Summary
1. Measuring physiological and behavioural parameters in free-ranging animals – and therefore under fully natural conditions – is of general biological concern but difficult to perform.
2. We have developed a minimally invasive telemetry system for ruminants that is capable of measuring heart rate (HR), body temperature (Tb) and locomotor activity (LA). A ruminal transmitter unit was per os placed into the reticulum and therefore located in close proximity to the heart. The unit detected HR by the use of an acceleration sensor and also measured Tb. HR and Tb signals were transmitted via short-distance UHF link to a repeater system located in a collar unit. The collar unit decoded and processed signals received from the ruminal unit, measured LA with two different activity sensors and transmitted pulse interval-modulated VHF signals over distances of up to 10 km.
3. HR data measured with the new device contained noise caused by reticulum contractions and animal movements that triggered the acceleration sensor in the ruminal unit. We have developed a software filter to remove this noise. Hence, the system was only capable of measuring HR in animals that showed little or no activity and in the absence of rumen contractions. Reliability of this ‘stationary HR’ measurement was confirmed with a second independent measurement of HR detected by an electrocardiogram in a domestic sheep (Ovis aries).
4. In addition, we developed an algorithm to correctly classify an animal as ‘active’ or ‘at rest’ during each 3-min interval from the output of the activity sensors. Comparison with direct behavioural observations on free-ranging Alpine ibex (Capra ibex) showed that 87% of intervals were classified correctly.
5. First results from applications of this new technique in free-ranging Alpine ibex underlined its suitability for reliable and long-term monitoring of physiological and behavioural parameters in ruminants under harsh field conditions. With the battery settings and measurement cycles used in this study, we achieved a system lifetime of approximately 2 years.
Keywords: body temperature, heart rate, locomotor activity, ruminant, telemetry
Introduction
Many questions in biology require telemetrical measurement of heart rate (HR) in free-ranging animals (Butler et al. 2004). Telemetry generally allows accurate measurements of physiological variables in the field and allows better understanding of the interaction of animals with their environment and with each other (Ropert-Coudert & Wilson 2005). However, until today, there have been considerable drawbacks when measuring HR in the field with the use of telemetry systems. Continuous transmission of HR is nearly impossible because free-ranging animals are not often found within the range of receiving antennas. Another disadvantage is the need to implant monitoring devices in order to reliably pick up an electrocardiogram (ECG) over long periods of time. Surgical implantation in the field is complicated and can cause problems. In particular, subcutaneous implantation of devices and ECG electrodes is surgically challenging and bears the risk of infection, repulsion or damage to the device (Woakes, Butler, & Bevan 1995; Wild et al. 1998; Giacometti et al. 2001; Arnold et al. 2004; Laske et al. 2005; Arnold, Ruf, & Kuntz 2006). Intra-abdominal implantation can lead to adhesions between the transmitter and rumen or intestines, or to peritonitis, skin necrosis and death (Eagle, Choromanski-Norris, & Kuechle 1984; Guynn, Davis, & Vonrecum 1987; Wagner 1991; Wallace et al. 1992; Moe et al. 1995). The deep anaesthesia required for surgical implantation also carries significant risks.
Here, we report a new approach for continuous measurement of HR and body temperature (Tb) in ruminants that avoids surgical implantation. The rationale for developing this device was the fact that foreign objects placed in the reticulum – the first compartment of the fore-stomach – are tolerated well by ruminants. This tolerance is widely used in veterinary medicine to render swallowed objects innocuous. If a ruminant has swallowed a potentially dangerous object like a nail, the common therapy is to insert a wire case with a magnet inside. The nail will get attached to the magnet and is thus rendered harmless. In the vast majority of cases the whole device remains in place causing no harm to the animal (Braun, Gansohr, & Flückiger 2003). Another common use of foreign objects introduced into the reticulum is the use of identification boli (Hanton 1981; Fallon & Rogers 2001; Garin, Caja, & Bocquier 2003).
Inspired by these routine methods in veterinary practice, we further developed our existing telemetry system for simultaneous measurement of physiological and behavioural parameters (Giacometti et al. 2001). We constructed an internal transmitter designed to persist in the reticulum of ruminants and to measure both HR and Tb. Administration occurs per os and therefore does not require surgical intervention, only mechanical or chemical immobilization. The ruminal unit communicates via UHF link with a repeater system located in a collar unit, where data are stored. The collar unit can perform additional measurements, such as recording locomotor activity (LA) and enables radio-tracking by integration of a VHF transmitter.
Below, we describe this new technology in detail and provide results from applications in cattle (Bos taurus), domestic sheep (Ovis aries) and free-ranging Alpine ibex (Capra ibex). Treatment of the animals in this study was approved by the institutional ethics committees (Veterinärmedizinische Universität Wien; Österreichisches Bundesministerium für Bildung, Wissenschaft und Kultur, Bereich Gentechnik und Tierversuche, GZ 68.205/25-BrGT/2003, GZ 68.205/7-BrGT/2004; Amt für Lebensmittelsicherheit und Tiergesundheit Graubünden, Switzerland, No. 24/2006) and followed the guiding principles for research involving animals and human beings. Furthermore, we describe filtering, validation and calibration procedures used to improve the quality of the telemetrically obtained data.
Technical design
RUMINAL UNIT
The ruminal unit was packed in a cylindrical housing, diameter 22 mm, length 80 mm and weight ca 100 g (Fig. 1). A stainless steel compartment contained the battery and provided the weight necessary for the device to remain in the reticulum. A glass ceramic compartment covered the electronic circuits and enabled the propagation of electromagnetic waves through the housing. The stainless steel and glass ceramic parts were bonded and sealed hermetically with an epoxy adhesive (Duralco 4525; Contronics Corp., Brooklyn, NY, USA).
Fig. 1.
