Summary
Knowledge of infection reservoir dynamics is critical for effective disease control, but identifying reservoirs of multi-host pathogens is challenging. Here, we synthesize several lines of evidence to investigate rabies reservoirs in complex carnivore communities of the Serengeti ecological region in northwest Tanzania, where the disease has been confirmed in 12 carnivore species.
Long-term monitoring data suggest that rabies persists in high-density domestic dog Canis familiaris populations (> 11 dogs km−2) and occurs less frequently in lower-density (< 5 dogs km−2) populations and only sporadically in wild carnivores.
Genetic data show that a single rabies virus variant belonging to the group of southern Africa canid-associated viruses (Africa 1b) circulates among a range of species, with no evidence of species-specific virus–host associations.
Within-species transmission was more frequently inferred from high-resolution epidemiological data than between-species transmission. Incidence patterns indicate that spill-over of rabies from domestic dog populations sometimes initiates short-lived chains of transmission in other carnivores.
Synthesis and applications. The balance of evidence suggests that the reservoir of rabies in the Serengeti ecosystem is a complex multi-host community where domestic dogs are the only population essential for persistence, although other carnivores contribute to the reservoir as non-maintenance populations. Control programmes that target domestic dog populations should therefore have the greatest impact on reducing the risk of infection in all other species including humans, livestock and endangered wildlife populations, but transmission in other species may increase the level of vaccination coverage in domestic dog populations necessary to eliminate rabies.
Keywords: carnivore, infectious disease, multi-host, phylogeny, rabies, reservoir, spill-over, transmission
Introduction
Pathogens that infect multiple host species are often (i) economically important, (ii) major threats to human health, (iii) risk factors for endangered wildlife populations, and (iv) causes of emerging human and livestock diseases (Daszak, Cunningham & Hyatt 2000; Cleaveland, Laurenson & Taylor 2001; Dobson & Foufopoulos 2001; Taylor, Latham & Woolhouse 2001). The generalist nature of these pathogens poses considerable challenges for understanding infection dynamics and designing effective control strategies (Haydon et al. 2002). Key questions relate to the identification of infection reservoirs, the mechanisms by which infections are sustained within reservoirs, and the sources and routes of transmission from reservoirs to species of concern. These problems are well-illustrated by bovine tuberculosis in Great Britain, where the role of cattle and badgers Meles meles in maintaining Mycobacterium bovis is not fully resolved despite numerous observational and intervention studies (Woodroffe et al. 2005; Independent Scientific Group on Cattle TB 2007). Many challenges posed by complex reservoir systems are encapsulated by rabies in the Serengeti, where the disease threatens human, wildlife and domestic animal health (Cleaveland et al. 2007). The complexity of Serengeti’s abundant and diverse host communities necessitates a multi-faceted approach involving multiple data sources and a suite of analytical tools for understanding reservoir dynamics.
Following the terminology of Haydon et al. (2002), we define a reservoir as a set of epidemiologically connected populations that permanently maintain a pathogen and transmit infection to particular target populations considered to require protection. The key components of reservoirs of directly transmitted microparasites are therefore the target populations (of concern) and maintenance populations (in which sustained transmission occurs). Some reservoir systems also involve source populations, which provide transmission links between maintenance and target populations. By definition, maintenance populations must exceed the minimum population size required for disease persistence [the critical community size (CCS) sensu Bartlett 1960]. Populations smaller than the CCS (non-maintenance populations) cannot maintain a pathogen independently, but together with other maintenance or non-maintenance populations can constitute part of a reservoir (Haydon et al. 2002). These definitions are illustrated in Fig. 1.
Fig. 1.
Potential rabies reservoir systems in the Serengeti. CCS, critical community size. In this figure, we use humans as the target population, but for rabies target populations include humans, livestock and endangered wildlife. If the epidemiological situation corresponds to (a), vaccinating domestic dogs would shift the situation first to (c) and finally to an overall community insufficient for rabies maintenance.
Rabies is a classic example of a multi-host pathogen for which the identification of reservoirs has proven challenging (Nel 1993; Cleaveland & Dye 1995; Bingham et al. 1999a,b; Johnson et al. 2003; Bernardi et al. 2005). In Africa and Asia, domestic dog rabies predominates among reported and confirmed cases and domestic dogs are the reported source of infection for over 90% of human cases (World Health Organization 1999); however, it has been argued that this may reflect surveillance bias and that the role of wildlife is poorly understood (Swanepoel et al. 1993; Wandeler et al. 1994). Certain mongoose species (Cynictis penicillata and Galerella sanguinea) maintain distinct rabies virus (RABV) variants in parts of southern Africa (Foggin 1988; King, Meredith & Thomson 1993; Swanepoel et al. 1993; Bingham et al. 2001; Nel et al. 2005), and wild canids such as bat-eared foxes Otocyon megalotis, side-striped jackals Canis adustus and black-backed jackals Canis mesomelas, appear able to sustain rabies cycles in some ecosystems (Thomson & Meredith 1993; Bingham et al. 1999a), but their role as independent maintenance hosts and infection reservoirs is debated (Cleaveland & Dye 1995; Rhodes et al. 1998; Bingham et al. 1999b).
Wild carnivore communities in the Serengeti comprise 26 species (Sinclair & Arcese 1995) including Canidae and Herpestidae species implicated as independent maintenance hosts of rabies in parts of southern Africa (i.e. slender mongooses, bat-eared foxes, side-striped and black-backed jackals). Cleaveland & Dye (1995) found that rabies appears to persist endemically in high-density domestic dog populations (> 5 km−2) to the west of Serengeti National Park (SNP), yet occurred only sporadically in lower-density domestic dog populations (< 1 km−2) to the east of SNP and in wild carnivore populations. They concluded that domestic dog populations to the west of SNP were likely to be the sole reservoir in the ecosystem (Fig. 1a); however, their study was based on limited data and did not consider the possibility of a multi-host reservoir. Furthermore, with rapid growth of human and associated domestic dog populations, it seems likely that in the meantime the lower-density domestic dog population, which previously could not support rabies cycles, has become large enough to maintain infection. Additionally, East et al. (2001) argue that, in the Serengeti, a distinct rabies virus variant is maintained in spotted hyaenas Crocuta crocuta, which undergo atypical infections with no evidence of mortality. Additional data now allow more detailed examination of the role of wild carnivores in potential rabies reservoir systems, (i) as maintenance populations independent of domestic dogs (Fig. 1b), (ii) as part of multi-host maintenance communities (Fig. 1c), or (iii) as one of several independent maintenance populations (Fig. 1d).
