Abstract
Pyrroloquinoline quinone (PQQ) is a water-soluble, anionic, quinonoid substance that has been established as an essential nutrient in animals. Owing to the inherent properties of PQQ as an antioxidant and redox modulator in various systems, PQQ is expected to be used in pharmacological applications in the near future. Although many recent studies have investigated its neuroprotective effects, the effect of PQQ on traumatic brain injury (TBI) has not been examined. In this study we employed Morris water maze (MWM) training, the results of which showed that PQQ led to improved behavioral performance in post-TBI animals. Considering that many experiments have suggested that β-1,4-galactosyltransferase I (β-1,4-GalT-I) and -V play significant roles in inflammation and the nervous system, in the present study we used Western blot analysis to study the effect of PQQ on the expression of β-1,4-GalT-I and -V. We found apparent expression upregulation of β-1,4-GalT-I and -V after PQQ was systemically administered. Lectin-fluorescent staining with RCA-I also revealed that PQQ contributed to expression upregulation of the galactosidase β-1 (Gal β-1), 4-galactosyltransferase N-acylsphingosine (4-GlcNAc) group in microglia and neurons of the cortex and hippocampal CA2 region. In summary, our experiment established that PQQ may play an important role in recovery post-TBI.
Key words: galactosidase β-1; β-1,4-galactosyltransferase I; 4-galactosyltransferase V; 4-galactosyltransferase N-acylsphingosine group; Morris water maze; pyrroloquinoline quinone; training; traumatic brain injury
Introduction
Pyrroloquinoline quinone (PQQ) is an anionic, water-soluble compound. It was initially isolated from cultures of methylotropic bacteria as a crystalline acetone adduct, and was proposed as a cofactor of many bacterial primary alcohol dehydrogenases (Salisbury et al., 1979). Its precise structure was identified by x-ray diffraction and confirmed by organic synthesis (Salisbury et al., 1979). PQQ has recently been discovered in various vegetables, fruits, milk, and tissues of mammals (Kumazawa et al., 1992, 1995). It has been reported that when PQQ is removed from the diet, growth impairment is observed in animals, particularly if it is prior to neonatal development (Kobayashi et al., 2006). PQQ has recently been classified as a new B vitamin (Kasahara and Kato, 2003). As an essential nutrient, PQQ has also been considered to have many other beneficial effects, such as anti-inflammatory (Hamagishi et al., 1990), hepatoprotective (Tsuchida et al., 1993), cardioprotective (Zhu et al., 2004), and antioxidative properties (Scanlon et al., 1997; He et al., 2003; Zhang et al., 2006; Nunome et al., 2008). PQQ is therefore expected to be applied pharmacologically in the near future. Many studies in recent years have shown that PQQ possesses potent neuroprotective effects. The neurotoxicity of aggregated β-amyloid (Aβ) has been considered to be a critical factor in the pathogenesis of Alzheimer's disease (AD). It can lead to neurotoxicity in AD by inducing a cascade of oxidation. It was reported that PQQ pretreatment could protect human neuroblastoma SH-SY5Y cells against Aβ-induced neurotoxicity (Zhang et al., 2009). One study established that PQQ may offer an effective therapeutic approach to Parkinson's disease (PD; Zhang et al., 2009) by preventing α-synuclein amyloid fibril formation (Kobayashi et al., 2006). Zhang and associates also investigated whether PQQ may be a beneficial neuroprotectant in stroke therapy (Zhang et al., 2006). It also has been reported that PQQ may inhibit N-methyl-D-aspartate (NMDA)-induced electrical responses and protect against NMDA-mediated neurotoxic injury (Aizenman et al., 1992; Alexandrova and Bochev, 2005). Although PQQ has exhibited neuroprotective activity in many experiments, no studies have been undertaken in relation to its effect on recovery from traumatic brain injury (TBI).
Glycosylation is regarded as one of the most important post-translational modifications, and each member of the β-1,4-galactosyltransferases (β-1,4-GalTs) may play a specific role in different tissues and cells. Glycosylational changes on neuronal surfaces have been considered a link with the development and regeneration of the mammalian nervous system, the mechanism of which involves lectins, anti-carbohydrate antibodies, and carbohydrate-specific toxins (Allendoerfer et al., 1999; Ronn et al., 2000). Nakamura and colleagues have cloned β-1,4-GalT-I, -II, and -V cDNA using mouse brain and found that both β-1,4-GalT-I and -V have different expression patterns in the development of the brain compared with that of other members of β-1,4-GalTs. During the development of mouse brain, it was found that β-1,4-GalT-I was mainly expressed in the mid-embryonic stage, and that the transcript level of β-1,4-GalT V increased after birth (Zhou et al., 1998; Nakamura et al., 2001). Both β-1,4-GalT-I and -V are thought to be involved in the biosynthesis of N-linked oligosaccharides (Furukawa and Sato, 1999). It has been shown that β-1,4-GalT-I is the major enzyme that galactosylates N-linked oligosaccharides in the mouse brain (Nakamura et al., 2001). Moreover, our previous study revealed that β-1,4-GalT-I was altered in the injured sciatic nerves of rats (Shen et al., 2002). It has been revealed that acute and chronic inflammatory responses are suppressed in β-1,4-GalT-I-deficient mice, and that neutrophil infiltration at inflammation sites is reduced (Asano et al., 2003). It has also been shown that the significant delay of skin-wound healing is concomitant with reduced leukocyte infiltration at the wound site in β-1,4-GalT-I-deficient mice (Mori et al., 2004). Our previous study reported that β-1,4-GalT-I and -V may play important roles in the regeneration of injured sciatic nerve (Yan et al., 2008). We also revealed that β-1,4-GalT-I is a key inflammation mediator in the initiation and maintenance of inflammatory reactions in spinal cord injury (SCI; Niu et al., 2008). All these findings suggest that β-1,4-GalT-I and -V play significant roles in inflammation and the nervous system. In this study, we established TBI models using mature rats to investigate the effect of PQQ on β-1,4-GalT-I and -V expression.
TBI model
All animals were kept under standardized laboratory conditions in an air-conditioned room with free access to food and water. All animal tests were conducted in accordance with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, published by the U.S. National Academy of Sciences (http://oacu.od.nih.gov/regs/index.htm), and approved by the Administration Committee of Experimental Animals, Jiangsu Province, China. A controlled cortical impact (CCI) model of TBI in the rat was utilized for the present study (Dixon et al., 1991). Male Sprague-Dawley (SD) rats were anesthetized intraperitoneally with 350 mg/kg body weight chloral hydrate. Rectal temperature was kept at 37°C with a feedback-regulated water-heating pad. A CCI device was used to induce the injury. The rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between the lambda and bregma. The second craniotomy allowed for movement of cortical tissue laterally. The dura was kept intact over the cortex. Injury was delivered by impacting the right cortex with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/sec and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer. After surgery, body temperature was monitored with a rectal probe and maintained between 37.0 and 37.5°C with a heating pad.