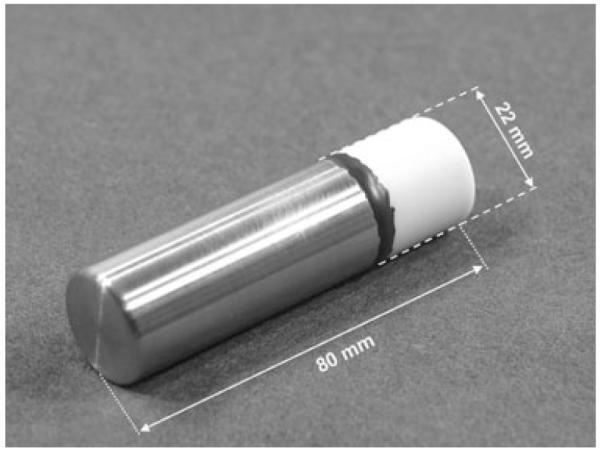
Ruminal unit; weight 100 g.
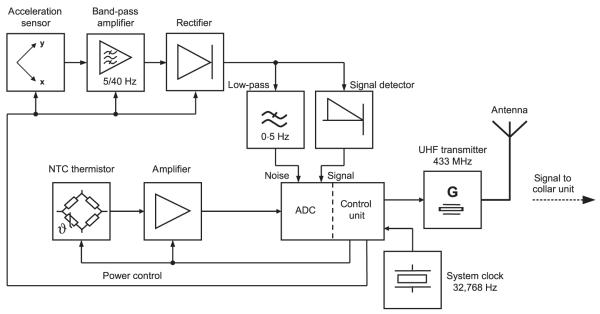
The ruminal unit recorded heart beats with a biaxial acceleration sensor for detection of mechanical shock waves (Fig. 2). The rationale for using a biaxial instead of a triaxial acceleration sensor was its high sensitivity (3–30 × 10−3g) combined with relatively low power consumption. Furthermore, because of the heaviness of the ruminal unit, we also expected no remarkable acceleration amplitude in the direction of its longitudinal axis. Due to its asymmetric weight distribution, it is likely that the ruminal unit is oriented vertically within the reticulum. In this case, both axes of the sensor would be oriented horizontally, which allows the most sensitive detection of mechanical shock waves deriving from heart beats. Nevertheless, it is obvious that consecutive heart beats cause different amplitudes in the sensor output whenever the angular orientation and position of the ruminal unit change. To address such situations, we used an adaptive trigger for detection of pulses. If a series of heart beats with high acceleration amplitudes was followed by a heart beat of much lower acceleration amplitude, the trigger level was shifted downwards in order to detect the subsequent signals. This trigger-level adaptation seemed the best solution to minimize effects of the inevitable conflict between eliminating noise and detecting as many real heart beats as possible.
Fig. 2.
Block diagram of the ruminal unit. The ruminal unit provides measurement of heart rate and body temperature and transmits data via UHF to the collar unit.
Acceleration signals from both axes were added, filtered by a band-pass filter (cut-off frequencies 5–40 Hz, which also eliminated static acceleration signals), amplified and rectified (Fig. 2). The rectifier output delivered a signal that concentrated on the typical spectral content of the acceleration signal found in heart beats (5–40 Hz, which is similar to the spectral content of the QRS complex in an ECG signal) and was highly independent from the angular orientation of the ruminal unit around the longitudinal axis. The rectified signal was: (i) filtered with a low-pass filter (cut-off frequency 0·5Hz) to measure noise level, and (ii) fed to a signal detector that formed an envelope signal. Both filtered and enveloped signals were analogue/digital converted and processed by the control unit (PIC microcontroller; Microchip Technology Inc., Chandler, AZ, USA). The microcontroller identified peaks in the acceleration signal by applying an adaptive trigger level that lies between the noise level and peaks in the acceleration signal.
For measurements of Tb, the microcontroller activated a temperature measurement circuit at programmable time intervals (e.g. every 3 min). Negative temperature coefficient (NTC) thermistors were used to measure Tb. As in HR detection, thermistor outputs were amplified, analogue/digital converted with a resolution of 0·1°C and thereafter processed by the same microcontroller.
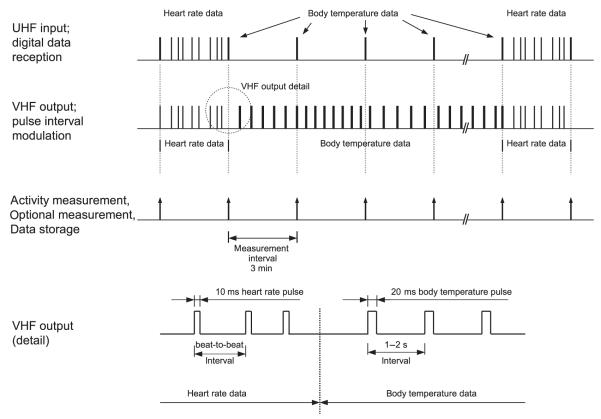
The microcontroller operated at a very low clock frequency of 32,768 Hz and consumed a permanent current of about 20 μA from a single 3·6-V lithium-thionyl chloride cell (nominal capacity 3400 mA h). Sensors and signal processing circuits could be switched ‘on’ and ‘off’ by the microcontroller to reduce power consumption (power control; Fig. 2). In our applications, we chose a HR detection period of 3 min every 21 min (Fig. 3). Therefore, for HR above approximately 40 beats per minute, we detected >120 heart beats per detection period. This quantity provided sufficient HR data points on which to apply our software filter for extraction of HR in stationary animals (see the Heart rate measurement section). The temperature circuit, however, needed only several milliseconds for one Tb measurement every 3 min (Fig. 3). With this cycle of HR and Tb measurement, the ruminal unit had a lifetime of approximately 21 months.
Fig. 3.
Itinerary of data acquisition and transmission in the ruminal unit.