Given the potential complexity of rabies reservoirs in communities comprising multiple hosts, it may not be possible to reach an exhaustive understanding of their structure. However, identifying maintenance populations and populations that act as sources of infection for target populations has important practical implications. Rabies is transmitted to several ‘spill-over’ hosts that might be considered target populations of concern, including humans (Knobel et al. 2005), endangered wildlife (Randall et al. 2006; Vial et al. 2006) and livestock. In the Serengeti, human rabies deaths occur, subsistence farmers suffer livestock losses from rabies and there is concern about risks to recovering populations of African wild dog Lycaon pictus [rabies was previously implicated in their local extinction (Cleaveland et al. 2007)]. There are long-term benefits to controlling rabies infection directly in maintenance rather than in target populations: human post-exposure prophylaxis (PEP) although effective, is costly and often unavailable; routine prophylactic vaccination of livestock is expensive; and vaccination of endangered canids has been controversial (Dye 1996; Woodroffe 2001; Cleaveland et al. 2007). Approaches to control will depend upon how rabies is maintained: if domestic dogs are the sole maintenance population, or neither domestic dogs nor wild carnivores maintain rabies independently (Fig. 1a,c), measures targeted at domestic dogs should in theory also eliminate human and animal rabies. However, if wild carnivores are the sole maintenance population (Fig. 1b), strategies will need to target wildlife. Similarly, if domestic dog and wild carnivore populations each maintain rabies (Fig. 1d), strategies will only be successful if infection in both populations is controlled.
Rabies in the Serengeti is therefore an important though challenging model for addressing questions relating to the structure and dynamics of complex reservoir systems. Here, we present several lines of evidence that advance our understanding of reservoir dynamics of rabies in multi-host carnivore communities of the Serengeti: we synthesize data from long-term epidemiological records, phylogenetic analyses of virus isolates from a range of domestic and wild species, and statistical analyses of high-resolution spatiotemporal patterns of rabies incidence.
Methods
STUDY AREA
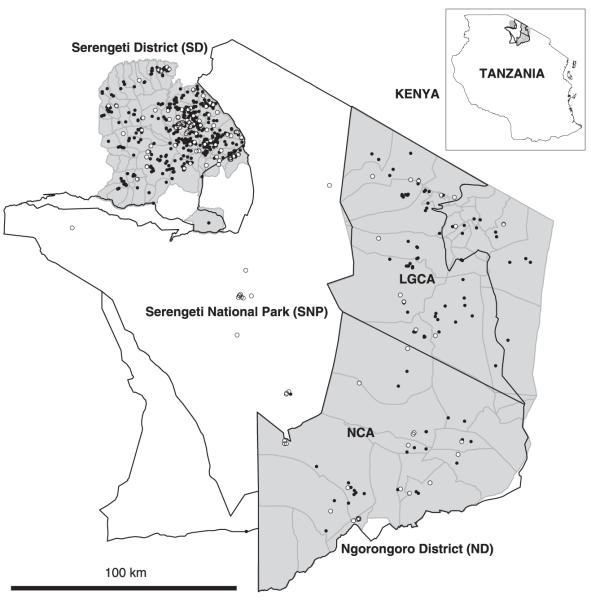
The study area in the Serengeti ecological region of northwest Tanzania was divided into three zones (Fig. 2). The first zone, SNP, comprises diverse wildlife communities, with domestic dogs found extremely rarely. The two other zones are districts adjacent to SNP, Serengeti District (SD) to the west and Ngorongoro District (ND), comprising the Loliondo Game Control Area (LGCA) and Ngorongoro Conservation Area (NCA), to the east. SD is inhabited by multi-ethnic, agro-pastoralist communities and high-density domestic dog populations (> 11 km−2). ND is a multiple-use controlled wildlife area, inhabited by low-density pastoralist communities, and lower-density domestic dog populations (< 5 km−2). We have observed a greater variety and abundance of wild carnivores in ND than SD. No physical barriers separate the wildlife-protected areas and human settlements.
Fig. 2.
Map of the Serengeti ecological region illustrating the distribution of suspected rabies cases (most were not laboratory-confirmed but diagnosed using epidemiological history and clinical criteria). Tables S2 and S3 in Supplementary material detail confirmed wildlife cases in Serengeti National Park (SNP) and Serengeti (SD) and Ngorongoro (ND) districts. Cases recorded in SNP from January 1995–January 2007 are mapped, whereas only cases from January 2002–January 2007 in SD and ND are mapped because GPS data were not available for the earlier period. Domestic dog cases are shown in black (SD: n = 833; ND: n = 128) and cases in other carnivores as white circles (SD: n = 96; ND: n = 27; SNP: n = 23). Village boundaries are indicated by grey lines. LGCA, Loliondo Game Control Area; NCA, Ngorongoro Conservation Area.
Estimated densities of human and domestic dog populations are presented in Table 1; these were extrapolated from human census data with projected annual growth rates of 2·6% in SD and 3·8% in ND (National Bureau of Statistics Tanzania 2005), and from human–domestic dog ratios (Wandeler et al. 1988; Brooks 1990) obtained from household questionnaires (from SD in 2003 and LGCA in 2004).
Table 1.
Estimated domestic dog population densities in Serengeti (SD) and Ngorongoro districts [ND – Loliondo Game Control Area (LGCA) only]. Confidence intervals about the means are indicated in brackets
| Area | Area (km2) |
Domestic dog–human ratio |
Average human density (km−2) at the village level |
Average domestic dog density (km−2) at the village level |
|---|---|---|---|---|
| SD urban (Mugumu town) | 18·8 | 6·6 | 619·8 | 93·9 |
| SD rural | 3128·0 | 6·6 | 67·9 (59·2–76·5) | 10·3 (9·0–11·6) |
| SD overall | 3146·8 | 6·6 | 75·2 (58·4–92·0) | 11·4 (8·9–13·9) |
| LGCA urban (Loliondo and Sakala) | 14·1 | 6·7 | 144·9 | 21·6 |
| LGCA rural | 8852·4 | 6·7 | 17·0 (11·1–23·0) | 2·5 (1·7–3·4) |
| LGCA overall | 8866·5 | 6·7 | 28·2 (9·2–47·1) | 4·2 (1·4–7·0) |
Several domestic dog vaccination programmes have been implemented in the study area. Details of vaccination campaigns are reported in Appendix S2 (Supplementary material), although their impact is not analysed here. The only vaccination of wildlife (African wild dogs) was conducted in 1990 (Gascoyne et al. 1993).
DISEASE MONITORING OPERATIONS
Data presented are the result of disease monitoring operations since the late 1980s (Table 2). Passive surveillance was used to detect rabies cases in SNP. Sightings of sick and dead carnivores were reported through a network of veterinarians from Tanzania National Parks (TANAPA) and Tanzania Wildlife Research Institute (TAWIRI), rangers, scientists, and tour-operators. Cases included animals that were snared, injured/wounded from other or unknown causes, observed with signs of disease or found dead with unknown history.