Experimental groups and treatment
Male SD rats (300–350 g, 8–9 weeks old) were provided by the Experimental Animal Center of Nantong University. The rats were randomly divided into five groups: a normal group (no treatment; n=6); a non-PQQ TBI group (craniotomy and TBI, but no PQQ; n=6); a TBI+PQQ 5 mg/kg per day group (craniotomy and TBI, with administration of PQQ 5 mg/kg per day; n=6); a TBI+PQQ 7 mg/kg per day group (craniotomy and TBI, with administration of PQQ 7 mg/kg per day; n=6); and a TBI+PQQ 10 mg/kg per day group (craniotomy and TBI, with administration of PQQ 10 mg/kg per day; n=6). PQQ was injected intraperitoneally for 3 days before TBI, and consecutively until termination of the Morris water maze training.
Nissl staining
In order to identify the basic neuronal structures of the cortex and hippocampal regions, a series of adjacent 6-μm-thick tissue sections were cut from each block in the coronal plane and Nissl stained. Frozen tissue sections were placed in xylene 2 or 3 times for 10 min each, and hydrated in 100% alcohol 2 times for 5 min each, 95% alcohol for 3 min, and 70% alcohol for 3 min. The sections were rinsed in tap water and then distilled water before staining in 0.1% cresyl violet solution for 3–10 min, after which they were quickly rinsed in distilled water. The sections were then differentiated in 95% ethyl alcohol for 2–30 min, examined microscopically for the best resulting sections, dehydrated in 100% alcohol 2 times for 5 min each, made transparent in xylene 2 times for 5 min each, and mounted with permanent mounting medium.
Hematoxylin and eosin (H&E) staining
Frozen tissue sections were washed using distilled water for about 5 min and stained with hematoxylin for 10–30 min. The stained tissue sections were washed with running water and color separated using 5% hydrochloric acid containing alcohol. The tissue sections were then placed in 50% alcohol for 5 min, 70% alcohol for 5 min, 80% alcohol for 5 min, and eosin staining solution for 60 sec. The tissue sections were then washed in running water and placed in 95% alcohol (I) for 5 min, 95% alcohol (II) for 5 min, anhydrous alcohol (I) for 5 min, anhydrous alcohol (II) for 5 min, xylene containing alcohol for 5 min, xylene (I) for 5 min, xylene (II) for 5 min, and xylene (III) for 5 min. Care was taken to ensure that the frozen tissue sections did not dehydrate throughout the process, and finally the sections were covered with a neutral gum.
MWM training
We used the standard hidden platform version of Morris water maze (MWM) training to investigate the spatial memory and learning performance of each group (Vorhees and Williams, 2006). Starting on day 4 following TBI, all animals were tested for 5 consecutive days and given 4 training trials per day. For data collection, a pool (1.8 m in diameter) was subdivided into four equal quadrants formed by imaging lines. The pool was located in a large test room, where there were many clues external to the maze (e.g., pictures and lamps); these clues were visible from the pool and presumably used by the rats for spatial orientation. The position of the cues remained unchanged throughout the training. The rats were placed randomly at one of four fixed starting points, facing toward the wall (designated north, south, east, and west), and allowed to swim for 120 sec or until they found the platform within 120 sec. If the animal found the platform, it was allowed to remain on it for 10 sec before the next training session. If the animal failed to find the platform within 120 sec, it was placed on the platform for 10 sec before the next training session. During the test period, the platform was located in the NE quadrant, 2 cm below the water, and remained unchanged until termination of the training. If the animal was unable to find the platform within 120 sec, the trial was terminated and a maximum score of 120 sec was assigned. If the animal reached the platform within 120 sec, the percentage of time traveled within the NE (correct) quadrant was calculated, relative to the total amount of time spent swimming before reaching the platform, and employed for statistical analysis. To further evaluate memory retention, at day 6 of the MWM training, we removed the safe platform to assess the final time each group spent at the site at which the platform had been located during training, and the number of platform crossings was recorded. A rat was given 1 trial on this last day and 120 sec was assigned.
Tissue preparations
Following termination of MWM training, the rats were anesthetized IP with chloral hydrate and perfused transcardially first with saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4. The brains were removed and post-fixed in 4% paraformaldehyde for 2 days at room temperature. The brain tissue was cut into a series of adjacent 6-μm-thick frozen tissue sections.
Western blot
The cortex and hippocampal tissue proteins were pooled as a mixture. We quantified the proteins using a BCA-100 Protein Quantitative Analysis Kit as described previously. After the addition of a sample loading buffer, protein samples were electrophoresed on a 12% SDS-PAGE gel, and subsequently transferred to a PVDF membrane (Millipore, Bedford, MA). The membrane was incubated in fresh blocking buffer (0.1% Tween 20 in Tris-buffered saline, pH 7.4, containing 5% nonfat dried milk) at room temperature for 30 min, and then probed with monoclonal mouse anti-β-1,4-GalT-I and -V antibody, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody in a blocking buffer at 4°C overnight. The membrane was washed 3 times for 5 min each using PBST (PBS and 0.1% Tween 20), and then incubated in IRDye 800-conjugated goat anti-mouse IgG at immunofluorescence staining room temperature for 2 h. The images were scanned with a GS800 Densitometer Scanner (Bio-Rad, Hercules, CA), and the optical density data were analyzed using PDQuest 7.2.0 software (Bio-Rad). GAPDH was used as an internal reference.
Lectin-fluorescence staining with RCA-I
To eliminate terminal sialic acid moieties, tissue sections were digested with sialidase for 5 h at 37°C. Frozen sections were blocked with 3% (w/v) bovine serum albumin (BSA) overnight at 4°C, then the sections were incubated with isothiocyanate FITC-conjugated RCA-I (1:400; Vector Labs, Burlingame, CA) in a dark room for 24 h at 4°C. For double-staining analysis, the sections were also incubated with monoclonal primary antibody for different cell markers, including CD-11b and NeuN (1:50; Vector Labs). After washing three times in PBS, the second antibodies of cell markers CD-11b and Nun were added in a dark room and incubated for 2 h at 4°C. After washing three times in PBS, the sections were covered. Fluorescence was detected using a Leica fluorescence microscope.
Statistical analysis
All data were calculated as means±standard error (SE). The statistical significance of the means was calculated using Student's t-test. One-way analysis of variance (ANOVA), followed by Tukey's post-hoc multiple comparison tests were used for statistical analysis of the immunoblotting data. p Values<0.05 were considered statistically significant. All statistical analyses were conducted with the STATA 7.0 software package (Systat Software Inc., San Jose, CA).