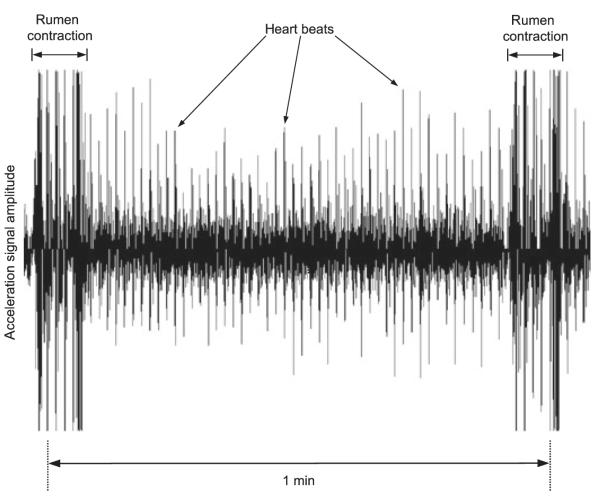
Data obtained from cattle equipped with an experimental ruminal unit that continuously transmitted unfiltered acceleration signals showed that heart beats produced distinct peaks in the sensor output (Fig. 4). However, other sources of mechanical shock waves like rumen contractions also produced sensor output peaks that rendered detection of heart beats during these periods virtually impossible.
Fig. 4.
An example of the signal output measured by the acceleration sensor in an experimental ruminal unit before further processing by the microcontroller.
Signals from the ruminal unit were transmitted to the collar unit via UHF that operated at the standardized public frequency of 433 MHz with an output power of about 2 mW and a power-saving modulation technique with carrier frequency pulses (on–off keying; Fig. 2). During each 3-min HR detection period, short data strings with duration of 36 ms were sent whenever the sensor output exceeded a preset trigger level (Fig. 3). Another string of the same length was sent for each Tb measurement every 3 min. Each signal string began with a preamble to adapt the UHF receiver in the collar unit to the specific signal strength. The preamble was followed by a header and 16 bit of data; a 3-bit descriptor that labelled the transmitted data as a HR or Tb string, a 5-bit identifier that enabled discrimination from interfering signals emitted by ruminal units of other animals close-by and, finally, 8 bits of binary data that either stated the actual noise level in HR detection or delivered a single Tb value. The noise level conveyed technical information for test purposes, such as signal-to-noise ratio but was ignored in standard applications.
COLLAR UNIT
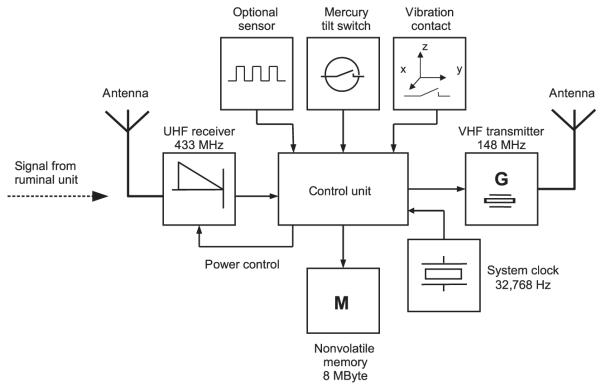
The collar unit consisted of an UHF receiver module, which picked up signals at 433 MHz from the ruminal unit (Fig. 5). Signals were demodulated and delivered to a control unit (PIC microcontroller). When an HR string was received from the ruminal unit, the VHF transmitter in the collar unit (unrestricted public frequencies, e.g. around 148 MHz, with an output power of about 8 mW) was switched on for 10 ms, resulting in a power-saving pulse interval-modulated signal (Fig. 6). By contrast, when a Tb string was received from the ruminal unit, a pulse duration of 20 ms (to distinguish between HR and Tb signals) and a temperature-dependent pulse interval of 1–2 s (which corresponds to temperatures of 18–42°C) were generated. Therefore, each Tb string resulted in a VHF signal with a constant pulse interval over 3 min, which facilitated radio-tracking of the animal. The theoretical maximum transmission distance of the VHF signal was approximately 10 km but practically declined to less than 5 km in the field application on Alpine ibex. This is because many factors – such as direct contact between transmitter and receiver, humidity, signal reflection, air temperature, ground cover, (forest) canopy, animal activity, animal position, etc. – influence the transmission of VHF signals. To save battery power in the collar unit, the UHF receiver was switched on during the whole 3 min of HR detection but only for 2 s every 3 min when Tb strings were expected (power control; Fig. 5).
Fig. 5.
Block diagram of the collar unit. The collar unit receives and processes signals from the ruminal unit, provides detection of locomotor activity and other optional measurements, stores all the data and continuously transmits VHF signals for radio-tracking.
Fig. 6.
Itinerary of data reception, processing, storage and transmission in the collar unit.
For measurement of LA, we used a triaxial vibration contact for detection of unspecific omnidirectional movements and a mercury tilt switch for detection of vertical head up/down movements. Three different activity parameters were measured every 3 min: (i) the duration of (omnidirectional) moving activity, (ii) the number of vertical head movements and (iii) the duration of ‘head down’.
All data were stored in an 8-MB non-volatile memory in the collar unit. After initial trials and due to this limited memory capacity, we programmed the collars to save only physiologically likely HR for phases at rest. We therefore ignored HR above 140 and 120 beats per minute in the sheep and ibex trial respectively. Data stored in the collar were read after retrieval. In addition, HR and Tb data could be obtained immediately because the pulse interval-modulated VHF signals contain this information. However, whenever a receiving station is used to receive transmitted data, there is the possibility that noise due to interference and data loss through fragmentary reception may occur. In our trials with storage of HR data in the collar and simultaneous transmission of HR data to a receiving station, data loss averaged 17%.
The collar electronics were also powered by a single 3·6-V lithium-thionyl chloride cell with a nominal capacity of 17,000 mA h. With this type of cell, the collar unit had a total weight of 450 g and an approximate lifetime of 2 years when using the recording cycle described above (Fig. 6).
SYSTEM SYNCHRONIZATION
As both collar unit and ruminal unit were switched on and off, synchronization was necessary to correctly run the system. This was achieved with a synchronization command transmitted by the ruminal unit at the beginning of every HR detection period (Fig. 3). The synchronization command was an adapted Tb string with a descriptor that reset the itinerary of the collar unit.