Table 2.
Timeline of disease monitoring operations in each zone in 6-month units. TAWIRI, Tanzania Wildlife Research Institute; TANAPA, Tanzania National Parks; NCAA, Ngorongoro Conservation Area Authority
| YEAR ZONE | 1987 | 1988 | 1989 | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | TANAPA Veterinary Unit | |||||||||||||||||||
| TAWIRI Veterinary Programme |
||||||||||||||||||||
| Viral Transmission Dynamics Project* |
||||||||||||||||||||
| SD | Passive surveillance (domestic dog-bite injury records from hospitals) |
Domestic dog vaccinations | ||||||||||||||||||
| Passive surveillance (Veterinary records, village reports, opportunistic carcass sampling) |
||||||||||||||||||||
| Active (livestock field-officers) | Active (livestock field-officers) | |||||||||||||||||||
| Contact tracing | ||||||||||||||||||||
| ND | Passive (domestic dog-bite injury records from hospitals) |
Domestic dog vaccinations |
||||||||||||||||||
| Passive (Veterinary records, opportunistic carcass sampling, TAWIRI, TANAPA and NCAA records) |
||||||||||||||||||||
| Contact tracing | ||||||||||||||||||||
A research project investigating infection dynamics of viral diseases.
Outside SNP, passive and active surveillance operations were employed. Passive surveillance data were available through veterinary office records (government offices, TAWIRI, TANAPA and NCA Authority), and district hospitals and medical dispensaries. Bite injuries to humans from suspected rabid dogs were defined based on evaluation by clinicians (from information on clinical signs reported by bite victims) and, when human vaccines were not widely available, on whether PEP had been administered. Bite injury incidence was calculated against human population sizes interpolated from census data (National Bureau of Statistics Tanzania 2005). Community-based active surveillance measures, based on previous studies in rural Kenya (Kitala et al. 2000) and Tanzania (Cleaveland et al. 2003), were implemented in SD, using livestock field officers stationed in randomly selected villages to collect information on rabies cases from key informants (village leaders, teachers, dispensary staff, local healers).
Contact tracing was used to collect high-resolution data on spatial and temporal patterns of disease. Visits to incidents involving biting animals (to bite victims and owners of biting and bitten animals) were initiated using hospital and medical dispensary records, and case reports from livestock offices and community-based surveillance. Incidents were mapped and witnesses interviewed to make a diagnosis (see below) and obtain case-history information (exposure source, onset of clinical signs and death, resulting exposures). The same procedure was followed for all exposures and preceding cases, where identified, so that transmission events between identified individual animals could be traced and confirmed. When multiple incidents involving suspected rabid wildlife were reported on the same/consecutive days within neighbouring homesteads, we assumed a single animal was involved.
Wherever possible, brain stem samples were collected from suspect rabies cases and carnivore carcasses, whatever the apparent cause of death. Sample collection was carried out by research personnel, veterinarians and livestock field officers; the preferred technique was removal via the occipital foramen according to World Health Organization recommendations (Barrat 1996). Specimens were frozen (−20 °C) immediately after collection or placed into phosphate-buffered 50% glycerol solution and preserved either cold (+4 °C/−20 °C) or at room temperature (25 ± 5 °C) when refrigeration facilities were not promptly available.
RABIES DIAGNOSIS AND VIRUS CHARACTERIZATION
Diagnostic tests on brains collected up to 2001 and virus isolation were carried out at the Agence Française de Sécurité Sanitaire des Aliments (AFSSA), Malzéville, France, using the fluorescent antibody test (Dean, Abelseth & Atanasiu 1996), inoculation of murine neuroblastoma cells and mouse inoculation (Barrat et al. 1988). Rabies diagnosis on more recent brain tissues was conducted at the Rabies Section of the Centers for Disease Control and Prevention (CDC), Atlanta, USA, by the fluorescent antibody test (http://www.cdc.gov/rabies/docs/standard_dfa_protocol_rabies.pdf). Virus samples were typed at CDC. RNA was extracted, reverse transcribed, amplified by polymerase chain reaction and nucleoprotein gene sequences generated as described in Lembo et al. (2007).
When brain tissues were not available for laboratory confirmation, diagnosis of suspected rabies cases was based on history of exposure and clinical evaluation (adapting the six criteria proposed by Tepsumethanon, Wilde & Meslin 2005) from information on clinical signs reported by villagers, livestock field officers, researchers and park veterinarians:
For owned domestic dogs: history of a bite, clinical signs (Tepsumethanon et al. 2005), disappearance or death within 10 days.
For domestic dogs of unknown origin: clinical signs (as above) and disappearance or death.
For wild carnivores: clinical signs (as above), plus tameness, loss of fear of humans, diurnal activity (for nocturnal species), and unprovoked biting (without eating) of objects and animals.
PHYLOGENETIC ANALYSIS
Nucleotide sequences were edited using bioedit 7·0·0 (Hall 1999) and aligned in clustal-x version 1·83 (Jeanmougin et al. 1998). Phylogenetic relationships among viruses from the study area (Table S1, Supplementary material) and sequence data for RABVs from other African countries (Kissi, Tordo & Bourhy 1995; Randall et al. 2004) were estimated using Bayesian Markov chain Monte Carlo (MCMC) methods using mrbayes 3·0b4 (Ronquist & Huelsenbeck 2003) as described in Lembo et al. (2007). Posterior probabilities of 0·95 or greater were considered significant. Graphic output was generated using TREEVIEW version 1·6·6 (Page 1996).
STATISTICAL ANALYSIS
Poisson regression was used to test for a relationship between rabies cases in domestic dogs and in other carnivores. Using maximum likelihood, a gamma-distributed spatial infection kernel for domestic dogs k(d) was estimated from the distances between source animals and their known contacts, and a probability distribution of the generation interval for domestic dogs, g(t), was estimated from observed dates of exposure, onset of clinical signs and death (full details in Appendix S2). An algorithm (detailed in Appendix S2) was developed to generate putative epidemic trees by assigning probabilities to links between possible progenitors and each suspected case according to their spatiotemporal proximity and the estimated spatial infection kernel k(d) and generation interval distribution g(t). This algorithm assumes all cases are observed and therefore cannot account for unobserved intermediates. Cases in all species were included in the analysis and all carnivore species were assumed equally likely to transmit rabies. Only limited data could be collected on transmission from wild carnivores, therefore progenitors of all species were assigned using parameters estimated for domestic dogs (k(d) and g(t)). One thousand bootstrapped trees were generated and the proportional contribution of transmission to each case from preceding cases was calculated. If the date of onset of clinical signs was uncertain, in each realization a date was assigned with uniform probability from the range of uncertainty.
