Abstract
Morphological data from life, protargol impregnation, and scanning electron microscopy were combined with genetic data not only to describe the marine plankton ciliates Pelagostrobilidium neptuni (Montagnes and Taylor, 1994) Petz, Song, and Wilbert, 1995 and Strombidium biarmatum nov. spec., but also to elucidate their phylogenetic relationships. Additionally, the ontogenesis of P. neptuni was studied and the diagnosis of the genus Pelagostrobilidium was improved due to further data from the newly affiliated species P. epacrum (Lynn and Montagnes, 1988) nov. comb. (basionym: Strobilidium epacrum Lynn and Montagnes, 1988). The phylogenetic analysis of the small subunit ribosomal RNA genes matched the morphologic and ontogenetic assigning of P. neptuni to the choreotrichid family Strobilidiidae. The considerable genetic distance of d = 0.074 between P. neptuni and Strobilidium caudatum corroborated the morphological differences and thus the maintenance of the genus Pelagostrobilidium. Strombidium biarmatum nov. spec. is a typical member of the genus, except for the two types of extrusomes (“trichites”): ~12 × 0.5 μm, needle-shaped ones attached anterior to the girdle kinety and ~6 × 0.5 μm, rod-shaped ones at the distal end of the intermembranellar ridges. Its flask-shaped resting cysts have several strong spines. In accordance with the morphologic data, S. biarmatum is placed within the order Oligotrichida by gene sequence analysis. The great genetic distances within the oligotrichids support the diversity found in morphologic and ontogenetic studies.
Keywords: Choreotrichida, Marine plankton, Oligotrichida, Protozoa, Resting cyst, SSrRNA
Introduction
Oligotrichids and aloricate choreotrichids are an important component of the marine energy flow due to their high abundances and growth rates (Pierce and Turner 1992). About 200 species are known, of which ~60% have been described, or redescribed, using modern techniques. Thus, there are still large gaps in our knowledge about these taxa, which become more obvious when investigating the phylogenetic relationships (Agatha 2004b). Likewise, few gene sequences are available for phylogenetic analyses (Agatha 2004b; Agatha et al. 2004; Modeo et al. 2003; Snoeyenbos-West et al. 2002; Strüder-Kypke and Lynn 2003). Since the combination of morphological and molecular data provides a means to better describe species (Modeo et al. 2003), the present paper provides not only morphologic and ontogenetic data, but also the gene sequences of a known choreotrichid and a new oligotrichid species.
Materials and methods
Collection and cultivation
Pelagostrobilidium neptuni and Strombidium biarmatum were isolated from a plankton sample taken in February 2002 at monitoring site C1 (~45°42′N, 13°42′E; Fonda Umani and Beran 2003), Gulf of Trieste, Mediterranean Sea, at a salinity of ~37% and a temperature of ~7 °C. Monoclonal cultures of the two species were grown at 10 °C on a 14:10 hlight:dark cycle with an irradiance of ~10 μEm−2 s−1. Pelagostrobilidium neptuni was fed with Isochrysis galbana, an unidentified cryptophyte, and Gyrodinium sp. Strombidium biarmatum was fed on Micromonas pusilla and seemed to be mixotrophic, as it died after 1 week in the absence of light. For more details on the isolation procedure and culture media see Agatha et al. (2004).
Morphological investigations
Live cells were studied in a Petri dish(~6 cm across; water depth ~2.5 cm) under a dissecting microscope at ~20 °C and a compound microscope equipped with a high-power oil immersion objective as well as bright-field and interference contrast optics. Protargol impregnation followed the protocol of Song and Wilbert (1995). For scanning electron microscopy, cells were fixed for 30 min in a modified Parducz' solution made of six parts of 2% OsO4 (w/v) in sea water and one part of saturated aqueous HgCl2 (Valbonesi and Luporini 1990); further steps followed Foissner (1991).
Counts and measurements on protargol-impregnated cells were performed at ×1000; in vivo measurements were made at × 40–1200. Cell sizes measured in vivo (5–6 individuals) provided an estimate of shrinkage due to fixation and staining.
Illustrations of live specimens summarize information and are based on mean measurements, while those of protargol-impregnated specimens were made with a camera lucida (see Foissner et al. 2002). Note that cells in micrographs are often compressed or shrunken, especially in scanning electron micrographs.