SPECIAL OPTIONS
It is also possible to add serial digital data sensors to the collar unit to collect information about environmental parameters, such as ambient temperature, light intensity or air pressure (Fig. 5). Furthermore, a stand-alone GPS receiver module with autonomous power supply and on board storage of data may be mounted on the collar. For short-range applications, the ruminal unit can be equipped with a VHF transmitter (instead of the UHF transmitter) that directly transmits data from inside the body to a receiving station. In this case, the modulation technique to encode HR and Tb information is performed by the ruminal unit. This option renders the use of a collar unit unnecessary for measurement of HR and Tb.
Applications
First administration of the ruminal unit was in a 4-year-old female domestic sheep. We chemically immobilized the animal and, after blindfolding and restraining, transported it to a crush cage where, after 25 min, anaesthesia was partially reversed. As soon as the animal was able to stand, the ruminal unit was introduced into the pharynx with an applicator used to introduce identification boli in cattle (Rumitag S.L., Barcelona, Spain). After successful application, anaesthesia was completely reversed. The ruminal unit remained in situ for 6 months and was removed after the animal was slaughtered. It was located in the sheep’s reticulum, the first compartment of the fore-stomach, and therefore situated in close vicinity to the heart. We can assume that during the previous 6 months, the location of the ruminal unit was stable because HR detection was continuous and of constant quality. The ruminal unit showed very little abrasion on its glass ceramic part, the epoxy adhesive still held tight and showed no dissolution effects.
The first long-term field study was conducted from 2006 to 2009 with 22 free-ranging adult Alpine ibex in the Swiss Alps. Individuals were caught and immobilized with dart application of anaesthetic drugs by the use of a gas-driven rifle (Abderhalden et al. 1998). Legs of immobilized animals were bound and eyes masked. Introduction of the ruminal unit into the pharynx occurred immediately after anaesthetic reversal, when animals had recovered from the state of deep dormancy and showed a pronounced swallowing reflex. For introduction of the ruminal unit we used the same device as for the sheep. We then forced the ibex to swallow by pouring water into their mouth, which facilitated the passage of the ruminal unit through the oesophagus and entry into the rumen.
Heart rate measurement
Heart rate data measured with a ruminal unit in an Alpine ibex (Fig. 7a) and a domestic sheep (Fig. 8a) showed two bands of dense data surrounded by noise. Noise was generated by the acceleration sensor when it detected rumen contractions or animal movement (Fig. 8). The wide band of dense data in the middle of the scale in Fig. 8a indicates real HR, whereas the narrow band at the lower end of the scale is the result of the adaptive trigger level used to cope with varying acceleration amplitudes. Each adaptation of the trigger level can lead to the loss of a single heart beat resulting in a twofold pulse interval, which for HR corresponds to half the real level. However, the resulting band of false data points was eliminated by the software routine used to filter real HR.
Fig. 7.
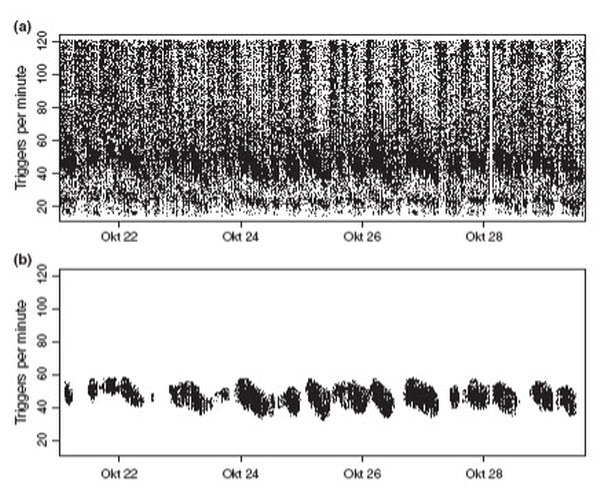
Extraction of real heart rate from the raw data detected by a ruminal unit in a free-ranging Alpine ibex. (a) Raw data including noise. (b) Filtered stationary heart rate data during phases when the animal was at rest or showed only weak activity.
Fig. 8.
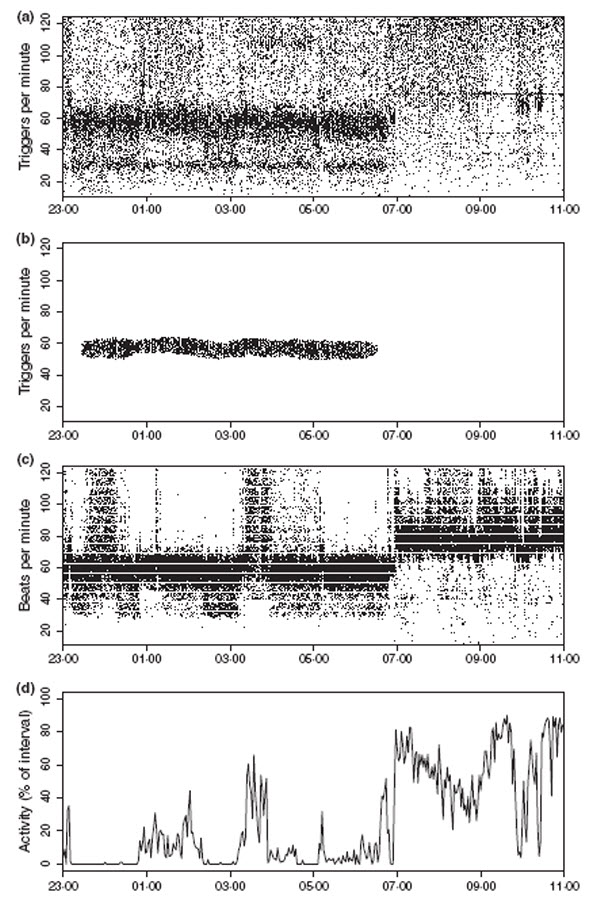
Comparison of heart rate detected by the acceleration sensor in the ruminal unit with simultaneously detected heart rate via ECG, in a domestic sheep. (a) Raw data from the ruminal unit. (b) Stationary heart rate data from the ruminal unit after filtering. (c) Heart rate from ECG. (d) Proportion of activation of the triaxial vibration contact during 2-min measurement intervals as a measure of the locomotor activity of the animal.