From the bootstrap trees, the amount of transmission within and between host types was estimated. We pooled all carnivores except domestic dogs because of small sample sizes. A null model of the expected frequency of within- and between-species transmission, assuming that intra- and interspecific transmission events occur with the same probability (i.e. transmission is non-assortative), was estimated as follows: the species identity of each case was randomly reassigned, retaining the original distribution of cases amongst species, and the resulting frequency of within- and between-species transmission was calculated. This procedure was iterated 1000 times to evaluate the probability that the difference between inferred within- and between-species transmission frequencies could have been due to chance. To investigate whether the spatial distribution of hosts influenced the estimated patterns of transmission, we repeated the analysis but randomly reassigned species identities within grid cells rather than across the entire district and we repeated the analysis for different sized grids (see Appendix S2).
Results
RABIES RECOGNITION PROBABILITY
The sensitivity, specificity and positive predictive value of clinical rabies diagnosis (i.e. recognition of rabies by villagers, livestock field officers, park veterinarians and research personnel) calculated against the gold standard fluorescent antibody test (sensitivity ~99%, specificity > 99%, Dean et al. 1996) are reported in Table 3. As a result of the relatively high rabies recognition probability (> 74% of animals reported as suspect rabies cases were confirmed positive) and the small number of confirmed cases, the analyses presented include all suspected cases unless otherwise stated.
Table 3.
Sensitivity, specificity and positive predictive value of rabies diagnosis calculated against the gold standard fluorescent antibody test. Data include only cases for which complete history was available (2002–2005)
| Laboratory diagnosis |
|||
|---|---|---|---|
| Clinical diagnosis | Positive | Negative | Total |
| Positive | 55 | 19 | 74 |
| Negative | 0 | 33 | 33 |
| Total | 55 | 52 | 107 |
| Sensitivity = 100·00% | |||
| Specificity = 63·46% (95% CI: 50·37–76·55%) | |||
| Positive predictive value = 74·32% (95% CI: 64·37–84·28%) | |||
RABIES INCIDENCE IN THE STUDY POPULATIONS
Wildlife monitoring activities in SNP are shown throughout the study period in Table 2 and Fig. 3a. A peak in wild carnivores reported dead and sick coincided with a canine distemper outbreak in late 1993 and 1994 (Roelke-Parker et al. 1996). Most rabies cases in wildlife were reported and confirmed in 1998 and 1999, with only sporadic detection in other years (Fig. 3b, Table S2 in Supplementary material). Low numbers of snared animals were reported in 1998 suggesting that the increase in carnivore carcasses recorded that year (Fig. 3a) was not due to increased monitoring. No wildlife cases have been confirmed in SNP since 1999, except for a leopard Panthera pardus on the SNP–NCA boundary in September 2004, when there was an outbreak among adjacent domestic dog populations (shown in Fig. 5b).
Fig. 3.
Wildlife disease monitoring in Serengeti National Park: (a) carnivore reports and (b) samples retrieved for rabies diagnosis. The peak in 1994 coincided with a canine distemper outbreak in lions (Roelke-Parker et al. 1996).
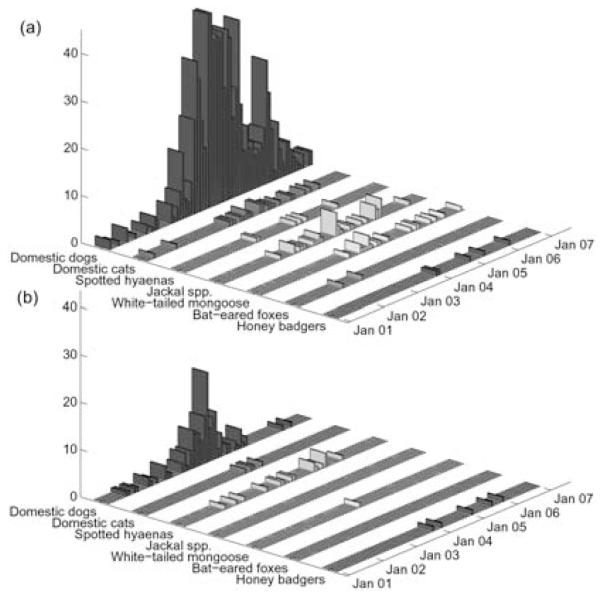
Fig. 5.
Suspected rabies cases among carnivore species in (a) Serengeti (n = 929) and (b) Ngorongoro districts (n = 155) monitored by contact tracing from January 2001 to January 2007.
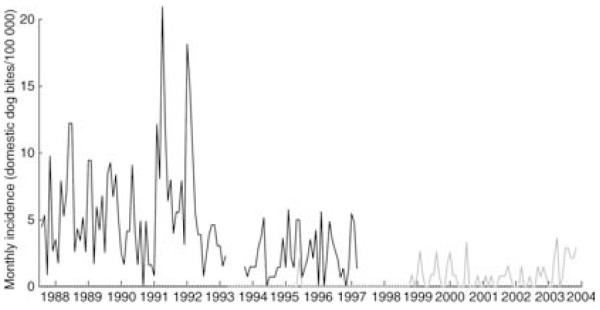
Animal-bite injuries in humans from suspected rabid domestic dogs were reported continuously in high-density domestic dog populations to the west of SNP before implementation of dog vaccination programmes, whereas in the lower-density populations to the east, incidence was lower and there were several periods when no bites were recorded (Fig. 4). Gaps in recorded bites by suspected rabid dogs may also reflect PEP shortages; however, when we included all domestic dog bite injuries, there were four periods of at least 5 months with no reported bite injuries in ND, and no equivalent pattern in SD (Fig. S1, Supplementary material).
Fig. 4.
Incidence of bites by suspected rabid domestic dogs in Serengeti (SD, black line) and Ngorongoro (ND, grey line) districts derived from hospital records. Incidence is shown only for years before the implementation of domestic dog vaccination programmes in the study populations (July 1987–January 1997 in SD and January 1994–December 2003 in ND). No data were available for 6 months during 1993 in SD.
We traced 1255 suspected rabid animals from January 2002 to January 2007 (1044 in SD, 211 in ND; see Fig. 5 for carnivore cases). In both districts, cases in wildlife were sporadic and coincided with outbreaks in domestic dogs (Figs 3b, 4 & 5). The spatial and temporal distribution of suspected cases is shown in Figs 2 and 5.