Results
H&E staining of brain tissues
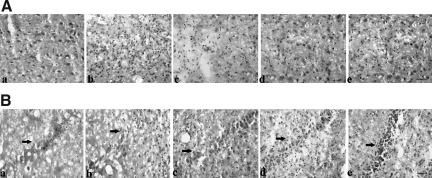
H&E staining was used to determine whether the TBI models were successful (Fig. 1). The results showed that TBI resulted in serious damage to brain tissues, and that PQQ significantly improved this state. In injured brain cortices (Fig. 1A), the nuclei were disordered and the morphology of nuclei changed significantly compared with those in normal brain cortices. Moreover, the number of nuclei increased. After administration of PQQ, this state was alleviated. In the hippocampus CA2 region (Fig. 1B), TBI resulted in the destruction, disordering, and disfiguring of nuclei, but PQQ improved this condition. The effect was dose-dependent. When PQQ was administered at 5 mg/kg per day, there was a slight improvement of the state compared with the non-PQQ TBI group. When PQQ was administered at 7 mg/kg per day, the state was further improved. When PQQ was administered at 10 mg/kg per day, the nuclei were well-arrayed and the morphology of the nuclei was similar to that seen in normal brain tissues. Thus we can initially speculate that PQQ is beneficial in recovery post-TBI in these animals.
FIG. 1.
Hematoxylin and eosin (H&E) staining of brain tissues. (A) Cortex. (B) Hippocampus (arrows indicate hippocampal CA2 region). (a) Normal group (n=6). (b) Non-PQQ TBI group (n=6). (c) TBI+PQQ 5 mg/kg per day group. (d) TBI+PQQ 7 mg/kg per day group. (e) TBI+PQQ 10 mg/kg per day group (n=6); scale bars=100 μm; PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury).
Nissl staining of brain tissues
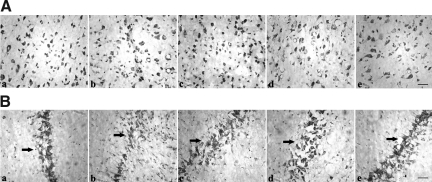
Nissl staining was used to observe neuronal morphology of the cortex and hippocampus CA2 regions (Fig. 2). In injured cortex and hippocampus CA2 regions, the Nissl bodies were disordered and their morphology significantly changed compared with those in normal brain tissues. Moreover, there was a loss of Nissl bodies in the hippocampus CA2 regions of injured brain tissues, which was improved by PQQ. When PQQ was administered at 5 mg/kg per day, the disordered arrangement and abnormal morphology of Nissl bodies was alleviated compared with those in the non-PQQ TBI group. When PQQ was administered at 7 mg/kg per day, further improvement was observed. To our surprise, when PQQ was administered at 10 mg/kg per day, the Nissl bodies were well-arrayed and the morphology of Nissl bodies was to those seen in normal brain tissues.
FIG. 2.
Nissl staining of brain tissues. (A) Cortex. (B) Hippocampus (arrows indicate hippocampal CA2 region). (a) Normal group (n=6). (b) Non-PQQ TBI group (n=6). (c) TBI+PQQ 5 mg/kg per day group. (d) TBI+PQQ 7 mg/kg per day group. (e) TBI+PQQ 10 mg/kg per day group (n=6; scale bars=100 μm; PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury).
PQQ improved spatial memory and learning performance of TBI animals
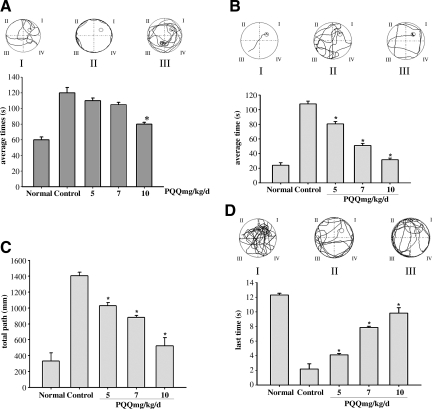
In order to assess the effect of PQQ on the spatial memory and learning performance of TBI animals, MWM training was performed for 5 consecutive days (beginning 4 days post-TBI). Statistical analysis showed that there was already a difference in the escape latency in the five groups on day 1 of MWM training (Fig. 3A). As the results show, normal animals were able to easily reach the platform within 120 sec, but non-PQQ TBI animals were unable to do so throughout training. As expected, animals administered PQQ found the platform within the expected time, although the time spent was longer than that of normal animals. When PQQ was administered at 5 mg/kg per day, the animals spent 110±3.28 sec searching for the platform. When PQQ was administered at 7 mg/kg per day, the spent time was 105±2.96 sec. When PQQ was administered at 10 mg/kg per day, the time spent searching was further shortened to 80±2.28 sec.
FIG. 3.
(A) The difference in spatial memory and learning performance on day 1 of Morris water maze (MWM) training [(I), typical path map that the normal group covered; (II), typical path map that the non-PQQ TBI group covered; (III), typical path map that the TBI+PQQ 10 mg/kg per day group covered; average time(s), mean times that each group spent searching for the platform; Normal, normal group; 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±standard error of the mean (SEM) and represent three independent experiments [#p<0.05 versus Normal (n=6); *p<0.05 versus Control (n=6)]. (B) The difference in spatial memory and learning performance on day 5 of MWM training [(I), typical path map that the normal group covered; (II), typical path map that the non-PQQ TBI group covered; (III), typical path map that the TBI+PQQ 10 mg/kg per day group covered; average time(s), mean times that each group spent searching for the platform; Normal, normal group; 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±SEM and represent three independent experiments [#p<0.05 versus Normal (n=6)]. (C) The difference in the total path that each group's animals covered on day 5 of MWM training [total path (mm), mean distance each group covered (within 120 sec) searching for the platform; Normal, normal group; 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±SEM and represent three independent experiments [#p<0.05 versus Normal (n=6); *p<0.05 versus Control (n=6)]. (D) The difference in memory retention when the platform was removed on day 6 of MWM training [(I), typical path map that the normal group covered; (II), typical path map that the non-PQQ TBI group covered; (III), typical path map that the TBI+PQQ 10 mg/kg per day group covered; last times(s), mean time that each group spent in the site where the platform was located; Normal, normal group; 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±SEM and represent three independent experiments [#p<0.05 versus Normal (n=6); PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury].
To determine if the difference might be attributed to pretreatment with PQQ, we prolonged the training (Fig. 3B). As anticipated, at day 5 of MWM training the time spent by normal animals in searching for safe platform was significantly shortened compared with non-PQQ TBI animals. In TBI groups administered PQQ, the time spent was further shortened and the effect was dose-dependent. When PQQ was administered at 5 mg/kg per day, the TBI animals spent 80±3.28 sec searching for the safe platform. When PQQ was administered at 7 mg/kg per day, the time spent was 51±2.96 sec. When PQQ was administered at 10 mg/kg per day, the time spent was further shortened to 31±2.28 sec, very close to that of normal animals.
Considering that the physical strength of the animals in each group might affect the mean time during which they searched for the platform, we measured the total path that the five groups covered at day 5 of MWM training (Fig. 3C). Accordingly, the path that the non-PQQ TBI animals covered was longer than that of normal animals, but the covered course gradually shortened with increasing dosages in the TBI groups administered PQQ. When PQQ was administered at 5 mg/kg per day, the covered course was 1031±38.17 mm, longer than the TBI+PQQ 7 mg/kg per day group. When PQQ was administered at 10 mg/kg per day, the covered course was further shortened to 525±102.51 mm.