Terminology
The terminology is according to Agatha (2004b) and the numbering of the somatic kineties follows Deroux (1974). The inner undulating membrane of the Oligotrichida is referred to as endoral (Agatha 2004a, b). The term “polykinetid” is applied to the infraciliary base of the compound ciliary organelle named membranelle. The peristomial field refers to the apical area surrounded by the external membranelles in choreotrichid ciliates. The internal membranelles are located in the oral cavity of choreotrichid ciliates. They are distinctly smaller than the external membranelles, especially the elongated ones extending into the oral cavity. The occurrence of polygonal polysaccharidic platelets is restricted to the posterior cell portion of the Oligotrichida (Agatha 2004b; Fauré-Fremiet and Ganier 1970; Laval-Peuto et al. 1994). For this layer of cortical platelets, the term “hemitheca” is introduced, following the terminology in dinoflagellates where polysaccharidic platelets form a so-called “theca” in the whole cell cortex. The frequently observed swelling posterior to the girdle kinety is probably correlated with the presence of polysaccharide platelets (Agatha 2004b). However, “distended cell surface” is an inappropriate term for the platelet layer, as it only denotes a state of the cortex caused by certain environmental conditions. Cortical granules are ~0.5–3 μm sized granules in the outer cell layer (cortex); their colour, size, and arrangement are useful species features, which are usually recognizable only in live specimens. Some granules are probably extrusomes. Kinetal lips are thin sheets of cytoplasm and plasma membrane covering the proximal portion of the somatic cilia (Grim 1987).
Slide deposition
The type material of P. neptuni (USNM #47748) is deposited in the Smithsonian Institution, Washington, DC, USA; voucher slides from the Antarctic population are in the Biology Centre of the Museum of Upper Austria (LI) in A-4040 Linz (Austria). The voucher slides of P. neptuni from Italy and the type slides of S. biarmatum are deposited with the relevant cells marked in the Biology Centre of the Museum of Upper Austria (LI) in A-4040 Linz (Austria).
DNA extraction and amplification
DNA of P. neptuni and S. biarmatum was extracted from ethanol-fixed cells, following a modified Chelex® extraction (Walsh et al. 1991), as described by Strüder-Kypke and Lynn (2003). A 10–15 μl of the supernatant was used in the PCR reactions. The PCR amplification was performed in a Perkin-Elmer GeneAmp 2400 thermocycler (PE Applied Biosystems, Mississauga, Ont., Canada), using the internal forward primer 300F (5′-AGGGTTCGATTCCGGAG-3′; Elwood et al. 1985) and the reverse primer C (5′-TTGGTCCGTGTTTCAAGACG-3′; Jerome and Lynn 1996) for S. biarmatum and the universal forward primer A (5′-AACCTGGTTGATCCTGCCAGT-3′) and the reverse primer B (5′TGATCCTTCTGCAGGTTCACCTAC-3′; Medlin et al. 1988) for P. neptuni. PCR products were purified with the GeneClean Kit (Qbiogen, Carlsbad, CA, USA) and sequenced in both directions in an ABI Prism 377 Automated DNA Sequencer (Applied Biosystems Inc., Foster City, CA, USA), using a dye terminator and Taq FS with two forward and two reverse internal small subunit ribosomal RNA (SSrRNA) primers (Elwood et al. 1985) and the amplification primers.