To remove noise from real HR, we: (i) deleted physiologically unlikely HR for phases at rest (i.e. data points above 120 and 100 triggers per minute in ibex and sheep respectively), and (ii) filtered the remaining data with a contrast-enhancing routine implemented in the software package R (R Development Core Team 2009). The filter was designed to assign each data point a weight based on the number of neighbouring points. This was implemented by looping through all data points and counting neighbours within a rectangular frame, adjustable in width and height and centred on a focal data point. Subsequently, we ranked all data points in a given data set according to their neighbour-counts and retained only those points with highest ranks (i.e. points within a dense band). By varying cut-off values and rectangle size, we found that retaining the highest 30% of all ranked data points and using a rectangle width of 1600 data points along the time axis and a rectangle height of 20 triggers per minute produced the best results. By applying this filtering routine to the ibex and sheep data, we were able to extract HR data in stationary animals (Fig. 7b; Fig. 8b), visible as a band within a large amount of noise produced by acceleration signals apparently not generated by heart beats (Fig. 7a; Fig. 8a). We use the term ‘stationary HR’ because reliable measurements of HR were limited to periods when the animals were at rest or showed only weak activity (Fig. 8).
We validated our method of HR detection and filtering by simultaneous acquisition of an ECG in the sheep. Over 7 days the ECG signal was detected by two electrodes and the resulting HR sent by a transmitter attached to a harness. For detection of HR by the ruminal unit, we used the type with a VHF transmitter for direct data transmission, as described under ‘special options’. Data from both transmitters were received and stored by a remote receiving station. In addition, we equipped the sheep with the activity sensors described above, mounted on a collar. Activity parameters were measured over intervals of 2 min and stored in the collar.
The wide band in the raw data from the ruminal unit, around 60 triggers per minute (Fig. 8a), corresponds well to the ECG data (Fig. 8c), which demonstrates that the ruminal unit accurately recorded HR. Our filtering routine separated this band effectively from surrounding noise, which indicates the suitability of the filter and its parameter settings (Fig. 8b). In contrast to the recordings during periods of rest and weak activity, measurements performed while the animal was highly active (e.g. after 07:00 hours; Fig. 8d) were vastly dispersed and essentially conveyed no information on HR (cf. Fig. 8a,b with Fig. 8c,d).
To correlate stationary HR with ECG data, the whole sheep data set with 7 days of simultaneously measured heart rates was considered for analysis. Raw data from the ruminal unit were filtered to give stationary HR and thereafter summarized to 2-min means. The resulting data set was reduced to the 95% CI of its range (56–82 beats per minute). Accordingly, ECG raw data were summarized to 2-min means and reduced to the 95% CI of the stationary HR (Fig. 9). This step was necessary to eliminate data points that lay outside the range of reliable HR detection by the ruminal unit. The medians of classes of one beat per minute correlated highly (R2=0·97), particularly at the lower range of the scale. Above 71 beats per minute, however, the correlation became weaker, which again reveals the limitations of our system in the detection of HR during elevated animal activity.
Fig. 9.
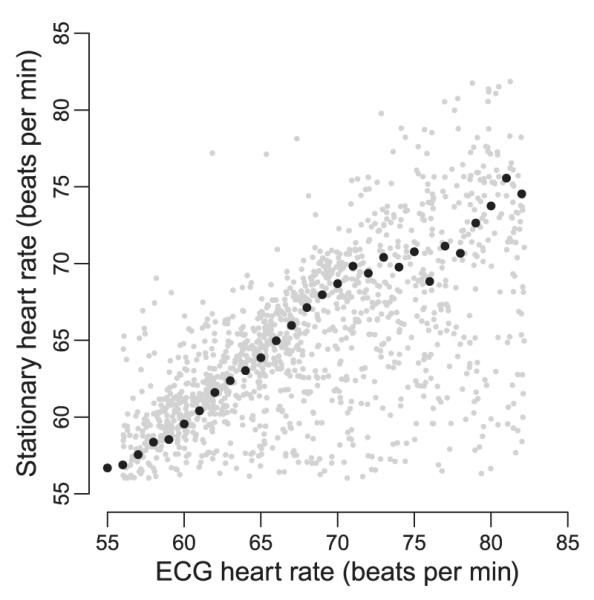
Correlation of heart rates measured simultaneously by a ruminal unit (stationary heart rate) and via ECG over 7 days in a domestic sheep. These continuously detected data sets were both summarized to 2-min means and reduced to the 95% CI of the stationary heart rate (grey dots). Class medians (black dots) are added to better visualize the overall trend. For further details, see text.
Body temperature measurement
The NTC thermistors of the ruminal units were calibrated individually after assembly and prior to application. Each unit was placed in a water bath and the temperature dependence of the pulse interval was measured in 5°C increments between 20 and 40°C. We were able to recalibrate thermistors from three devices recovered from ibex that were found dead. Deviation from the first calibration was, in all cases, <0·1°C over the entire measurement range after having been in situ for up to 19 months.
Activity measurement
Inferring an animal’s behaviour from the output of the two activity sensors in the collar unit also required calibration. This was done through direct observation of the behaviour of two free-ranging, collared adult Alpine ibex (a 7-year-old male and a 5-year-old female). Observations were carried out between 7 November 2006 and 23 April 2007 for a total of 32·6 h by focal-animal sampling (Altmann 1974). Our attempts to extract detailed information regarding an animal’s behaviour from the sensors’ output were unsuccessful; therefore, we restricted our analysis to discriminate ‘active’ from ‘at rest’. An animal was classified as ‘at rest’ when it was lying on the ground, for all other behaviours it was classified as ‘active’.