To further evaluate memory retention, at day 6 of MWM training, we removed the platform to assess the time that each group spent in the site at which the platform was located during training. As shown in Figure 3D, the time spent by TBI animals administered PQQ was longer than that spent by the non-PQQ TBI animals. At the same time, the frequency with which each group crossed the platform region was also different. When PQQ was administered at 5 mg/kg per day, the time was 4±0.19 sec, slightly shorter than that of the TBI+PQQ 7 mg/kg per day group. Further, when PQQ was administered at 10 mg/kg per day, the TBI animals wandered for nearly 9±0.74 sec at the location where the platform had been located, a period approaching that of normal animals.
When the PQQ was systemically administered, we observed an improved neurobehavioral outcome in the TBI groups.
The expression of β-1,4-GalT-I and -V in cortex and hippocampus
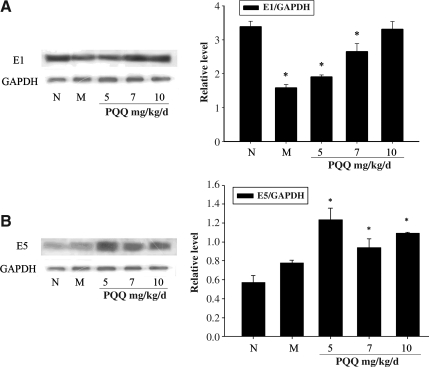
Because the cortex and hippocampus regions play crucial roles in spatial memory and learning performance, we extracted the protein of the cortex and hippocampal regions to detect the effect of PQQ on β-1,4-GalT-I and -V expression (Fig. 4A and B). Western blot analysis showed that the expression of β-1,4-GalT-I and -V was increased after PQQ administration in TBI rats compared with that seen in the non-PQQ TBI group. As shown in Figure 4A, the effect of PQQ on the expression of β-1,4-GalT-I was dose-dependent. The expression of β-1,4-GalT-I in the TBI+PQQ 5 mg/kg per day group was increased compared to that seen in the non-PQQ TBI group. When PQQ was administered at 7 mg/kg per day, the expression of β-1,4-GalT-I was further increased. Remarkably, in the TBI+PQQ 10 mg/kg per day group, the expression of β-1,4-GalT-I approached that of the normal group. Figure 4B also shows that PQQ led to expression changes in β-1,4-GalT-V in each group compared with that in the non-PQQ TBI group, but there was no apparent dose-dependent effect. Because the gray scale analysis also showed that such expression changes were statistically significant, we concluded that PQQ might improve spatial memory learning performance of TBI rats by regulating the expression of β-1,4-GalT-I and -V in the cortex and hippocampus.
FIG. 4.
(A) The expression of β-1,4-GalT-I in cortex and hippocampus [E1, β-1,4-GalT-I; N, normal group (n=6); M, non-PQQ TBI group (n=6); 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was the internal reference. Results are shown as the mean±standard error of the mean (SEM) and represent three independent experiments [#p<0.05 versus normal (n=6)]. (B) The expression of β-1,4-GalT-V in cortex and hippocampus [E5, β-1,4-GalT-V; N, normal group (n=6); M, non-PQQ TBI group (n=6); 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. GAPDH was the internal reference. Results are shown as the mean±SEM and represent three independent experiments [#p<0.05 versus normal (n=6); PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury; β-1,4-GalT-I, β-1,4-galactosyltransferase I; β-1,4-GalT-V, β-1,4-galactosyltransferase V].
The expression of the Gal β-1,4-GlcNAc group in microglia and the neurons of the cortex and hippocampus
As we know, β-1,4-GalT-I and -V are involved in the synthesis of the Gal β-1,4-GlcNAc group. To determine whether the levels of the Gal β-1,4-GlcNAc group are altered after TBI, we used lectin-fluorescence staining with FITC-RCA-I to detect the expression change of the Gal β-1,4-GlcNAc group in the microglia and neurons in the cortex and hippocampal CA2 regions. Cell-surface galactose-containing glycans were detected by using the galactose-specific lectin RCA-I, which interacts with oligosaccharides terminating with the Galb1→4GlcNAc (Venka-tesh and Lambert, 1997), conjugated with fluorescein isothiocyanate (FITC). The results showed that expression changes of the Gal β-1,4-GlcNAc group in the microglia of the cortex and hippocampal regions in each group were increased after administration of PQQ, compared with those seen in the non-PQQ TBI group (Figs. 5 and 6 and 5S and 6S). Moreover, this effect was dose-dependent. As Figures 5 and 6 show, TBI resulted in upregulated expression of the Gal β-1,4-GlcNAc group compared with that seen in the normal group. When PQQ was administered at 5 and 7 mg/kg per day, the expression of the Gal β-1,4-GlcNAc group was upregulated compared with that seen in the non-PQQ TBI group. When PQQ was administered at 10 mg/kg per day, the expression of the Gal β-1,4-GlcNAc group increased further. At the same time, we found that there were similar expression changes in the Gal β-1,4-GlcNAc group in the neurons of the cortex and hippocampal regions (Figs. 7 and 8 and 7S and 8S).
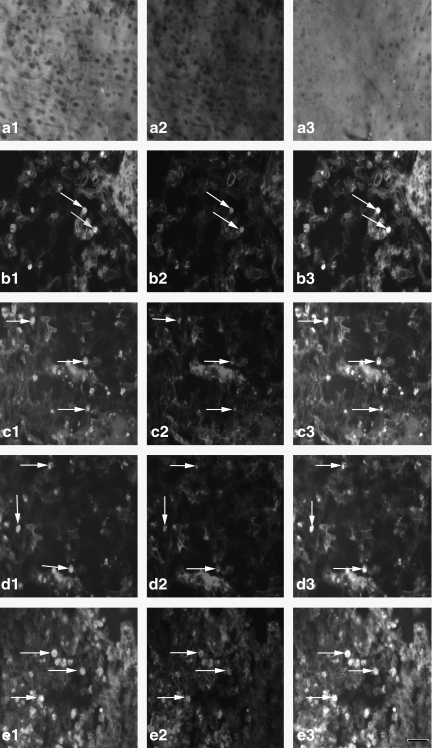
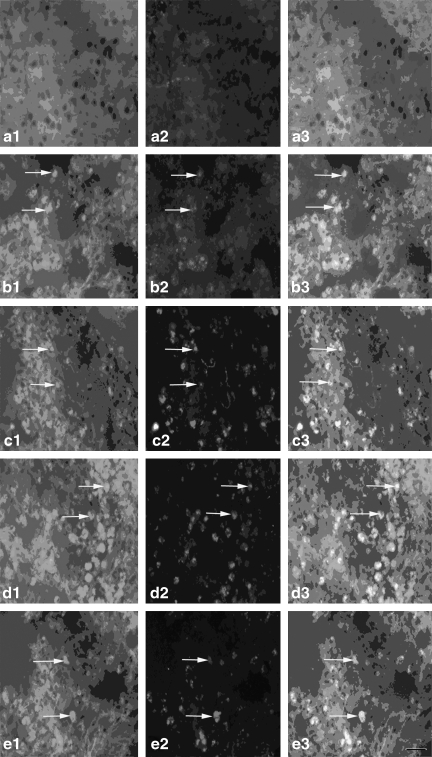
FIG. 5.
