Abstract
Chromogenic in situ hybridization (ISH) is a commonly used tool in diagnostic pathology to detect pathogens in formalin-fixed, paraffin-embedded (FFPE) tissue sections. Prolonged formalin fixation time was identified to be a limiting factor for the successful detection of nucleic acid from different pathogens, most probably due to the cross-linking activity of formalin between RNA, DNA, and proteins. Therefore, in the current study, the influence of formalin fixation time on ISH signal intensity of 2 viral (Porcine circovirus-2 [PCV-2], Porcine respiratory and reproductive virus [PRRSV]) and 2 protozoal agents (Cryptosporidium serpentis and Tritrichomonas sp.) was evaluated. Tissue samples were fixed in 7% neutral buffered formaldehyde solution, and at defined intervals, pieces were embedded in paraffin wax and subjected to pathogen-specific ISH. For all 4 pathogens, the signal intensity remained comparable to the starting ISH signal for different periods of fixation (PCV-2: 6 weeks, PRRSV: 23 weeks, Cryptosporidium serpentis: 55 weeks, Tritrichomonas sp.: 53 weeks). Thereafter, the signal started to decline until loss of nucleic acid detection. The influence of increased proteinase K concentrations for inverting the formalin-induced cross-linking activity was examined compared to the standard protocol. With all 4 infectious agents, a 4-fold proteinase K concentration restored the ISH signals to a level comparable to 1 day of fixation. In conclusion, the influence of prolonged formalin fixation on the intensity of detected ISH signal highly depends on the analyzed infectious agent and the pretreatment protocol.
Keywords: Infectious agents, in situ hybridization, prolonged formalin fixation time
Chromogenic in situ hybridization (ISH), a commonly used technique in diagnostic pathology, is used to identify different pathogens such as viruses,1,3,11,15 bacteria,16 or protozoa2,6,8,9 in tissue sections. Furthermore, ISH combines modern molecular biology techniques with traditional histopathological examination allowing for correlation of detected pathogens with tissue morphology and microscopic lesions. This method is primarily performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections, which can be stored for decades without deterioration and are a frequent source for retrospective studies.
Formalin is a commonly used fixative agent that effectively kills most infectious agents and inhibits autolysis. Fixation is based on the cross-linking activity of formalin, which guarantees a good preservation of cellular morphology, but in the same way reduces the accessibility of nucleic acids. The process of cross-linking takes approximately 24-48 hr, depending on the size of the tissue sample, and occurs between RNA, DNA, and proteins via hydroxymethylene bridges.12,17 For immunohistochemistry (IHC), prolonged formalin fixation has been shown to lead to masking of epitopes due to the occurring cross-linking, which subsequently reduces or even abolishes antigen detection.5,7,12,13
Formalin is a suitable fixative agent for chromogenic ISH.14 As a result of the decreased accessibility of nucleic acids, reduced signal intensity due to prolonged formalin fixation time should be taken into account as a limiting factor. A previous study of the effects of prolonged formalin fixation time for the detection of Porcine circovirus-2 (PCV-2) showed that after a 730-day fixation the specific signal intensity was significantly reduced using a DNA ISH probe of 473 bp.3 At present, the easily synthesized much shorter oligonucleotide probes are a convenient and increasingly popular alternative to DNA and RNA probes, and thus further data on the maximal fixation time for assays using this probe type are desirable. In the current study, the influence of prolonged formalin fixation on the functionality of chromogenic ISH for the detection of 4 different pathogenic agents, namely PCV-2, Porcine respiratory and reproductive syndrome virus (PRRSV), Tritrichomonas sp., and Cryptosporidium serpentis, using digoxigenin-labeled oligonucleotide probes is investigated.
The samples originated from 3 pigs and 1 snake (Pantherophis guttatus). Pig 1 died suddenly and was found to be highly positive for PCV-2 using ISH of FFPE lymph node tissue. Pig 2 was experimentally infected with PRRSV, and ISH of the right cranial lung lobe revealed positively stained cells. Pig 3 showed wasting, diarrhea, and respiratory symptoms. At histopathological examination, a moderate colitis was found, and subsequent testing for Tritrichomonas sp. by ISH showed a severe trichomonad infection of the colon. The snake had died suddenly, and Cryptosporidium serpentis was found in the stomach. In situ hybridization specific for Cryptosporidium sp. confirmed the histopathological findings.
