmans G.C. Joosten E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Heiss E. Herhaus C. Klimo K. Bartsch H. Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001;276:32,008–32,015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Innamorato N.G. Rojo A.I. Garcia–Yague A.J. Yamamoto M. de Ceballos M.L. Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Ishii T. Itoh K. Takahashi S. Sato H. Yanagawa T. Katoh Y. Bannai S. Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16,023–16,029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Jin W. Wang H. Yan W. Xu L. Wang X. Zhao X. Yang X. Chen G. Ji Y. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008a;725:174–180. doi: 10.1155/2008/725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W. Wang H. Yan W. Zhu L. Hu Z. Ding Y. Tang K. Role of Nrf2 in protection against traumatic brain injury in mice. J. Neurotrauma. 2009;26:131–139. doi: 10.1089/neu.2008.0655. [DOI] [PubMed] [Google Scholar]
- Jin W. Zhu L. Guan Q. Chen G. Wang Q.F. Yin H.X. Hang C.H. Shi J.X. Wang H.D. Influence of Nrf2 genotype on pulmonary NF–kappaB activity and inflammatory response after traumatic brain injury. Ann. Clin. Lab. Sci. 2008b;38:221–227. [PubMed] [Google Scholar]
- Keane R.W. Kraydieh S. Lotocki G. Bethea J.R. Krajewski S. Reed J.C. Dietrich W.D. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J. Neuropathol. Exp. Neurol. 2001;60:422–429. doi: 10.1093/jnen/60.5.422. [DOI] [PubMed] [Google Scholar]
- Khodagholi F. Eftekharzadeh B. Maghsoudi N. Rezaei P.F. Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaB. Mol. Cell. Biochem. 2010;337:39–51. doi: 10.1007/s11010-009-0284-1. [DOI] [PubMed] [Google Scholar]
- Kotlo K.U. Yehiely F. Efimova E. Harasty H. Hesabi B. Shchors K. Einat P. Rozen A. Berent E. Deiss L.P. Nrf2 is an inhibitor of the Fas pathway as identified by Achilles' Heel Method, a new function-based approach to gene identification in human cells. Oncogene. 2003;22:797–806. doi: 10.1038/sj.onc.1206077. [DOI] [PubMed] [Google Scholar]
- Lee J.M. Johnson J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Li W. Kong A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Khor T.O. Xu C. Shen G. Jeong W.S. Yu S. Kong A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian K.C. Chuang J.J. Hsieh C.W. Wung B.S. Huang G.D. Jian T.Y. Sun Y.W. Dual mechanisms of NF-kappaB inhibition in carnosol-treated endothelial cells. Toxicol. Appl. Pharmacol. 2010;245:21–35. doi: 10.1016/j.taap.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Lin W. Wu R.T. Wu T. Khor T.O. Wang H. Kong A.N. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008;76:967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.Y. Li C.Y. Bu H. Li Z. Sun M. M. Guo Y.S. Zhang L. Ren W.B. Fan Z.L. Wu D.X. Wu S.Y. The neuroprotective potential of phase Ii enzyme inducer on motor neuron survival in traumatic spinal cord injury in vitro. Cell Mol. Neurobiol. 2008;28:769–779. doi: 10.1007/s10571-007-9219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Tachibana T. Dai Y. Kondo E. Fukuoka T. Yamanaka H. Noguchi K. Heme oxygenase-1 expression after spinal cord injury: the induction in activated neutrophils. J. Neurotrauma. 2002;19:479–490. doi: 10.1089/08977150252932424. [DOI] [PubMed] [Google Scholar]
- Lu S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L. Wang H. Qiao L. Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010;238:321–331. doi: 10.1155/2010/238321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z. Geh E. Chen L. Meng Q. Fan Y. Sartor M. Shertzer H.G. Liu Z.G. Puga A. Xia Y. Inhibitor of kappaB kinase beta regulates redox homeostasis by controlling the constitutive levels of glutathione. Mol. Pharmacol. 2010;77:784–792. doi: 10.1124/mol.109.061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledgie–Tracy A. Sobolewski M.D. Davidson N.E. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol. Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Ramos–Gomez M. Kwak M.K. Dolan P.M. Itoh K. Yamamoto M. Talalay P. Kensler T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.V. Lungu G. Kuang X. Stoica G. Wong P.K. Neuroprotective effects of the drug GVT (monosodium luminol) are mediated by the stabilization of Nrf2 in astrocytes. Neurochem. Int. 2010;56:780–788. doi: 10.1016/j.neuint.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A.Y. Johnson D.A. Wong G. Kraft A.D. Jiang L. Erb H. Johnson J.A. Murphy T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane L. Li Dai W. Fiskum G. Bambrick L.L. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J. Neurosci. Res. 2010;88:1355–1363. doi: 10.1002/jnr.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov P. Adamovich Y. Elliot E. Shaul Y. E3 ligase STUB/CHIP regulates NAD(P)H:quinone oxidoreductase 1 (NQO1) accumulation in aged brain, a process impaired in certain Alzheimer disease patients. J. Biol. Chem. 2011;286:8839–8845. doi: 10.1074/jbc.M110.193276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M.R. Johnson D.A. Sirkis D.W. Messing A. Johnson J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008;28:13,574–13,581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M.R. Pehar M. Cassina P. Marinez-Palma L. Thompson J.A. Beckman J.S. Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J. Biol. Chem. 2005;280:25,571–25,579. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- Wang J. Fields J. Zhao C. Langer J. Thimmulappa R.K. Kensler T.W. Yamamoto M. Biswal S. Dore S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic. Biol. Med. 2007;43:408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W. Wang H.D. Hu Z.G. Wang Q.F. Yin H.X. Activation of Nrf2-ARE pathway in brain after traumatic brain injury. Neurosci. Lett. 2008;431:150–154. doi: 10.1016/j.neulet.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Zhao J. Kobori N. Aronowski J. Dash P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Zhao J. Moore A.N. Redell J.B. Dash P.K. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 2007a;27:10,240–10,248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Song S. Sun G. Strong R. Zhang J. Grotta J.C. Aronowski J. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J. Neurosci. 2009;29:15,819–15,827. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Sun G. Zhang J. Strong R. Dash P.K. Kan Y.W. Grotta J.C. Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007b;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]