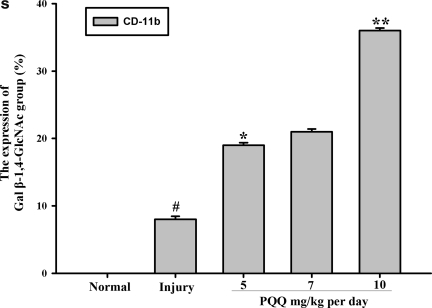
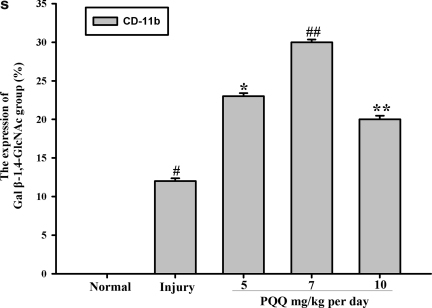
The expression of the Gal β-1,4-GlcNAc group in cortex microglia [a1–e1, Gal β-1,4-GlcNAc group; a2–e2, cortex microglia; a3–e3, co-localization; a1, a2, a3, the expression of the Gal β-1,4-GlcNAc group in cortex microglia of the normal group (n=6); b1, b2, b3, the expression of the Gal β-1,4-GlcNAc group in cortex microglia of the non-PQQ TBI group (n=6); c1, c2, c3, the expression of the Gal β-1,4-GlcNAc group in cortex microglia of the TBI+PQQ 5 mg/kg per day group (n=6); d1, d2, d3, the expression of the Gal β-1,4-GlcNAc group in cortex microglia of the TBI+PQQ 7 mg/kg per day group (n=6); e1, e2, e3, the expression of the Gal β-1,4-GlcNAc group in cortex microglia of the TBI+PQQ 10 mg/kg per day group (n=6); arrows, co-localization; a1–e3, ×400]. (S) Quantitative expression of the Gal β-1,4-GlcNAc group in cortex microglia [Normal, normal group (n=6); Injury, non-PQQ TBI group (n=6); 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±standard error of the mean (SEM) and represent three independent experiments [#p<0.05 versus Normal (n=6); *p<0.05 versus Injury (n=6); **p<0.05 versus 7 mg/kg per day (n=6); PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury; Gal β-1,4-GlcNAc, galactosidase β-1,4-galactosyltransferase N-acylsphingosine].
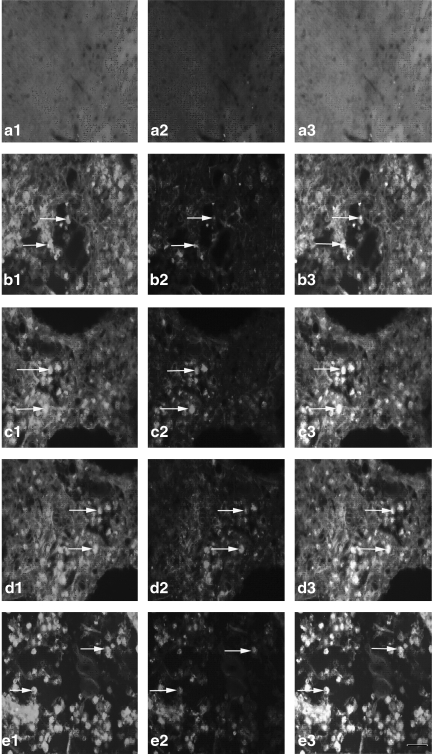
FIG. 6.
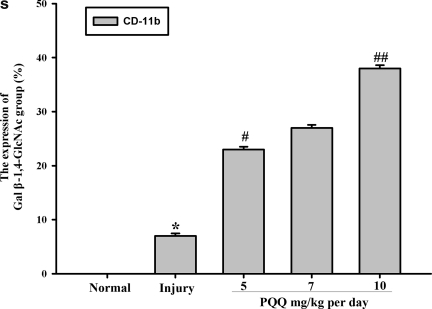
The expression of the Gal β-1,4-GlcNAc group in hippocampal microglia [a1–e1, Gal β-1,4-GlcNAc group; a2–e2, hippocampal microglia; a3–e3, co-localization; a1, a2, a3, the expression of the Gal β-1,4-GlcNAc group in hippocampal microglia of the normal group (n=6); b1, b2, b3, the expression of the Gal β-1,4-GlcNAc group in hippocampal microglia of the non-PQQ TBI group (n=6); c1, c2, c3, the expression of the Gal β-1,4-GlcNAc group in hippocampus microglia of the TBI+PQQ 5 mg/kg per day group (n=6); d1, d2, d3, the expression of the Gal β-1,4-GlcNAc group in hippocampal microglia of the TBI+PQQ 7 mg/kg per day group (n=6); e1, e2, e3, the expression of the Gal β-1,4-GlcNAc group in hippocampal microglia of the TBI+PQQ 10 mg/kg per day group (n=6); arrows, co-localization; a1–e3, ×400]. (S) Quantitative expression of the Gal β-1,4-GlcNAc group in hippocampal microglia [Normal, normal group (n=6); Injury, non-PQQ TBI group (n=6); 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±standard error of the mean (SEM) and represent three independent experiments [*p<0.05 versus Normal (n=6); #p<0.05 versus Injury (n=6); ##p<0.05 versus 7 mg/kg per day (n=6); PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury; Gal β-1,4-GlcNAc, galactosidase β-1,4-galactosyltransferase N-acylsphingosine].
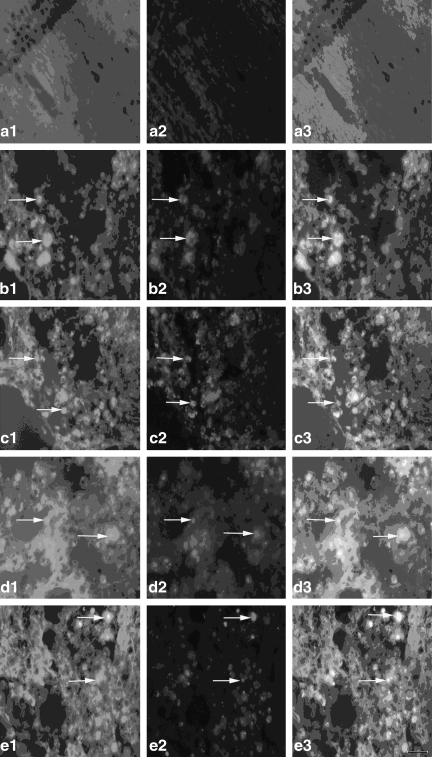
FIG. 7.