To discriminate between ‘at rest’ and ‘active’ from sensor output only, we selected those 569 intervals when an animal had either been resting or active for an entire 3-min interval. We used a logistic regression approach that accounts for repeated measurements of the two individuals to predict the observationally determined binomial response variable ‘active’ vs. ‘at rest’ from the activity sensor output (function glmmPQL using ‘family binomial’ within package R; R Development Core Team 2009). We started with a full model including all telemetrically measured variables, ‘duration of moving activity’, ‘number of vertical head movements’, ‘duration of head down’, as well as their interaction terms as predictors and thereafter eliminated non-significant terms. The final model only included ‘duration of moving activity’ (P <0·001), ‘number of vertical head movements’ (P <0·001) and the interaction between these terms (P <0·001), while ‘duration of head down’ was insignificant (P =0·33).
The reliability of predictions using the parameter estimates of our final model was tested by bootstrapping. We estimated the parameters for the predictors with randomly generated subsets of our complete data set (80% of all 3-min intervals). With these parameter estimates, we classified the remaining 20% of the 3-min intervals as ‘at rest’ or ‘active’. By repeating this procedure 1000 times, we found that we correctly classified 87% (95% CI ± 13%) of all 3-min intervals from telemetric data as ‘active’ or ‘at rest’.
Discussion
APPLICATIONS
Our telemetry system offers a minimally invasive method to acquire physiological and behavioural data in ruminants. Anaesthesia is not mandatory for application and can even be omitted in confined animals, such as cattle, or smaller ungulates, with the latter restrained sufficiently in a crush cage or a box trap. However, in cases where anaesthesia is required, ruminal units should be introduced to mechanically restrained animals during the early recovery phase of the anaesthesia when the swallowing reflex has resumed. The simple administration of the ruminal unit together with the well-known tolerance to foreign objects in the reticulum greatly reduces the impact on experimental animals.
For long-term retention in the reticulum, the weight of the ruminal unit was a critical feature. Our first series of ruminal units, used in a study of red deer (Cervus elaphus) housed under semi-natural conditions, weighed only 80 g. These units remained in the reticulum over winter, but were regurgitated in spring, presumably because of the massive increase in food intake and ruminal activity. We were able to inhibit regurgitation by increasing the mass of the unit to 100 g while keeping its dimensions stable. This heavier unit was retained in red deer (up to 4 years), domestic sheep (6 months) and Alpine ibex (up to 2 years) without any signs of a negative impact on the animals. However, our experiences show that, if desired, spontaneous regurgitation of the ruminal unit can be accomplished by a reduction in its weight.
In future, our system may also be applied to monogastric species. Stomach temperature data loggers and transmitters with dimensions comparable with those of our ruminal unit have already been used in different species of free-ranging seabirds (Wilson, Cooper, & Plötz 1992; Wilson et al. 1995) as well as in northern elephant seals (Kuhn et al. 2009). As these species appear to tolerate such foreign objects within their stomachs, it is likely that we may also be able to successfully measure HR in monogastric animals by the incorporation of our ruminal unit.
HEART RATE MEASUREMENT
Telemetrical recording of HR enables continuous measurements over long periods of time (Schober 1992; Schober, Fluch, & Bögel 1997). This renders HR telemetry extremely suitable for field applications (Butler et al. 2004), particularly when long-term estimates of metabolic rate and its relative changes are of interest. However, the surgical implantation of recording devices bears inherent risks and is limited in application. The new telemetry system introduced here circumvents these problems. Compared with surgical interventions, the system is easy to apply and hence truly suitable for field studies.
The major drawback of the new system is the frequent triggering of the acceleration sensor by mechanical shock waves that derive from movements other than heart beats. Although our filtering routine efficiently removed most of this noise, reliable measurements of HR with the device are limited to periods when study animals are at rest or show only weak activity (Fig. 8). During such phases, stationary HR and HR deriving from an ECG correlated well (Fig. 9). However, measurements at the beginning of a rest phase may pass the filter, even though HR may still be elevated due to preceding high activity. Thus, the filtered stationary HR presumably represents resting HR in most, but not all, cases.
BODY TEMPERATURE MEASUREMENT
As with HR detection, our telemetry system offers an effective and almost non-invasive method to continuously determine Tb in ruminants over long periods of time. However, there are limitations in using rumen temperature as a measure of Tb. In cattle, average rumen temperature is usually 0·8–1·6°C higher than abdominal or rectal temperature (Cunningham, Martz, & Merilan 1964; Beatty et al. 2008). Temporal declines in rumen temperature due to water consumption have been reported for sheep (Brod, Bolsen, & Brent 1982), cattle (Dale, Stewart, & Brody 1954; Cunningham et al. 1964) and musk oxen (Ovibus moschatus; Crater & Barboza 2007). Similar patterns may arise through ingestion of very cold food, particularly if the food is wet or covered by snow. On the other hand, temporal increases in rumen temperature due to microbial fermentation after feeding are also known to occur (Barnes, Comline, & Dobson 1983; Dehority 2003). These short-term changes should be considered in the interpretation of Tb measured with a ruminal unit. However, changes in rumen temperature affect abdominal or rectal temperature by direct conduction to adjacent tissues and by convection to perfusing blood (Cunningham et al. 1964; Gengler et al. 1970; Beatty et al. 2008). Therefore, Tb measured with a ruminal unit will be highly correlated with long-term variation in core Tb. If short-term changes of core Tb are of interest, appropriate filtering of Tb data is necessary to exclude confounding effects of water consumption and food intake.