INTER- AND INTRA-SPECIFIC TRANSMISSION
The virus
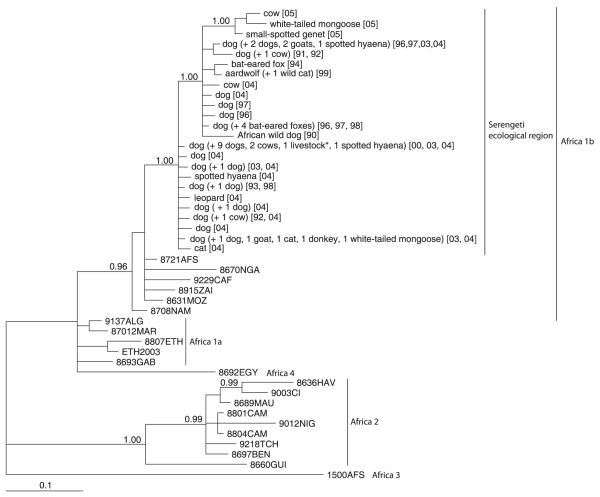
All of the 57 RABV specimens recovered from a range of species from the study area (including spotted hyaenas) fell within the Africa 1b group of canid-associated viruses and revealed no clustering by host species (Fig. 6). The evident intermingling of viral lineages from domestic dogs and wildlife indicates frequent interspecific transmission. Analyses using statistical parsimony techniques were also consistent with both within- and between-species transmission events (Lembo et al. 2007).
Fig. 6.
Phylogenetic tree of nucleoprotein gene sequences (282 bp, 94 deduced amino acids, nucleotide positions 1139–1420 on the SAD B19 genome (Conzelmann et al. 1990) for RABV samples from the study area compared with isolates from other areas of Africa. The tree is constructed using Bayesian phylogenetics under the transitional model of nucleotide evolution with a gamma-shaped distribution of rates across sites (TIM + Γ Posada & Crandall 1998; base frequencies = 0·3253, 0·2134, 0·2360, 0·2253; nucleotide substitution rates = 1·0000, 3·6723, 0·4393, 0·4393, 8·1773, 1·0000; Γ = 0·3390). For samples from the study area, the year of collection is indicated within square brackets. Previously published sequences are designated by strain names (Kissi et al. 1995; Randall et al. 2004). The tree is rooted with isolate 1500AFS, defined as outgroup, representative of the lineage Africa 3. Nodal posterior probabilities > 0·95 are shown. The scale indicates branch-length expressed as the expected number of substitutions per site. *Species not definitively identified.
Spatiotemporal incidence patterns in hosts
Suspected rabies cases in domestic dogs were significant predictors of those in other carnivores (pooled cases in domestic cats and wildlife: P < 0·001 in both districts). Credibility intervals (95%) of the expected frequency of within- and between-species transmission events, based upon the null assumption of non-assortative transmission (derived from the bootstrap analysis), are shown in Fig. 7. More within-species transmission than expected was estimated in both districts (domestic dog-to-domestic dog transmission: P = 0·052 in SD and P = 0·008 in ND; transmission within other carnivores, i.e. wild carnivores and domestic cats Felis catus: P < 0·0001 in both districts). Estimated between-species transmission was less than expected (P < 0·03 in SD and P < 0·001 in ND) except for transmission from domestic dogs to livestock. These patterns were consistent and significant when the randomization procedure was carried out on a 10 × 10 km grid, while on a 5 × 5 km grid the patterns were evident but no longer significant, probably because of reduced statistical power.
Fig. 7.
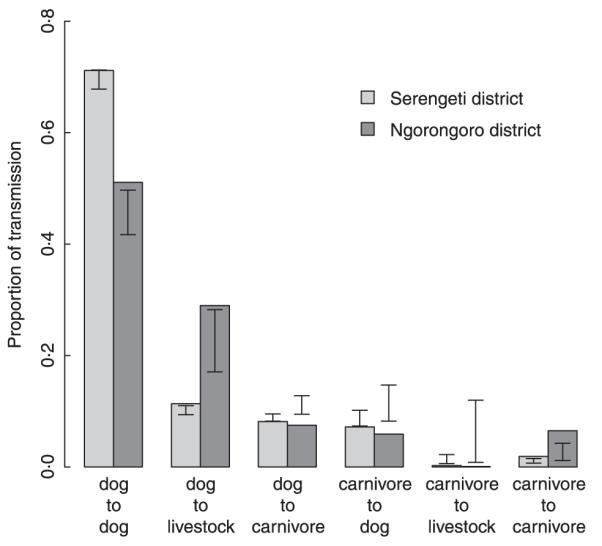
The proportion of estimated transmission within and between host types (associated with 1255 suspected rabies cases, 1044 in Serengeti and 211 in Ngorongoro) in Serengeti (light grey) and Ngorongoro (dark grey) districts, with ‘dog’ corresponding to domestic dogs and ‘carnivore’ to domestic cats and wild carnivores. Credibility intervals (95%) are shown for expected transmission within and between host types assuming inter- and intraspecific transmission were equally likely and that livestock are dead-end hosts.
The majority of human exposures to suspected rabid animals were from domestic dogs (Table 4). Similarly, domestic dogs were the predominant source of infection for livestock (Fig. 7).
Table 4.
Human exposures to rabies as a result of bites by suspected rabid animals in Serengeti and Ngorongoro districts (January 2002–December 2006). Data were obtained by contact tracing
| Species | Serengeti district (%) |
Ngorongoro district (%) |
|---|---|---|
| Domestic dogs | 466 (85·5) | 84 (70·0) |
| Domestic cats | 25 (4·6) | 6 (5·0) |
| Livestock | 7(1·3) | 3 (2·5) |
| Human | 2 (0·4) | 1 (0·8) |
| Spotted hyaena, Crocuta crocuta | 4 (0·7) | 16 (13·3) |
| Honey badger, Mellivora capensis | 11 (2·0) | 6 (5·0) |
| African wildcat, Felis lybica | 0 (0) | 2 (1·7) |
| White-tailed mongoose, Ichneumia albicauda |
2 (0·4) | 1 (0·8) |
| Bat-eared fox, Otocyon megalotis | 2 (0·4) | 0 (0) |
| Small-spotted genet, Genetta genetta | 1 (0·2) | 1 (0·8) |
| Jackal spp., Canis spp. | 23 (4·2) | 0 (0) |
| Leopard, Panthera pardus | 2 (0·4) | 0 (0) |
| Total | 545 (100) | 120 (100) |
Discussion
Demonstrating persistence of infection is a prerequisite for identifying reservoir(s). Domestic dogs living at high densities (within the SD) are the only species in which rabies has been recorded continuously throughout the study period, whereas cases in other species (inside and outside SNP) have been sporadic. Although disease monitoring presumably detects a lower proportion of cases in wildlife than domestic dogs, it seems likely that infection in wildlife populations, had it been present continuously, would have been detected in more recent years, where the number of samples collected and tested was higher than in previous years when rabies was recorded (Table 2; Fig. 3b). These observations provide circumstantial evidence for rabies persistence in domestic dog populations and lack of persistence in other carnivores. Clusters of wildlife cases that coincided with outbreaks in domestic dog populations (Figs 3b, 4 & 5) and explosive but transient (< 2 months) epidemics previously reported in bat-eared foxes (Maas 1993) are also consistent with chains of transmission that are not sustained, and contrast with incidence patterns in wild carnivores that are believed to maintain rabies through independent cycles elsewhere in Africa. For example, rabies was recorded sporadically in bat-eared fox populations in South Africa from the 1950s to 1970, then continuously, suggesting a shift to persistent infection (Thomson & Meredith 1993), and in Zimbabwe, outbreaks in jackals, although often initiated by domestic dog cases, appear to be sustained over several years (Bingham et al. 1999a,b).