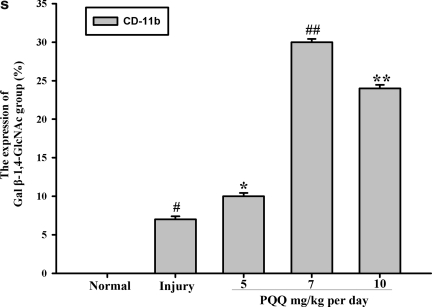
The expression of the Gal β-1,4-GlcNAc group in cortex neurons [a1–e1, Gal β-1,4-GlcNAc group; a2–e2, cortex neurons; a3–e3, co-localization; a1, a2, a3, the expression of the Gal β-1,4-GlcNAc group in cortex neurons of the normal group (n=6) ; b1, b2, b3, the expression of the Gal β-1,4-GlcNAc group in cortex neurons of the non-PQQ TBI group (n=6); c1, c2, c3, the expression of the Gal β-1,4-GlcNAc group in cortex neurons of the TBI+PQQ 5 mg/kg per day group (n=6); d1, d2, d3, the expression of the Gal β-1,4-GlcNAc group in cortex neurons of the TBI+PQQ 7 mg/kg per day group (n=6); e1, e2, e3, the expression of the Gal β-1,4-GlcNAc group in cortex neurons of the TBI+PQQ 10 mg/kg per day group (n=6); arrows, co-localization; a1–e3, ×400]. (S) Quantitative expression of the Gal β-1,4-GlcNAc group in cortex neurons [Normal, normal group (n=6); Injury, non-PQQ TBI group (n=6); 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±standard error of the mean and represent three independent experiments [#p<0.05 versus Normal (n=6); *p<0.05 versus Injury (n=6); ##p<0.05 versus Injury (n=6);**p<0.05 versus 7 mg/kg per day (n=6); PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury; Gal β-1,4-GlcNAc, galactosidase β-1,4-galactosyltransferase N-acylsphingosine].
FIG. 8.
The expression of the Gal β-1,4-GlcNAc group in hippocampal neurons [a1–e1, Gal β-1,4-GlcNAc group; a2–e2, hippocampal neurons; a3–e3, co-localization; a1, a2, a3, the expression of the Gal β-1,4-GlcNAc group in hippocampal neurons of the normal group (n=6); b1, b2, b3, the expression of the Gal β-1,4-GlcNAc group in hippocampal neurons of the non-PQQ TBI group (n=6); c1, c2, c3, the expression of the Gal β-1,4-GlcNAc group in hippocampal neurons of the TBI+PQQ 5 mg/kg per day group (n=6); d1, d2, d3, the expression of the Gal β-1,4-GlcNAc group in hippocampal neurons of the TBI+PQQ 7 mg/kg per day group (n=6); e1, e2, e3, the expression of the Gal β-1,4-GlcNAc group in hippocampal neurons of the TBI+PQQ 10 mg/kg per day group (n=6); arrows, co-localization; a1–e3, ×400]. (S) Quantitative expression of the Gal β-1, 4-GlcNAc group in cortex neurons [Normal, normal group (n=6); Injury, non-PQQ TBI group (n=6); 5, TBI+PQQ 5 mg/kg per day group (n=6); 7, TBI+PQQ 7 mg/kg per day group (n=6); 10, TBI+PQQ 10 mg/kg per day group (n=6)]. Results are shown as the mean±standard error of the mean and represent three independent experiments [*p<0.05 versus Normal (n=6); #p<0.05 versus Injury (n=6); **p<0.05 versus Injury (n=6); ##p<0.05 versus 7 mg/kg per day (n=6); PQQ, pyrroloquinoline quinine; TBI, traumatic brain injury; Gal β-1,4-GlcNAc, galactosidase β-1,4-galactosyltransferase N-acylsphingosine].
Discussion
PQQ is an anionic, water-soluble compound with free-radical-scavenging properties. It also serves as a redox factor in various systems such as the brain, heart, and lung (Gallop et al., 1989a, 1989b; Paz et al., 1989; Gallop et al., 1993). Many studies have examined PQQ for its nutritional and pharmacological properties (Killgore et al., 1989). Several experiments, particularly in recent years, have revealed that PQQ may possess potent neuroprotective functions. It has been well established that PQQ can function as a protective agent in experimental stroke models (Alexandrova and Bochev, 2005). It has also been suggested that PQQ alleviates symptoms in animals suffering from epilepsy (Sanchez et al., 2000). Nunome and associates found that PQQ prevented oxidative, stress-induced neuronal death by altering the oxidative status of DJ-1 (Nunome et al., 2008) and more recently, Kim and colleagues demonstrated that PQQ inhibits the amyloid fibril formation and cytotoxicity of the C-truncated alpha-synuclein variants, which suggests that PQQ may be a good candidate for treating PD (Kim et al., 2010). Although PQQ exerts many beneficial effects on the nervous system and other organs, thus far the effect of PQQ in TBI has not been studied.
A better understanding of the recovery process seen post-TBI is critical to developing effective treatment, and based on the aforementioned neuroprotective effects that PQQ possesses, here we tried to further affirm its efficacy in recovery from TBI in rats.
We first administered PQQ post-TBI, but this approach was not efficacious. As many studies have confirmed the neuroprotective effect of PQQ, we believed our original results were due to the serious damage caused by TBI compared with other experimental models. Additionally, a study reported that PQQ pretreatment could protect human neuroblastoma SH-SY5Y cells against Aβ-induced neurotoxicity (Zhang et al., 2009); therefore we injected PQQ intraperitoneally for 3 days before TBI and consecutively until termination of MWM training. Considering that a previous study had already demonstrated that PQQ administered at 10 mg/kg reduced brain infarct size caused by reversible middle cerebral artery occlusion and significantly increased behavioral scores, but that when PQQ was given at a dose of 1 mg/kg it failed to act (Zhang et al., 2006), in our experiments we administered PQQ intraperitoneally at 5, 7, and 10 mg/kg per day for 3 days (Dixon et al., 1991). At 4 days post-injury, we performed MWM training, the results of which showed that PQQ improved the neurobehavioral outcome of TBI animals, and that these effects were dose-dependent. A similar effect was also observed in another experiment in which animals were subjected to brain injury via hypoxia, and their memory function was maintained by PQQ (Ohwada et al., 2008). At the termination of MWM training, we conducted Nissl staining and H&E staining of brain tissue sections to test the reliability of our model and to detect the protective effect of PQQ on brain tissues. As anticipated, TBI resulted in serious injury to nerve tissues, which was apparently improved with the administration of PQQ. Based on these results, we initially speculated that PQQ was beneficial in recovery post-TBI.