Sequence availability and phylogenetic analysis
The nucleotide sequences used in this study are available from the GenBank/EMBL databases and have the following accession numbers: Aspidisca steini AF305625 (Chen and Song 2002), Codonellopsis americana AY143571 (Strüder-Kypke and Lynn 2003), Engelmanniella mobilis AF508757 (Hewitt et al. 2003), Euplotidium arenarium Y19166 (Petroni et al. 2000), Eutintinnus pectinis AY143570 (Strüder-Kypke and Lynn 2003), Favella panamensis AY143572 (Strüder-Kypke and Lynn 2003), Gastrostyla steinii AF508758 (Hewitt et al. 2003), Gonostomum strenuum AJ310493 (Bernhard et al. 2001), Halteria grandinella AF194410 (Shin et al. 2000), Laboea strobila AY302563 (Agatha et al. 2004), Laurentiella strenua AJ310487 (Bernhard et al. 2001), Metacylis angulata AY143568 (Strüder-Kypke and Lynn 2003), Novistrombidium testaceum AJ488910 (Modeo et al. 2003), Onychodromus quadricornutus X53485 (Schlegel et al. 1991), Oxytricha granulifera X53486 (Schlegel et al. 1991), Rhabdonella hebe AY143566 (Strüder-Kypke and Lynn 2003), Strobilidium caudatum AY143573 (Strüder-Kypke and Lynn 2003), Strobilidium sp. AF399123, AF399124 (Snoeyenbos-West et al. 2002), Strombidinopsis sp. AF399132 (Snoeyenbos-West et al. 2002), Strombidium inclinatum AJ488911 (Modeo et al. 2003), Strombidium purpureum U97112 (Hirt et al., unpubl.), Strombidium sp. (strain SBB99-1) AY143565 (Strüder-Kypke and Lynn 2003), Strombidium sp. (strain SNB99-2) AY143564 (Strüder-Kypke and Lynn 2003), Strombidium sp. (clone Stromb00ssu_4) AF399116 (Snoeyenbos-West et al. 2002), Tetmemena pustulata (as Stylonychia pustulata) X03947, M14600 (Elwood et al. 1985), Tintinnidium mucicola AY143563 (Strüder-Kypke and Lynn 2003), Tintinnopsis fimbriata AY143560 (Strüder-Kypke and Lynn 2003), and Uronychia transfuga XY123456 (Chen and Song 2001).
The two new SSrRNA gene sequences were added to the existing dedicated comparative sequence editor (DCSE; De Rijk and De Wachter 1993) database and automatically aligned to other choreotrichid and oligotrichid sequences. The alignment was further refined, considering secondary structural features of the SSrRNA molecule.
For the phylogenetic analysis, MODELTEST (Posada and Crandall 1998) was employed to find the model of DNA substitution that best fits our data. The parameters were implemented into MrBayes ver. 2.01 (Huelsenbeck and Ronquist 2001). A maximum likelihood (ML) analysis was performed over 1,000,000 generations, determining the maximum posterior probability of a phylogeny out of 15,001 trees, approximating it with the Markov Chain Monte Carlo (MCMC). A maximum parsimony (MP) analysis was performed with PAUP* ver. 4.0b10 (Swofford 2002). Genetic distances of the included sequences were calculated by DNADIST of the Phylip ver. 3.6a2 package (Felsenstein 1993), and neighbor-joining (NJ) analysis (Saitou and Nei 1987) was performed to compute a tree. In both, the parsimony and genetic distance analyses, the data were bootstrap resampled 1000 times. In all analyses, the species U. transfuga, A. steini, and E. arenarium were used as out-group.
Results and discussion
Order: Choreotrichida Small and Lynn, 1985.
Family: Strobilidiidae Kahl in Doflein and Reichenow, 1929.
Pelagostrobilidium Petz, Song, and Wilbert, 1995
Improved diagnosis
Strobilidiidae with kineties 3 and 4 posteriorly shortened and abutting on curved kinety 2. Oral primordium develops left laterally anterior to kinety 2 and right of kinety 3.
Type species
Strobilidium neptuni Montagnes and Taylor, 1994 (by original designation).
Discussion of genus
According to Montagnes and Taylor (1994), the strobilidiid pattern of the somatic ciliature is distinctive, but possibly shows convergences; thus, they rejected the ciliary pattern as a distinguishing feature at genus level, and morphological analyses of strombidiids supported their conclusion (Agatha 2004a, c). Nevertheless, Petz et al. (1995) established the genus Pelagostrobilidium for species “with longitudinal and transversely arched somatic kineties which do not form a spiral at posterior pole”. Since the genus was established, one further species has been described, and now the genus comprises three species: P. spirale (Leegaard, 1915) Petz, Song, and Wilbert, 1995; P. neptuni (Montagnes and Taylor, 1994) Petz, Song, and Wilbert, 1995; and P. simile Song and Bradbury, 1998. Additionally, congeneric populations withsix instead of five somatic kineties are known (S.A.; Ota and Taniguchi 2003). Compared with these populations and the three species mentioned above, the curvature of somatic kinety 2 is less prominent in Strobilidium epacrum Lynn and Montagnes, 1988, suggesting that it represents a transitional stage to Rimostrombidium with its more or less meridional kineties. However, S. epacrum shows the main feature of the genus, viz., the posteriorly shortened kineties 3 and 4 that abut on the curved kinety 2. Therefore, it is transferred to Pelagostrobilidium: P. epacrum (Lynn and Montagnes, 1988) nov. comb. The remaining kineties extend longitudinally in Pelagostrobilidium, except for the distinctly curved kinety 5 in P. neptuni and P. spirale (Lynn and Montagnes 1988; Petz et al. 1995; Song and Bradbury 1998). The consistency in the pattern created by the somatic kineties 3 and 4 and the posterior portion of kinety 2 supports the maintenance of the genus Pelagostrobilidium with a refined diagnosis.