After necropsy, all tissue samples were immediately transferred to 7% neutral buffered formaldehyde solution for fixation. After 24 hr, a part of the tissue was embedded in paraffin wax, and histopathological examination was carried out. This time-point was defined as week 1. With some exceptions, tissue samples were processed on a weekly basis for 24 weeks (PCV-2), 29 weeks (PRRSV), 77 weeks (Cryptosporidium serpentis), and 79 weeks (Tritrichomonas sp.) for evaluation of prolonged fixation effects by ISH.
Four different published ISH probes were used for the detection of PCV-2 (probe: PCV-2), PRRSV (probe: PRRSV), Tritrichomonas sp. (probe: Tritri), and Cryptosporidium sp. (probe: Crypto; Table 1). The PCV-2 and PRRSV probes targeted a specific region in the viral genome, whereas the Tritri and Crypto probes were designed complementary to a specific region in the 18S ribosomal RNA (rRNA) gene. All probes were labeled with a digoxigenin molecule at the 3′ end.
Table 1.
List of all in situ hybridization probes with nucleotide sequence, probe concentration, and corresponding publication used in the current study.*
| Probe name |
Nucleotide sequence and length | Concentration (ng/ml) |
Reference |
|---|---|---|---|
| PCV-2 | 5′- CAGTAAATACGACCAGGACTACAATATC CGTGTAACCATG-3′ (40 nt) |
10 | 11 |
| PRRSV | 5′- TGAGGTGGTGCCGGATGTCATCTTCAGCA GCCAGG-3′ (35 nt) |
100 | 1 |
| Crypto | 5′- GTGCTGAAGGAGTAAGGAACAACCTCCA ATCTCTAGTTGG-3′ (40 nt) |
25 | 2 |
| Tritri | 5′- AGCTGTAGCCTTTTCAGGACAGCATCTCT -3′ (29 nt) |
20 | 6 |
Porcine circovirus-2 (probe: PCV-2), Porcine respiratory and reproductive syndrome virus (probe: PRRSV), Cryptosporidium sp. (probe: Crypto), and Tritrichomonas sp. (probe: Tritri).
Chromogenic ISH was carried out according to a previously published protocol.2 Three-μm thick FFPE tissue sections were dewaxed and rehydrated. Proteolysis was carried out using proteinase Ka (2.5 μg/ml) in Tris buffered saline for 30 min at 37°C. Additionally, 2 ISH runs were performed using either 5 μg/ml or 10 μg/ml proteinase K to analyze the influence of proteinase K concentration on the formalin-induced cross-linking. Subsequently, the slides were rinsed in distilled water, dehydrated in alcohol (95% and 100%), and air-dried. The hybridization mixture was applied, slides were heated up to 95°C for 6 min, and hybridization was carried out overnight at 40°C in a humid chamber. One hundred microliters of hybridization mix were composed of 50 μl of formamide, 20 μl of 20× standard sodium citrate (SSC), 10 μl of dextran sulfate (50%, w/v), 2 μl of 50× Denhardt solution, and 1 μl (Tritri and Crypto) or 2 μl (PCV-2 and PRRSV) of probe using the corresponding concentration (Table 1). The concentrations had been previously determined as those which gave optimal specific labeling with minimal background. Additionally, an ISH run using double probe concentration was performed to evaluate the influence on ISH signal intensity. On the second day the slides were washed with decreasing concentrations of SSC (2× SSC, 1× SSC, 0.1× SSC; 10 min each) to remove unbound probe. Afterward, the slides were incubated with anti-digoxigenin-alkaline phosphatase Fab fragmentsa (1:200). Visualization was carried out using the color substrate 5-bromo-4-chloro-3-indolyl phosphate and 4-nitro blue tetrazolium chloride.a Color development was stopped with Tris-ethylenediamine tetra-acetic acid buffer (pH 8.0) after 1 hr for the probes PCV-2, Tritrichomonas, and Cryptosporidium, and after 2 hr for the PRRSV probe. The slides were slightly counterstained with hematoxylin and mounted under coverslips using Aquatex.b
The influence of prolonged formalin fixation could clearly be shown using different probes specific for 2 viruses (PCV-2 and PRRSV) and 2 protozoal agents (Tritrichomonas sp. and Cryptosporidium serpentis; Fig. 1). There were significant differences of the detectability of the 4 tested pathogens after certain time points.