In order to identify the possible mechanisms and molecules involved in the improved cognitive function seen in animals suffering from TBI, we used Western blot analysis to probe the expression changes of β-1,4-GalT-I and -V in the cortex and hippocampal regions of each group. Glycosylation is regarded as one of the most important post-translational modifications, and each member of the β-1,4-GalTs may play a specific role in different tissues and cells. β-1,4-GalT-I and -V are thought to be involved in the biosynthesis of N-linked oligosaccharides. The changes of glycosylation that occur on the neuronal surface are thought to be a link with the development and regeneration of the mammalian nervous system (Allendoerfer et al., 1999; Nunome et al., 2008). One study reported that the expression levels of the β1,4-GalT-V transcript increase during mouse brain development after birth, but that the β1,4-GalT-I gene is expressed primarily in the mid-embryonic stages (Liedtke et al., 2001; Nakamura et al., 2001). A related study, however, found that both β-1,4-GalT-I and -V are expressed at low levels in normal sciatic nerve, and play an important role in the regeneration of the injured sciatic nerve, which differed from the expression of β-1,4-GalT-I and -V seen in the central nervous system. These results further confirmed that β-1,4-GalT- I and -V are expressed differently in different tissues (Yan et al., 2008). In the present study, we found the expression of β-1,4-GalT-I in the normal brain, which also differed from previous conclusions. We supposed that this difference might be the consequence of MWM training. In our study, we also established the expression of β-1,4-GalT-V in normal brain tissue, which was consistent with previous experimental results.
It is well established that several properties of PQQ can be attributed to its neuroprotective effects. For one thing, PQQ may suppress peroxynitrite formation because it is a free-radical scavenger and a cofactor for quinoprotein enzymes (Kim et al., 2010). For another, PQQ can potentially oxidize the NMDA receptor redox site and thereby decrease activity of the NMDA receptor (Aizenman et al., 1992). Finally, PQQ may serve as an effective antioxidant in protecting mitochondrial lipid and protein, and it has been shown to protect mitochondrial functions against oxidative damage (He et al., 2003). In our experiment, PQQ increased the expression of β-1,4-GalT-I and -V in the cortex and hippocampal regions compared with those in the non-PQQ TBI group, and gray scale analysis results also showed that this increased expression was statistically significant. Considering both β-1,4-GalT-I and -V play key roles in inflammation and the nervous system (Asano et al., 2003; Mori et al., 2004; Yan et al., 2008; Niu et al., 2008), we presumed that PQQ may also, through regulating the inflammatory response and neuroregeneration, improve spatial memory and learning performance in TBI animals.
As we know, β-1,4-GalT-I and -V are involved in the synthesis of the Gal β-1,4-GlcNAc group, and this synthesis of is the basis of HNK-1 carbohydrate and polysialic acid, which are essential for the modification of several nerve-regenerative-associated molecules such as L1 and N-CAM (Furukawa and Sato, 1999; Liedtke et al., 2001; Nakamura et al., 2001). We used lectin-fluorescence staining with RCA-I to further detect the expression changes of the Gal β-1,4-GlcNAc group in microglia and neurons of the cortex and hippocampal regions, which showed that PQQ increased the expression of the Gal β-1,4-GlcNAc group in microglia of the cortex and hippocampal regions compared with that seen in the non-PQQ TBI group. At the same time, we also found that there were expression changes of the Gal β-1,4-GlcNAc group in neurons of the cortex and hippocampal regions, but that the total number apparently lower than that seen in microglia. In the present study, we have not seen expression of the Gal β-1,4-GlcNAc group in microglia and neurons of normal brain tissue, which is not consistent with the expression of β-1,4-GalT-I and -V. We think this is because the cortex and hippocampal tissue proteins were pooled as a mixture, and the Gal β-1,4-GlcNAc group may be expressed in the local cortex or hippocampus separately. Or, this difference may be because the galactose-specific lectin, RCA-I, which interacts with oligosaccharides terminating with the Galb1→4GlcNAc (Venka-tesh and Lambert, 1997), when conjugated with fluorescein isothiocyanate (FITC), only shows cell-surface galactose-containing glycans. Whether or not the Gal β-1,4-GlcNAc group exists in other nerve cells requires further investigation.
Taken together, our results support the theory that PQQ may be a potential candidate for treating TBI, but the specific mechanisms and molecules involved require further study.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (grant nos. 81070275, 31070723, and 30970996), the College and University Natural Scientific Research Program of Jiangsu Province (08KJB320011), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Natural Science Foundation of Nantong University (10ZY013).
References
- Aizenman E. Hartnett K.A. Zhong C. Gallop P.M. Rosenberg P.A. Interaction of the putative essential nutrient pyrroloquinoline quinone with the N-methyl-D-aspartate receptor redox modulatory site. J. Neurosci. 1992;12:2362–2369. doi: 10.1523/JNEUROSCI.12-06-02362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova M.L. Bochev P.G. Oxidative stress during the chronic phase after stroke. Free Radic. Biol. Med. 2005;39:297–316. doi: 10.1016/j.freeradbiomed.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Allendoerfer K.L. Durairaj A. Matthews G.A. Patterson P.H. Morphological domains of Lewis-X/FORSE-1 immunolabeling in the embryonic neural tube are due to developmental regulation of cell surface carbohydrate expression. Dev. Biol. 1999;211:208–219. doi: 10.1006/dbio.1999.9308. [DOI] [PubMed] [Google Scholar]
- Asano M. Nakae S. Kotani N. Shirafuji N. Nambu A. Hashimoto N. Kawashima H. Hirose M. Miyasaka M. Takasaki S. Iwakura Y. Impaired selectin-ligand biosynthesis and reduced inflammatory responses in beta-1,4-galactosyltransferase-I-deficient mice. Blood. 2003;102:1678–1685. doi: 10.1182/blood-2003-03-0836. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Furukawa K. Sato T. Beta-1,4-galactosylation of N-glycans is a complex process. Biochim. Biophys. Acta. 1999;1473:54–66. doi: 10.1016/s0304-4165(99)00169-5. [DOI] [PubMed] [Google Scholar]
- Gallop P.M. Henson E. Paz M.A. Greenspan S.L. Fluckiger R. Acid-promoted tautomeric lactonization and oxidation-reduction of pyrroloquinoline quinone (PQQ) Biochem. Biophys. Res. Commun. 1989a;163:755–763. doi: 10.1016/0006-291x(89)92287-0. [DOI] [PubMed] [Google Scholar]
- Gallop P.M. Paz M.A. Fluckiger R. Henson E. Is the antioxidant, anti-inflammatory putative new vitamin, PQQ, involved with nitric oxide in bone metabolism? Connect. Tissue Res. 1993;29:153–161. doi: 10.3109/03008209309014242. [DOI] [PubMed] [Google Scholar]