ACTIVITY MEASUREMENT
Diurnal and seasonal activity patterns are important factors in understanding the behavioural ecology and habitat use of a species, and hence are important for wildlife management. However, it is often difficult to assess an animal’s activity, especially in species that are elusive, forest-dwelling or range over large distances (Gervasi, Brunberg, & Swenson 2006). The use of radio telemetry is therefore a promising tool to study activity patterns in free-ranging animals. However, calibration by simultaneous visual observations is necessary to infer an animal’s behaviour from activity sensor output (Wagner, Hightower, & Pace 2001). With our logistic regression approach, we were able to correctly predict ‘active’ and ‘at rest’ in 87% of cases. This result is in line with other calibration studies on collared animals (Hansen, Garner, & Fancy 1992; Moen, Pastor, & Cohen 1996; Baumann, Struch, & Pfluger 2000; Adrados et al. 2003; Gervasi et al. 2006).
In recent studies on several mammal and bird species, external acceleration sensors were used to identify different behaviours (Yoda et al. 1999, 2001) as well as to estimate energy expenditure and speed during locomotion (Wilson et al. 2006; Halsey et al. 2008, 2009). In the latter studies, when properly calibrated, ‘overall dynamic body acceleration’ correlated well with the rate of oxygen consumption and therefore has great potential in estimating the energy expenditure involved in locomotion. In future, this method may be improved by using a three-dimensional version of our ruminal unit, rather than mounting external acceleration sensors on animals.
Conclusion
In summary, the new telemetry system is a suitable tool for measurement of physiological parameters and to quantify activity levels under difficult field conditions. Per os administration of the ruminal unit is simple and demands no surgical intervention. With simultaneous measurement of stationary HR, Tb and LA, we have developed a system that may significantly contribute to an increased understanding of physiological adjustments. Moreover, our system allows continuous measurements over long periods of time and may also be useful in the investigation of broader physiological questions, such as seasonal changes in the physiology of free-ranging animals.
Acknowledgements
We thank the Hunting and Fishing Services of Grisons, Chur, Switzerland, and especially Daniel Godli, Georg Brosi and Hannes Jenny for their great support in the ibex field work. This study was funded by the Swiss Federal Office for the Environment, Bern with a stipend to Claudio Signer. Many thanks to Rebecca Drury, Wolfgang Zenker, ‘Skywards’, the Swiss National Park and the community of Pontresina.
References
- Abderhalden W, Buchli C, Ratti P, Godli D. Capture and immobilization of alpine ibex (Capra i. ibex) Zeitschrift für Jagdwissenschaft. 1998;44:123–132. [Google Scholar]
- Adrados C, Verheyden-Tixier H, Cargnelutti B, Pepin D, Janeau G. GPS approach to study fine-scale site use by wild red deer during active and inactive behaviors. Wildlife Society Bulletin. 2003;31:544–552. [Google Scholar]
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus) American Journal of Physiology-Regulatory and Integrative Comparative Physiology. 2004;286:R174–R181. doi: 10.1152/ajpregu.00593.2002. [DOI] [PubMed] [Google Scholar]
- Arnold W, Ruf T, Kuntz R. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. Journal of Experimental Biology. 2006;209:4566–4573. doi: 10.1242/jeb.02536. [DOI] [PubMed] [Google Scholar]
- Barnes RJ, Comline RS, Dobson A. Changes in the blood flow to the digestive organs of sheep induced by feeding. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1983;68:77–88. doi: 10.1113/expphysiol.1983.sp002704. [DOI] [PubMed] [Google Scholar]
- Baumann M, Struch M, Pfluger D. Äsen oder Ruhen? – Eine neue Methode zur Verhaltensbestimmung von Huftieren anhand mehrfach pulsmodulierter Sendersignale. In: Baumann M, Struch D, editors. Waldgemsen. WildARK; Bern: 2000. pp. 1–13. [Google Scholar]
- Beatty DT, Barnes A, Taylor E, Maloney SK. Do changes in feed intake or ambient temperature cause changes in cattle rumen temperature relative to core temperature? Journal of Thermal Biology. 2008;33:12–19. [Google Scholar]
- Braun U, Gansohr B, Flückiger M. Effects of atropine, scopolamine and xylazine on the placement of an orally administered magnet in cows. Veterinary Record. 2003;152:258–260. doi: 10.1136/vr.152.9.258. [DOI] [PubMed] [Google Scholar]
- Brod DL, Bolsen KK, Brent BE. Effect of water temperature on rumen temperature, digestion and rumen fermentation in sheep. Journal of Animal Science. 1982;54:179–182. doi: 10.2527/jas1982.541179x. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Green JA, Boyd IL, Speakman JR. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Functional Ecology. 2004;18:168–183. [Google Scholar]
- Crater AR, Barboza PS. The rumen in winter: cold shocks in naturally feeding muskoxen (Ovibos moschatus) Journal of Mammalogy. 2007;88:625–631. [Google Scholar]
- Cunningham MD, Martz FA, Merilan CP. Effect of drinking-water temperature upon ruminant digestion, intraruminal temperature, and water consumption of nonlactating dairy cows. Journal of Dairy Science. 1964;47:382–385. [Google Scholar]
- Dale HE, Stewart RE, Brody S. Rumen temperature. 1. Temperature gradients during feeding and fasting. Cornell Veterinarian. 1954;44:369–374. [PubMed] [Google Scholar]
- Dehority BA. Rumen Microbiology. Nottingham University Press; Nottingham: 2003. [Google Scholar]
- Eagle TC, Choromanski-Norris J, Kuechle VB. Implanting radio transmitters in Mink and Franklin ground squirrels. Wildlife Society Bulletin. 1984;12:180–184. [Google Scholar]
- Fallon RJ, Rogers PAM. Evaluation of rumen boluses as a method of electronic animal identification. Irish Journal of Agricultural and Food Research. 2001;40:161–168. [Google Scholar]