Specific demographic attributes (high densities and/or turnover) are thought to be required for sustained rabies transmission. A threshold density of > 5 dogs km−2 has been suggested for persistence of rabies in domestic dog populations in Africa (Foggin 1988; Brooks 1990; Cleaveland & Dye 1995; Kitala et al. 2001). Domestic dog populations in the study region have grown considerably, and even in the lower-density area (within the ND), the estimated average density has approached this threshold (Table 1), and in 2003 population sizes were sufficient for a sizable epidemic to occur (Fig. 5b). Rabies-control measures make it hard to determine whether current densities in ND are high enough for infection to persist in the absence of vaccination, but they may now be close.
We detected only one canid-associated variant (Africa 1b) from a range of species in the Serengeti and were unable to replicate East et al.’s (2001) finding of a distinct virus variant circulating atypically in spotted hyaenas (Lembo et al. 2007). The lack of species-specific virus–host associations in the canid-associated variant that we detected and the high degree of genetic relatedness between virus samples from different species indicate frequent cross-species transmission. This is consistent with results from southern Africa where phylogenetic branches from a single geographic area include several host species (Sabeta, Bingham & Nel 2003).
The statistical association between rabies cases in domestic dogs and other species also indicates cross-species transmission. Unfortunately, the length of our time series and paucity of cases detected in species other than domestic dogs preclude analyses of ‘spill-over’ risk and reduce the interpretative power of time-lagged regressions, like those conducted for rabies in North America, that could otherwise provide insight (Guerra et al. 2003; Gordon et al. 2004). In general, as the amount of between-species transmission increases, the more difficult it becomes to tease apart the role of different potentially independent maintenance hosts. We find greater within-species and less between-species transmission than would be expected if species interacted randomly. This pattern could occur as a consequence of the spatial distribution of host species and/or preferential contact between conspecifics. Our randomization indicates that large-scale patterns in the distribution of hosts (> 10 × 10 km) do not explain this ‘assortative’ transmission, but we cannot make inference on a smaller scale. These analyses suggest that occasional ‘spill-over’ of infection from domestic dog populations into alternative hosts sometimes results in short-lived chains of transmission as contact is more common among conspecifics (Dobson 2004).
Definitive identification of infection reservoirs is extremely challenging and rarely possible. Despite intensive effort, understanding of reservoir dynamics in many complex multi-host systems remains incomplete (Woodroffe et al. 2005; Biek et al. 2006; Independent Scientific Group on Cattle TB 2007). Here the balance of evidence implicates domestic dogs as the only maintenance population of rabies in the Serengeti, whereas other carnivores are nonessential to maintenance. However, because they transmit disease to target populations (and frequent transmission within and between such species may occur) they do constitute part of the reservoir (Table 4, Fig. 1a). A single maintenance-host species is consistent with broad patterns of rabies infection observed elsewhere (Smith 1989; Rupprecht et al. 1991; Wandeler et al. 1994; Smith, Orciari & Yager 1995).
A lesson from this study is that even with considerable data from various sources, we cannot exhaustively quantify the contribution of transmission from all non-essential reservoir components. However, unravelling every thread of reservoir dynamics may not be essential. Our key finding that wildlife are not able to maintain rabies cycles or distinct genetic variants provides new and critical information allowing rabies control measures in the Serengeti to be targeted more confidently at domestic dog populations alone. The long-term implication is that control of domestic dog rabies in the Serengeti will eliminate rabies in all other species including humans, livestock and wildlife. The absence of wildlife maintenance hosts, even in an area with such abundant wildlife, raises the expectation that canine rabies elimination is a feasible objective throughout much of Africa.
Interventions to prevent transmission from domestic dog maintenance populations would also provide convincing evidence with which to identify reservoirs. Such approaches have demonstrated that cattle were the reservoir for rinderpest: after cattle vaccinations, rinderpest disappeared from wildlife and the wildebeest Connochaetes taurinus population increased nearly ten-fold (Sinclair 1979; Plowright 1982; Dobson 1995). We are currently ring-vaccinating domestic dogs around SNP, which should provide critical data to evaluate the impact of alternative hosts on long-term control efforts, in particular their effects on the level of vaccination required for disease control.
In conclusion, resolving reservoir dynamics in any complex system is likely to prove challenging, and our experiences in the Serengeti suggest that consideration needs to be given to identifying (i) the potential role of different components of the system, (ii) the key information needed for different disease control strategies, (iii) the epidemiological approaches likely to yield most useful information, and (iv) the range of analytical tools available.
Supplementary Material
Acknowledgements
We are grateful to Tanzania Government ministries, TANAPA, TAWIRI, NCA Authority, Tanzania Commission for Science and Technology, and National Institute for Medical Research for permissions; and TANAPA and TAWIRI Veterinary Units, Viral Transmission Dynamics Project, Serengeti Lion and Cheetah Projects, Frankfurt Zoological Society, livestock field-officers of the Ministry of Water and Livestock Development in Mara and Arusha Regions, M. Magoto, P. Tiringa, M. Roelke-Parker, R. Fyumagwa, H. Wiick and Mwanza and Arusha Veterinary Investigation Centres for assistance with sample collection. We are indebted to J. Barrat, AFSSA, for performing diagnostic testing and virus isolation and the staff of the Rabies Section of the CDC, especially C.E. Rupprecht, A. Velasco-Villa, L.A. Orciari, I.V. Kuzmin and M. Niezgoda, and P.E. Brandão (Universidade de São Paulo) for technical assistance with diagnostic tests and virus characterization. We thank K.Laurenson, D. Bennett, J. Pulliam and H. Horn for valuable discussions.
This work was supported by the joint National Institutes of Health (NIH)/National Science Foundation (NSF) Ecology of Infectious Diseases Program under grant no. NSF/DEB0225453. Opinions, findings, conclusions and recommendations are those of the authors and do not necessarily reflect the views of NIH or NSF. K.H. was supported by NSF grant: DEB-0513994, the Pew Charitable Trusts award 2000-002558 to Princeton University, the Department of Ecology and Evolution, Princeton University and the Heinz Foundation. T.L. was supported by the Royal (Dick) School of Veterinary Studies, University of Edinburgh and the University of Edinburgh Development Trust (visits to CDC) and S.C. by the Wellcome Trust and the Department for International Development Animal Health Programme.