- Gallop P.M. Paz M.A. Fluckiger R. Kagan H.M. PQQ, the elusive coenzyme. Trends Biochem. Sci. 1989b;14:343–346. doi: 10.1016/0968-0004(89)90169-2. [DOI] [PubMed] [Google Scholar]
- Hamagishi Y. Murata S. Kamei H. Oki T. Adachi O. Ameyama M. New biological properties of pyrroloquinoline quinone and its related compounds: inhibition of chemiluminescence, lipid peroxidation and rat paw edema. J. Pharmacol. Exp. Ther. 1990;255:980–985. [PubMed] [Google Scholar]
- He K. Nukada H. Urakami T. Murphy M.P. Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): implications for its function in biological systems. Biochem. Pharmacol. 2003;65:67–74. doi: 10.1016/s0006-2952(02)01453-3. [DOI] [PubMed] [Google Scholar]
- Kasahara T. Kato T. Nutritional biochemistry: A new redox-cofactor vitamin for mammals. Nature. 2003;422:832. doi: 10.1038/422832a. [DOI] [PubMed] [Google Scholar]
- Killgore J. Smidt C. Duich L. Romero-Chapman N. Tinker D. Reiser K. Melko M. Hyde D. Rucker R.B. Nutritional importance of pyrroloquinoline quinone. Science. 1989;245:850–852. doi: 10.1126/science.2549636. [DOI] [PubMed] [Google Scholar]
- Kim J. Harada R. Kobayashi M. Kobayashi N. Sode K. The inhibitory effect of pyrroloquinoline quinone on the amyloid formation and cytotoxicity of truncated alpha-synuclein. Mol. Neurodegener. 2010;5:20. doi: 10.1186/1750-1326-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M. Kim J. Kobayashi N. Han S. Nakamura C. Ikebukuro K. Sode K. Pyrroloquinoline quinone (PQQ) prevents fibril formation of alpha-synuclein. Biochem. Biophys. Res. Commun. 2006;349:1139–1144. doi: 10.1016/j.bbrc.2006.08.144. [DOI] [PubMed] [Google Scholar]
- Kumazawa T. Seno H. Urakami T. Matsumoto T. Suzuki O. Trace levels of pyrroloquinoline quinone in human and rat samples detected by gas chromatography/mass spectrometry. Biochim. Biophys. Acta. 1992;1156:62–66. doi: 10.1016/0304-4165(92)90096-d. [DOI] [PubMed] [Google Scholar]
- Kumazawa T. Sato K. Seno H. Ishii A. Suzuki O. Levels of pyrroloquinoline quinone in various foods. Biochem. J. 1995;307(Pt. 2):331–333. doi: 10.1042/bj3070331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke S. Geyer H. Wuhrer M. Geyer R. Frank G. Gerardy-Schahn R. Zahringer U. Schachner M. Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology. 2001;11:373–384. doi: 10.1093/glycob/11.5.373. [DOI] [PubMed] [Google Scholar]
- Mori R. Kondo T. Nishie T. Ohshima T. Asano M. Impairment of skin wound healing in beta-1,4-galactosyltransferase-deficient mice with reduced leukocyte recruitment. Am. J. Pathol. 2004;164:1303–1314. doi: 10.1016/s0002-9440(10)63217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N. Yamakawa N. Sato T. Tojo H. Tachi C. Furukawa K. Differential gene expression of beta-1,4-galactosyltransferases I, II and V during mouse brain development. J. Neurochem. 2001;76:29–38. doi: 10.1046/j.1471-4159.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- Niu S. Fei M. Cheng C. Yan M. Gao S. Chen M. Wang H. Li X. Yu X. Qian J. Qin J. Zhao J. Gu J. Shen A. Altered beta-1,4-galactosyltransferase I expression during early inflammation after spinal cord contusion injury. J. Chem. Neuroanat. 2008;35:245–256. doi: 10.1016/j.jchemneu.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Nunome K. Miyazaki S. Nakano M. Iguchi-Ariga S. Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol. Pharm. Bull. 2008;31:1321–1326. doi: 10.1248/bpb.31.1321. [DOI] [PubMed] [Google Scholar]
- Ohwada K. Takeda H. Yamazaki M. Isogai H. Nakano M. Shimomura M. Fukui K. Urano S. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J. Clin. Biochem. Nutr. 2008;42:29–34. doi: 10.3164/jcbn.2008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz M.A. Fluckiger R. Torrelio B.M. Gallop P.M. Methoxatin (PQQ), coenzyme for copper-dependent amine and mixed-function oxidation in mammalian tissues. Connect. Tissue Res. 1989;20:251–257. doi: 10.3109/03008208909023895. [DOI] [PubMed] [Google Scholar]
- Ronn L.C. Berezin V. Bock E. The neural cell adhesion molecule in synaptic plasticity and ageing. Int. J. Dev. Neurosci. 2000;18:193–199. doi: 10.1016/s0736-5748(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Salisbury S.A. Forrest H.S. Cruse W.B. Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979;280:843–844. doi: 10.1038/280843a0. [DOI] [PubMed] [Google Scholar]
- Sanchez R.M. Wang C. Gardner G. Orlando L. Tauck D.L. Rosenberg P.A. Aizenman E. Jensen F.E. Novel role for the NMDA receptor redox modulatory site in the pathophysiology of seizures. J. Neurosci. 2000;20:2409–2417. doi: 10.1523/JNEUROSCI.20-06-02409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon J.M. Aizenman E. Reynolds I.J. Effects of pyrroloquinoline quinone on glutamate-induced production of reactive oxygen species in neurons. Eur. J. Pharmacol. 1997;326:67–74. doi: 10.1016/s0014-2999(97)00137-4. [DOI] [PubMed] [Google Scholar]
- Shen A. Wang H. Zhang Y. Yan J. Zhu D. Gu J. Expression of beta-1,4-galactosyltransferase II and V in rat injured sciatic nerves. Neurosci. Lett. 2002;327:45–48. doi: 10.1016/s0304-3940(02)00381-6. [DOI] [PubMed] [Google Scholar]
- Tsuchida T. Yasuyama T. Higuchi K. Watanabe A. Urakami T. Akaike T. Sato K. Maeda H. The protective effect of pyrroloquinoline quinone and its derivatives against carbon tetrachloride-induced liver injury of rats. J. Gastroenterol. Hepatol. 1993;8:342–347. doi: 10.1111/j.1440-1746.1993.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y.P. Lambert J.M. Galactose–induced dimerization of blocked ricin at acidic pH: evidence for a third galactose–binding site in ricin β–chain. Glycobiology. 1997;7:329–335. doi: 10.1093/glycob/7.3.329. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V. Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M. Cheng C. Shao X. Qian J. Shen A. Xia C. Expression change of beta-1,4 galactosyltransferase I, V mRNAs and Galbeta1,4GlcNAc group in rat sciatic nerve after crush. J. Mol. Histol. 2008;39:317–328. doi: 10.1007/s10735-008-9168-z. [DOI] [PubMed] [Google Scholar]
- Zhang J.J. Zhang R.F. Meng X.K. Protective effect of pyrroloquinoline quinone against Abeta-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2009;464:165–169. doi: 10.1016/j.neulet.2009.08.037. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Feustel P.J. Kimelberg H.K. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006;1094:200–206. doi: 10.1016/j.brainres.2006.03.111. [DOI] [PubMed] [Google Scholar]
- Zhou D. Chen C. Jiang S. Shen Z. Chi Z. Gu J. Expression of b1,4-galactosyltransferase in the development of mouse brain. Biochim. Biophys. Acta. 1998;1425:204–208. doi: 10.1016/s0304-4165(98)00070-1. [DOI] [PubMed] [Google Scholar]
- Zhu B.Q. Zhou H.Z. Teerlink J.R. Karliner J.S. Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size and improves cardiac function in rat models of ischemia and ischemia/reperfusion. Cardiovasc. Drugs Ther. 2004;18:421–431. doi: 10.1007/s10557-004-6219-x. [DOI] [PubMed] [Google Scholar]