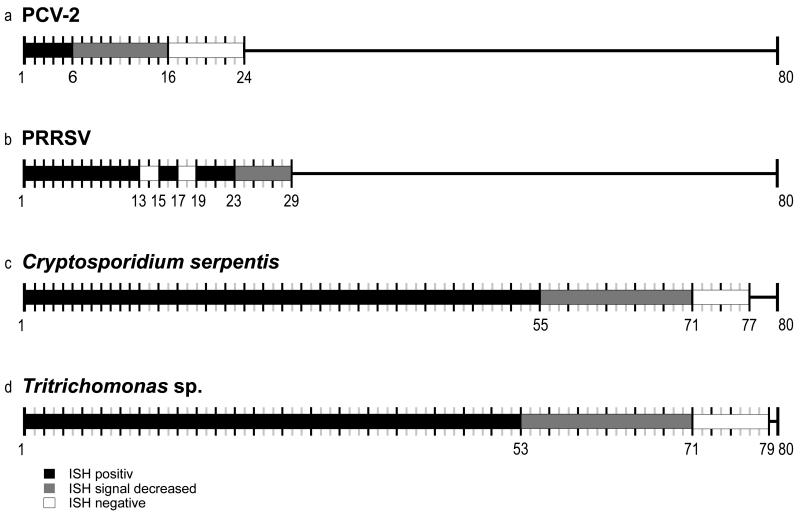
Figure 1.
Schematic overview of fixation periods and time points of sample examination. Each timeline marks 1 week starting with week 1 and ends after 24 weeks (Porcine circovirus-2 [PCV-2]), 29 weeks (Porcine respiratory and reproductive syndrome virus [PRRSV]), 77 weeks (Cryptosporidium serpentis), and 79 weeks (Tritrichomonas sp.). Timelines in gray symbolize weeks without sampling. In situ hybridization (ISH)-positive signal in black thick bars; ISH decreased signal in gray thick bars; and ISH with lack of signal in clear thick reaction.
With PCV-2, signal intensity was stable within the first 6 weeks; afterward, the detectable signal started to decrease, and after 16 weeks, no positive cells could be observed (Fig. 2a-c). The experiment was stopped at week 24. The results of a previous study3 claiming reactivity up to 730 days of fixation could not be reproduced. This might be due to the fact that in the present study an oligonucleotide probe (40 nt) labeled with 1 digoxigenin molecule was used, whereas a 473-bp DNA probe labeled with numerous digoxigenin molecules was used in the previous study, thus increasing the signal intensity per bound probe.
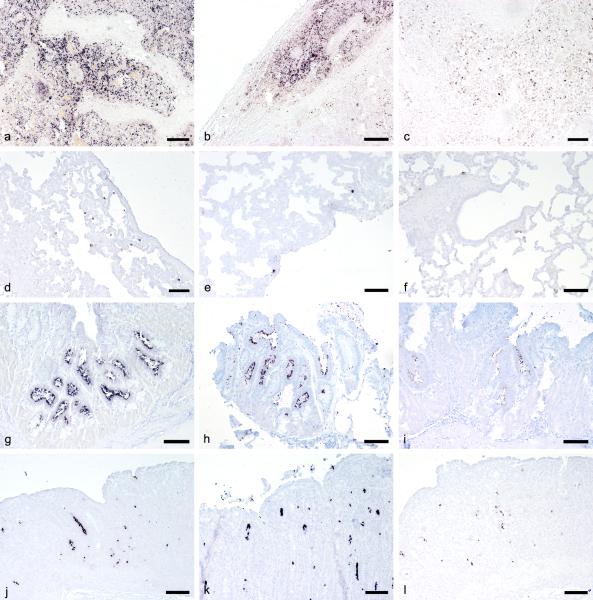
Figure 2.
In situ hybridization (ISH) performed after different time spans of prolonged formalin fixation. Positive cells are labeled dark purple. a-c, lymph node, pig. ISH for Porcine circovirus- 2: 1 week (a); 8 weeks (b); 10 weeks (c). d-f, lung, pig. ISH for Porcine respiratory and reproductive syndrome virus: 1 week (d); 10 weeks (e); 29 weeks (f). g-i, stomach, snake. ISH for Cryptosporidium serpentis: 1 week (g); 48 weeks (h); 62 weeks (i). j-l, colon, pig. ISH for Tritrichomonas sp.: 1 week (j); 47 weeks (k); 62 weeks (l). Bar = 400 μm.