- Garin D, Caja G, Bocquier F. Effects of small ruminal boluses used for electronic identification of lambs on the growth and development of the reticulorumen. Journal of Animal Science. 2003;81:879–884. doi: 10.2527/2003.814879x. [DOI] [PubMed] [Google Scholar]
- Gengler WR, Martz FA, Johnson HD, Krause GF, Hahn L. Effect of temperature on food and water intake and rumen fermentation. Journal of Dairy Science. 1970;53:434. doi: 10.3168/jds.S0022-0302(70)86226-9. [DOI] [PubMed] [Google Scholar]
- Gervasi V, Brunberg S, Swenson JE. An individual-based method to measure animal activity levels: a test on brown bears. Wildlife Society Bulletin. 2006;34:1314–1319. [Google Scholar]
- Giacometti M, Janovsky M, Fluch G, Arnold W, Schober F. A technique to implant heart rate transmitters in red deer. Wildlife Society Bulletin. 2001;29:586–593. [Google Scholar]
- Guynn DC, Davis JR, Vonrecum AF. Pathological potential of intraperitoneal transmitter implants in beavers. Journal of Wildlife Management. 1987;51:605–606. [Google Scholar]
- Halsey LG, Shepard EL, Hulston CJ, Venables MC, White CR, Jeukendrup AE, Wilson RP. Acceleration versus heart rate for estimating energy expenditure and speed during locomotion in animals: tests with an easy model species, Homo sapiens. Zoology. 2008;111:231–241. doi: 10.1016/j.zool.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Halsey LG, Shepard EL, Quintana F, Gomez Laich A, Green JA, Wilson RP. The relationship between oxygen consumption and body acceleration in a range of species. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2009;152:197–202. doi: 10.1016/j.cbpa.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Hansen MC, Garner GW, Fancy SG. Comparison of 3 methods for evaluating activity of Dall’s sheep. Journal of Wildlife Management. 1992;56:661–668. [Google Scholar]
- Hanton JP. Rumen-implantable electronic identification of livestock. Proceedings of the United States Animal Health Association. 1981;85:342–350. [Google Scholar]
- Kuhn CE, Crocker DE, Tremblay Y, Costa DP. Time to eat: measurements of feeding behaviour in a large marine predator, the northern elephant seal Mirounga angustirostris. Journal of Animal Ecology. 2009;78:513–523. doi: 10.1111/j.1365-2656.2008.01509.x. [DOI] [PubMed] [Google Scholar]
- Laske TG, Harlow HJ, Werder JC, Marshall MT, Iaizzo PA. High capacity implantable data recorders: system design and experience in canines and denning black bears. Journal of Biomechanical Engineering. 2005;127:964–971. doi: 10.1115/1.2049340. [DOI] [PubMed] [Google Scholar]
- Moe RO, Bakken M, Haga O, Smith A. Techniques for surgical implantation of radio transmitters in the silver fox (Vulpes vulpes) Journal of Zoo and Wildlife Medicine. 1995;26:422–429. [Google Scholar]
- Moen R, Pastor J, Cohen Y. Interpreting behavior from activity counters in GPS collars on moose. Alces. 1996;32:101–108. [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2009. [Google Scholar]
- Ropert-Coudert Y, Wilson RP. Trends and perspectives in animal-attached remote sensing. Frontiers in Ecology and the Environment. 2005;3:437–444. [Google Scholar]
- Schober F. Biotelemetriesystem für die physiologische und ethologische Forschung an Wildtieren. University of Technology; Vienna: 1992. PhD thesis. [Google Scholar]
- Schober F, Fluch G, Bögel R. Multichannel microcontroller-based repeater telemetry system with digital radio link. Biotelemetry XIV, Proceedings of the XIV International Conference on Biotelemetry; Marburg. 1997. pp. 77–82. [Google Scholar]
- Wagner J. EKG-Ableitungsorte bei Wildwiederkäuern zur Herzfrequenzübertragung mittels implantierbarer Telemetriesender. University of Veterinary Medicine; Vienna: 1991. PhD thesis. [Google Scholar]
- Wagner RO, Hightower DA, Pace RM. Measuring levels and patterns of activity in black bears. Ursus. 2001;12:181–188. [Google Scholar]
- Wallace MC, Krausman PR, De Young DW, Weisenberger ME. Problems associated with heart rate telemetry implants. Transactions Desert Bighorn Council. 1992;36:51–53. [Google Scholar]
- Wild MA, Piermattei DL, Heath RB, Baker DL. Surgical implantation and evaluation of heart rate transmitters in captive bighorn sheep. Journal of Wildlife Diseases. 1998;34:547–554. doi: 10.7589/0090-3558-34.3.547. [DOI] [PubMed] [Google Scholar]
- Wilson RP, Cooper J, Plötz J. Can we determine when marine endotherms feed? A case study with seabirds. Journal of Experimental Biology. 1992;167:267–275. [Google Scholar]
- Wilson RP, Pütz K, Grémillet D, Culik BM, Kierspel M, Regel J, Bost CA, Lage J, Cooper J. Reliability of stomach temperature changes in determining feeding characteristics of seabirds. Journal of Experimental Biology. 1995;198:1115–1135. doi: 10.1242/jeb.198.5.1115. [DOI] [PubMed] [Google Scholar]
- Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. Journal of Animal Ecology. 2006;75:1081–1090. doi: 10.1111/j.1365-2656.2006.01127.x. [DOI] [PubMed] [Google Scholar]
- Woakes AJ, Butler PJ, Bevan RM. Implantable data logging system for heart rate and body temperature: its application to the estimation of field metabolic rates in Antarctic predators. Medical and Biological Engineering and Computing. 1995;33:145–151. doi: 10.1007/BF02523032. [DOI] [PubMed] [Google Scholar]
- Yoda K, Sato K, Niizuma Y, Kurita M, Bost C-A, Le Maho Y, Naito Y. Precise monitoring of porpoising behaviour of Adélie penguins determined using acceleration data loggers. Journal of Experimental Biology. 1999;202:3121–3126. doi: 10.1242/jeb.202.22.3121. [DOI] [PubMed] [Google Scholar]
- Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, Kurita M, Le Maho Y. A new technique for monitoring the behaviour of free-ranging Adélie penguins. Journal of Experimental Biology. 2001;204:685–690. doi: 10.1242/jeb.204.4.685. [DOI] [PubMed] [Google Scholar]