Pelagostrobilidium neptuni (Montagnes and Taylor, 1994) Petz, Song, and Wilbert, 1995
1994 Strobilidium neptuni n. sp.—Montagnes and Taylor, J. Euk. Microbiol. 41: 572.
1995 Pelagostrobilidium neptuni (Montagnes and Taylor, 1994) nov. comb.—Petz et al., Stapfia 40:139 (combining authors; redescription from life and after protargol impregnation).
Improved diagnosis
Size ~55–60 × 50–60 μm in vivo and ~50–55 × 45–50 μm in protargol preparations; subspherical with slightly oblique flattening of left posterior cell half. Two ellipsoidal micronuclei in dorsal indentations of C-shaped macronucleus. Cortex with possibly extrusive granules. Five somatic kineties: kineties 1, 3, and 4 almost longitudinal; kinety 5 inverted L-shaped and parallel to kinety 1 in anterior half; kinety 2 distinctly transversely arched and enclosing a roughly semicircular area together with kinety 1. Usually 35–38 external and one or two internal membranelles.
Description of Italian population
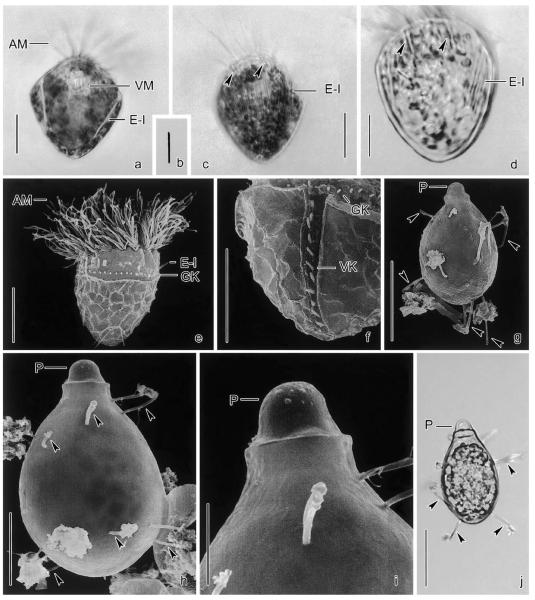
Size in vivo 45–60 × 45–55 μm, usually ~55 × 50 μm; after protargol impregnation 40–53 × 40–48 μm, usually ~46 × 43 μm (Table 1). Overall shape subspherical; it depends, however, on the side viewed, as kinety 2 causes an oblique flattening of the left posterior half. Flattening usually lost under cover glass pressure and in protargol preparations, but well preserved in scanning electron micrographs and Lugol-fixed specimens (Figs. 1a, c, 2b, and c; Montagnes pers. comm.). Macronucleus transversely orientated underneathexternal membranelles, C-shaped with ventral gap; contains numerous globular nucleoli 1–2 μm across (Fig. 1h). Invariably two ellipsoidal, usually faintly impregnated micronuclei in dorsal indentations of macronucleus. Neither a contractile vacuole nor a cytopyge recognized. Cortex with shallow ridges caused by the kinetal lips covering proximal portion of somatic cilia. Cells very fragile and thus easily burst. Cortical granules mainly along somatic kineties, and also scattered in remaining cell periphery, not recognized in vivo but distinct in several protargol preparations, where they are 1.5–2.5 × 0.5–1 μm and fusiform with a black spot in distal end; likely extrusive as ejection stages were observed in some protargol-impregnated cells and one scanning electron micrograph (Figs. 1f and 3d). Cell surface not distended in vivo and only slightly and rarely in protargol preparations, except for the peristomial field which is usually distinctly domed in impregnated cells (Fig. 1c). Cytoplasm packed with colourless lipid droplets 1–2 μm across and food vacuoles. Swims in spirals (~150 μm across) or straight at ~1mms−1 over distances of ~3 mm by rotating about main cell axis; escapes by up to 1 mm forward jumps (rough estimates); frequently rotates at oblique angles on the spot, with membranelles spread perpendicularly to main cell axis, occasionally producing a helicopter-like appearance in top view (Figs. 1b, e, and 2d). Membranelles overlap like an iris diaphragm in motionless cells (Fig. 3b).