In the case of PRRSV, positive cells could be visualized within the first 23 weeks; subsequently, the signal intensity started to decline (Fig. 2d-f). The signal was mostly found in the cytoplasm of a few alveolar macrophages of a circumscribed region in the lung sections. However, positively stained cells were not detected in each examined tissue section. In weeks 13, 14, and 17, no positively stained cells were detectable. It was likely that these tissue sections did not contain virus-infected cells. At week 29, the experiment had to be stopped due to the lack of tissue material, thus no endpoint of time for the detection of PRRSV could be determined.
For Cryptosporidium serpentis, intensely stained parasites could be identified by ISH within the first 55 weeks. The signal started to decrease after 55 weeks, and after week 71, no positive cells were detectable. The experiment was stopped after 77 weeks (Fig. 2g-i).
In the case of Tritrichomonas sp., the signal was stable for 53 weeks and then started to decline, until in week 71, no positively stained trichomonads could be visualized. At week 79, the experiment was stopped (Fig. 2j-l).
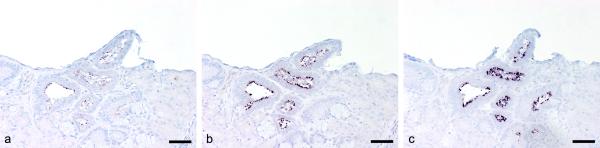
The ability of higher proteinase K concentration to abrogate the formalin-induced cross-linking was tested by comparing standard to double and 4-fold enzyme concentrations (tested weeks: PCV-2, 10 and 16; PRRSV, 27 and 29; Cryptosporidium serpentis, 62 and 71; Tritrichomonas sp., 68 and 71). For all 4 tested infectious agents, the signal intensity could be strongly augmented by increasing the proteinase K concentration without raised background staining. As an example, ISH performed to detect Cryptosporidium serpentis in week 71 is shown (Fig. 3). No or only weakly stained parasites could be seen with standard enzyme concentration (Fig. 3a). Signal intensity started to increase with double proteinase K quantity (Fig. 3b). With a 4-fold amount of enzyme, the observed signal intensity was comparable with week 1 of fixation (Fig. 3c). This effect of reduced cross-linking has already been demonstrated for IHC17 and reverse transcription polymerase chain reaction.4,10 No influence of double probe concentration could be detected in any of the infectious agents examined (data not shown).
Figure 3.
Influence of proteinase K concentration as seen in stomach tissue of a snake infected with Cryptosporidium serpentis. In situ hybridization was performed after 71 weeks of prolonged formalin fixation. Parasites are stained dark purple. a, standard proteinase K concentration, b, double proteinase K concentration, c, 4-fold proteinase K concentration. Bar = 400 μm.
In general, both viral pathogens lost ISH reactivity after shorter fixation periods than the protozoan agents. A possible explanation may be the targeting region of the probes and the number of accessible copies of these regions within the tissue. In the case of PCV-2, the probe binds to a region within the capsid protein gene, resulting mainly in detection of messenger RNA (mRNA). For PRRSV, both the viral-positive, single-strand RNA and the viral mRNA are recognized by the probe. For both protozoal agents, the probes were designed complementary to a region within the rRNA gene. It is known that rRNA has the highest expression levels of all RNA found in the cell. Therefore, it may be assumed that with progressive formalin-induced cross-linking, more accessible rRNA molecules are still available compared to other types of RNA.
In summary, prolonged formalin fixation has a significant influence on the detectability of different pathogenic agents using ISH. The current study demonstrated that the endpoint of detection of pathogenic agents varies between pathogens and the structure of the probe. Detection of agents after prolonged formalin fixation could be enhanced and even recovered using higher proteinase K concentrations.
Acknowledgements
The authors wish to thank Nora Nedorost, Karin Fragner, and Klaus Bittermann for their excellent technical support.
Funding
This work was funded by the Austrian Science Fund (FWF) grant P20926.
Footnotes
Sources and manufacturers
a. Roche, Basel, Switzerland.