Footnotes
Data deposition. The sequences of rabies viruses produced in this study have been deposited in the GenBank data base (Accession nos EU296434-EU296446).
References
- Barrat J. Simple technique for the collection and shipment of brain specimens for rabies diagnosis. In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory Techniques in Rabies. World Health Organization; Geneva, Switzerland: 1996. pp. 425–432. [Google Scholar]
- Barrat J, Barrat MJ, Picard M, Aubert MFA. Diagnosis of rabies by cell-culture. Comparative Immunology Microbiology and Infectious Diseases. 1988;11:207–214. doi: 10.1016/0147-9571(88)90039-2. [DOI] [PubMed] [Google Scholar]
- Bartlett MS. Stochastic Population Models in Ecology and Epidemiology. Methuen; London: 1960. [Google Scholar]
- Bernardi F, Nadin-Davis SA, Wandeler AI, Armstrong J, Gomes AAB, Lima FS, Nogueira FRB, Ito FH. Antigenic and genetic characterization of rabies viruses isolated from domestic and wild animals of Brazil identifies the hoary fox as a rabies reservoir. Journal of General Virology. 2005;86:3153–3162. doi: 10.1099/vir.0.81223-0. [DOI] [PubMed] [Google Scholar]
- Biek R, Walsh PD, Leroy EM, Real LA. Recent common ancestry of Ebola Zaire virus found in a bat reservoir. Public Library of Science, Pathogens. 2006;2:885–886. doi: 10.1371/journal.ppat.0020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham J, Foggin CM, Wandeler AI, Hill FWG. The epidemiology of rabies in Zimbabwe. 2. Rabies in jackals (Canis adustus and Canis mesomelas. Onderstepoort Journal of Veterinary Research. 1999a;66:11–23. [PubMed] [Google Scholar]
- Bingham J, Foggin CM, Wandeler AI, Hill FWG. The epidemiology of rabies in Zimbabwe. 1. Rabies in dogs (Canis familiaris) Onderstepoort Journal of Veterinary Research. 1999b;66:1–10. [PubMed] [Google Scholar]
- Bingham J, Javangwe S, Sabeta CT, Wandeler AI, Nel LH. Report of isolations of unusual lyssaviruses (rabies and Mokola virus) identified retrospectively from Zimbabwe. Journal of the South African Veterinary Association. 2001;72:92–94. doi: 10.4102/jsava.v72i2.624. [DOI] [PubMed] [Google Scholar]
- Brooks R. Survey of the dog-population of Zimbabwe and its level of rabies vaccination. Veterinary Record. 1990;127:592–596. [PubMed] [Google Scholar]
- Cleaveland S, Dye C. Maintenance of a microparasite infecting several host species: rabies in the Serengeti. Parasitology. 1995;111:S33–S47. doi: 10.1017/s0031182000075806. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Kaare M, Tiringa P, Mlengeya T, Barrat J. A dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog-bite injuries. Vaccine. 2003;21:1965–1973. doi: 10.1016/s0264-410x(02)00778-8. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Laurenson MK, Taylor LH. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaveland S, Mlengeya T, Kaare M, Haydon DT, Lembo T, Laurenson MK, Packer C. The conservation relevance of epidemiological research into carnivore viral diseases in the Serengeti. Conservation Biology. 2007;21:612–622. doi: 10.1111/j.1523-1739.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Wildlife ecology – Emerging infectious diseases of wildlife – Threats to Biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Dean DJ, Abelseth MK, Atanasiu P. The fluorescent antibody test. In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory Techniques in Rabies. World Health Organization; Geneva, Switzerland: 1996. pp. 88–95. [Google Scholar]
- Dobson A. The ecology and epidemiology of rinderpest virus in Serengeti and Ngorongoro Conservation Area. In: Sinclair ARE, Arcese P, editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press; Chicago: 1995. pp. 485–505. [Google Scholar]
- Dobson A. Population dynamics of pathogens with multiple host species. American Naturalist. 2004;164:S64–S78. doi: 10.1086/424681. [DOI] [PubMed] [Google Scholar]
- Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C. Serengeti wild dogs: what really happened? Trends in Ecology & Evolution. 1996;11:188–189. doi: 10.1016/0169-5347(96)30021-9. [DOI] [PubMed] [Google Scholar]
- East ML, Hofer H, Cox JH, Wulle U, Wiik H, Pitra C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proceedings of the National Academy of Sciences, USA. 2001;98:15026–15031. doi: 10.1073/pnas.261411898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foggin CM. Rabies and Rabies-related Viruses in Zimbabwe: Historical, Virological and Ecological Aspects. University of Zimbabwe; Harare, Zimbabwe: 1988. PhD thesis. [Google Scholar]
- Gascoyne SC, King AA, Laurenson MK, Borner M, Schildger B, Barrat J. Aspects of rabies infection and control in the conservation of the African wild dog (Lycaon pictus) in the Serengeti region, Tanzania. Onderstepoort Journal of Veterinary Research. 1993;60:415–420. [PubMed] [Google Scholar]
- Gordon ER, Curns AT, Krebs JW, Rupprecht CE, Real LA, Childs JE. Temporal dynamics of rabies in a wildlife host and the risk of cross-species transmission. Epidemiology and Infection. 2004;132:515–524. doi: 10.1017/s0950268804002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra MA, Curns AT, Rupprecht CE, Hanlon CA, Krebs JW, Childs JE. Skunk and raccoon rabies in the eastern United States: temporal and spatial analysis. Emerging Infectious Diseases. 2003;9:1143–1150. doi: 10.3201/eid0909.020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerging Infectious Diseases. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Independent Scientific Group on Cattle TB . Bovine TB: The Scientific Evidence. Defra Publications; London: 2007. PB12634. http://www.defra.gov.uk/animalh/tb/isg/index.htm. [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with clustal-x. Trends in Biochemical Sciences. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Johnson N, Black C, Smith J, Un H, McElhinney LM, Aylan O, Fooks AR. Rabies emergence among foxes in Turkey. Journal of Wildlife Diseases. 2003;39:262–270. doi: 10.7589/0090-3558-39.2.262. [DOI] [PubMed] [Google Scholar]
- King AA, Meredith CD, Thomson GR. Canid and viverrid rabies viruses in South Africa. Onderstepoort Journal of Veterinary Research. 1993;60:295–299. [PubMed] [Google Scholar]
- Kissi B, Tordo N, Bourhy H. Genetic polymorphism in the rabies virus nucleoprotein gene. Virology. 1995;209:526–537. doi: 10.1006/viro.1995.1285. [DOI] [PubMed] [Google Scholar]