b. VWR International, Vienna, Austria
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bukovsky C, Schmoll F, Revilla-Fernández S, Weissenböck H. Studies on the aetiology of non-suppurative encephalitis in pigs. Vet Rec. 2007;161:552–558. doi: 10.1136/vr.161.16.552. [DOI] [PubMed] [Google Scholar]
- 2.Chvala S, Fragner K, Hackl R, et al. Cryptosporidium infection in domestic geese (Anser anser f. domestica) detected by in-situ hybridization. J Comp Pathol. 2006;134:211–218. doi: 10.1016/j.jcpa.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Ha Y, Chae C. Optimal probe size and fixation time for the detection of Porcine circovirus-2 DNA by in situ hybridization in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest. 2009;21:649–654. doi: 10.1177/104063870902100509. [DOI] [PubMed] [Google Scholar]
- 4.McKinney MD, Moon SJ, Kulesh DA, et al. Detection of viral RNA from paraffin-embedded tissues after prolonged formalin fixation. J Clin Virol. 2009;44:39–42. doi: 10.1016/j.jcv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Miller MA, Ramos-Vara JA, Kleiboeker SB, Larson RL. Effects of delayed or prolonged fixation on immunohistochemical detection of bovine viral diarrhea virus type I in skin of two persistently infected calves. J Vet Diagn Invest. 2005;17:461–463. doi: 10.1177/104063870501700509. [DOI] [PubMed] [Google Scholar]
- 6.Mostegl MM, Richter B, Nedorost N, et al. Investigations on the prevalence and potential pathogenicity of intestinal trichomonads in pigs using in situ hybridization. Vet Parasitol. 2011;178:58–63. doi: 10.1016/j.vetpar.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pikkarainen M, Martikainen P, Alafuzoff I. The effect of prolonged fixation time on immunohistochemical staining of common neurodegenerative disease markers. J Neuropathol Exp Neurol. 2010;69:40–52. doi: 10.1097/NEN.0b013e3181c6c13d. [DOI] [PubMed] [Google Scholar]
- 8.Richter B, Fragner K, Weissenböck H. Simultaneous detection of protozoa in the tissues of snakes by double in situ hybridization. Microsc Res Tech. 2008;71:257–259. doi: 10.1002/jemt.20546. [DOI] [PubMed] [Google Scholar]
- 9.Richter B, Kübber-Heiss A, Weissenböck H, Schmidt P. Detection of Cryptosporidium spp., Entamoeba spp. and Monocercomonas spp. in the gastrointestinal tract of snakes by in-situ hybridization. J Comp Pathol. 2008;138:63–71. doi: 10.1016/j.jcpa.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Scicchitano MS, Dalmas DA, Bertiaux MA, et al. Preliminary comparison of quantity, quality, and microarray performance of RNA extracted from formalin-fixed, paraffin-embedded, and unfixed frozen tissue samples. J Histochem Cytochem. 2006;54:1229–1237. doi: 10.1369/jhc.6A6999.2006. [DOI] [PubMed] [Google Scholar]
- 11.Segalés J, Calsamiglia M, Rosell C, et al. Porcine reproductive and respiratory syndrome virus (PRRSV) infection status in pigs naturally affected with post-weaning multisystemic wasting syndrome (PMWS) in Spain. Vet Microbiol. 2002;85:23–30. doi: 10.1016/s0378-1135(01)00474-6. [DOI] [PubMed] [Google Scholar]
- 12.Webster JD, Miller MA, Dusold D, Ramos-Vara J. Effects of prolonged formalin fixation on the immunohistochemical detection of infectious agents in formalin-fixed, paraffin-embedded tissues. Vet Pathol. 2010;47:529–535. doi: 10.1177/0300985809359607. [DOI] [PubMed] [Google Scholar]
- 13.Webster JD, Miller MA, Dusold D, Ramos-Vara J. Effects of prolonged formalin fixation on diagnostic immunohistochemistry in domestic animals. J Histochem Cytochem. 2009;57:753–761. doi: 10.1369/jhc.2009.953877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss LM, Chen YY. Effects of different fixatives on detection of nucleic acids from paraffin-embedded tissues by in situ hybridization using oligonucleotide probes. J Histochem Cytochem. 1991;39:1237–1242. doi: 10.1177/39.9.1918942. [DOI] [PubMed] [Google Scholar]
- 15.Weissenböck H, Fragner K, Nedorost N, et al. Localization of avian bornavirus RNA by in situ hybridization in tissues of psittacine birds with proventricular dilatation disease. Vet Microbiol. 2010;145:9–16. doi: 10.1016/j.vetmic.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Weissenböck H, Mrakovcic M, Ladinig A, Fragner K. In situ hybridization for Lawsonia intracellularis-specific 16s rRNA sequence in paraffin-embedded tissue using a digoxigenin-labeled oligonucleotide probe. J Vet Diagn Invest. 2007;19:282–285. doi: 10.1177/104063870701900309. [DOI] [PubMed] [Google Scholar]
- 17.Werner M, Chott A, Fabiano A, Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24:1016–1019. doi: 10.1097/00000478-200007000-00014. [DOI] [PubMed] [Google Scholar]