- Kitala PM, McDermott JJ, Kyule MN, Gathuma JM. Community-based active surveillance for rabies in Machakos District, Kenya. Preventive Veterinary Medicine. 2000;44:73–85. doi: 10.1016/s0167-5877(99)00114-2. [DOI] [PubMed] [Google Scholar]
- Kitala PM, McDermott J, Kyule M, Gathuma JM, Perry B, Wandeler A. Dog ecology and demography information to support the planning of rabies control in Machakos District, Kenya. Acta Tropica. 2001;78:217–230. doi: 10.1016/s0001-706x(01)00082-1. [DOI] [PubMed] [Google Scholar]
- Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin F-X. Re-evaluating the burden of rabies in Africa and Asia. Bulletin of the World Health Organization. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- Lembo T, Haydon DT, Velasco-Villa A, Rupprecht CE, Packer C, Brandão PE, Kuzmin IV, Fooks AR, Barrat J, Cleaveland S. Molecular epidemiology identifies only a single rabies virus variant circulating in complex carnivore communities of the Serengeti. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2123–2130. doi: 10.1098/rspb.2007.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas B. Behavioural Ecology and Social Organisation of the Bat-eared Fox in the Serengeti National Park, Tanzania. University of Cambridge; Cambridge, UK: 1993. [Google Scholar]
- National Bureau of Statistics Tanzania . 2002 Population and Housing Census. President’s Office, Planning and Privatization; Dar es Salaam, Tanzania: 2005. [Google Scholar]
- Nel JAJ. The bat-eared fox-a prime candidate for rabies vector? Onderstepoort Journal of Veterinary Research. 1993;60:395–397. [PubMed] [Google Scholar]
- Nel LH, Sabeta CT, von Teichman B, Jaftha JB, Rupprecht CE, Bingham J. Mongoose rabies in southern Africa: a re-evaluation based on molecular epidemiology. Virus Research. 2005;109:165–173. doi: 10.1016/j.virusres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Page RDM. treeview: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Plowright W. The effects of rinderpest and rinderpest control on wildlife in Africa. Symposia of the Zoological Society of London. 1982;50:1–28. [Google Scholar]
- Posada D, Crandall KA. modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Randall DA, Marino J, Haydon DT, Sillero-Zubiri C, Knobel DL, Tallents LA, Macdonald DW, Laurenson MK. An integrated disease management strategy for the control of rabies in Ethiopian wolves. Biological Conservation. 2006;131:151–162. [Google Scholar]
- Randall DA, Williams SD, Kuzmin IV, Rupprecht CE, Tallents LA, Tefera Z, Argaw K, Shiferaw F, Knobel DL, Sillero-Zubiri C, Laurenson MK. Rabies in endangered Ethiopian wolves. Emerging Infectious Diseases. 2004;10:2214–2217. doi: 10.3201/eid1012.040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ, Atkinson RPD, Anderson RM, Macdonald DW. Rabies in Zimbabwe: reservoir dogs and the implications for disease control. Philosophical Transactions of the Royal Society B: Biological Sciences. 1998;353:999–1010. doi: 10.1098/rstb.1998.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, Obrien SJ, Pospischil A, HofmannLehmann R, Lutz H, Mwamengele GLM, Mgasa MN, Machange GA, Summers BA, Appel MJG. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rupprecht CE, Dietzschold B, Wunner WH, Koprowsky H. Antigenic relationships of lyssaviruses. In: Baer GM, editor. The Natural History of Rabies. CRC Press; Boca Raton: 1991. pp. 69–100. [Google Scholar]
- Sabeta CT, Bingham J, Nel LH. Molecular epidemiology of canid rabies in Zimbabwe and South Africa. Virus Research. 2003;91:203–211. doi: 10.1016/s0168-1702(02)00272-1. [DOI] [PubMed] [Google Scholar]
- Sinclair ARE. The eruption of the ruminants. In: Sinclair ARE, Norton-Griffiths M, editors. Serengeti: Dynamics of an Ecosystem. University of Chicago Press; Chicago: 1979. pp. 82–103. [Google Scholar]
- Sinclair ARE, Arcese P. Serengeti II: Dynamics, Management and Conservation of an Ecosystem. University of Chicago Press; Chicago: 1995. [Google Scholar]
- Smith JS. Rabies virus epitopic variation: use in ecological studies. Advances in Virus Research. 1989;36:215–253. doi: 10.1016/s0065-3527(08)60586-2. [DOI] [PubMed] [Google Scholar]
- Smith JS, Orciari LA, Yager PA. Molecular epidemiology of rabies in the United States. Seminars in Virology. 1995;6:387–400. [Google Scholar]
- Swanepoel R, Barnard BJH, Meredith CD, Bishop GC, Bruckner GK, Foggin CM, Hubschle OJB. Rabies in Southern Africa. Onderstepoort Journal of Veterinary Research. 1993;60:325–346. [PubMed] [Google Scholar]
- Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepsumethanon V, Wilde H, Meslin FX. Six criteria for rabies diagnosis in living dogs. Journal of the Medical Association of Thailand. 2005;88:419–422. [PubMed] [Google Scholar]
- Thomson GR, Meredith CD. Rabies in bat-eared foxes in South Africa. Onderstepoort Journal of Veterinary Research. 1993;60:399–403. [PubMed] [Google Scholar]
- Vial F, Cleaveland S, Rasmussen G, Haydon DT. Development of vaccination strategies for the management of rabies in African wild dogs. Biological Conservation. 2006;131:180–192. [Google Scholar]
- Wandeler AI, Budde A, Capt S, Kappeler A, Matter H. Dog ecology and dog rabies control. Reviews of Infectious Diseases. 1988;10:S684–S688. doi: 10.1093/clinids/10.supplement_4.s684. [DOI] [PubMed] [Google Scholar]
- Wandeler AI, Nadin-Davis SA, Tinline RR, Rupprecht CE. Rabies epidemiology: some ecological and evolutionary perspectives. In: Rupprecht CE, Dietzschold B, Koprowski H, editors. Lyssaviruses. Springer-Verlag; Berlin: 1994. pp. 297–324. [DOI] [PubMed] [Google Scholar]
- Woodroffe R. Assessing the risks of intervention: immobilization, radio-collaring and vaccination of African wild dogs. Oryx. 2001;35:234–244. [Google Scholar]
- Woodroffe R, Donnelly CA, Johnston WT, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, Cox DR, Gettinby G, Hewinson RG, Le Fevre AM, McInerney JP, Morrison WI. Spatial association of Mycobacterium bovis infection in cattle and badgers Meles meles. Journal of Applied Ecology. 2005;42:852–862. [Google Scholar]
- World Health Organization . World Survey of Rabies No. 34 for the Year 1998. Geneva; Switzerland: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.