Table 1.
Morphometric data on Pelagostrobilidium neptuni
| Characteristicsa | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|
| Cell, lengthb | 46.1 | 45.0 | 3.6 | 0.9 | 7.9 | 40.0 | 53.0 | 15 |
| Cell, width | 42.9 | 43.0 | 2.4 | 0.6 | 5.7 | 40.0 | 48.0 | 15 |
| Cell, length:width ratio | 44.6 | 44.0 | 4.0 | 0.9 | 8.9 | 40.0 | 53.0 | 19 |
| Anterior cell end to macronucleus, distanceb | 10.6 | 10.0 | 2.6 | 0.7 | 24.9 | 5.0 | 14.0 | 15 |
| Anterior cell end to somatic kineties 1, 3–5, distanceb | 19.7 | 20.0 | 3.6 | 0.9 | 18.5 | 13.0 | 25.0 | 15 |
| Anterior cell end to somatic kinety 2, distanceb | 31.1 | 30.0 | 4.1 | 1.1 | 13.2 | 25.0 | 38.0 | 15 |
| Posterior cell end to kinety 5, distance | 12.0 | 13.0 | 1.9 | 0.5 | 15.4 | 9.0 | 15.0 | 15 |
| Macronucleus, lengthc | 79.7 | 81.0 | 9.7 | 2.5 | 12.2 | 62.0 | 96.0 | 15 |
| Macronucleus, width | 7.4 | 8.0 | 1.4 | 0.4 | 19.0 | 5.0 | 9.0 | 15 |
| Micronuclei, length | 2.5 | 3.0 | 0.9 | 0.2 | 36.1 | 1.0 | 4.0 | 15 |
| Micronuclei, width | 1.9 | 2.0 | 0.7 | 0.2 | 37.5 | 1.0 | 3.0 | 15 |
| Somatic kinety 1, lengthc | 33.8 | 33.0 | 4.2 | 1.1 | 12.4 | 28.0 | 44.0 | 15 |
| Somatic kinety 2, lengthc | 54.5 | 55.0 | 6.3 | 1.6 | 11.5 | 41.0 | 65.0 | 15 |
| Somatic kinety 3, length | 21.4 | 21.0 | 3.1 | 0.8 | 14.6 | 16.0 | 26.0 | 15 |
| Somatic kinety 4, length | 26.3 | 25.0 | 3.0 | 0.8 | 11.5 | 23.0 | 34.0 | 15 |
| Somatic kinety 5, lengthc | 34.7 | 33.5 | 4.0 | 1.2 | 11.6 | 28.0 | 41.0 | 12 |
| Macronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 15 |
| Micronuclei, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 15 |
| External membranelles, numberd | 35.3 | 36.0 | 1.0 | 0.3 | 3.0 | 33.0 | 37.0 | 15 |
| Elongated external membranelles, number | 9.1 | 9.0 | 0.4 | 0.1 | 3.9 | 9.0 | 10.0 | 15 |
| Internal membranelles, numberd | 1.7 | 2.0 | 0.5 | 0.1 | 29.3 | 1.0 | 2.0 | 15 |
| Somatic kineties, number | 5.0 | 5.0 | 0.0 | 0.0 | 0.0 | 5.0 | 5.0 | 15 |
| Cortical granules, length | 2.1 | 2.0 | 0.3 | 0.1 | 16.1 | 1.5 | 2.5 | 15 |
| Cortical granules, width | 0.6 | 0.5 | 0.2 | 0.1 | 36.1 | 0.5 | 1.0 | 15 |
Data are based on protargol-impregnated, mounted, and randomly selected specimens from clonal cultures. Measurements in micron. CV, coefficient of variation in %; M, median; Max, maximum; Min, minimum; n, number of individuals investigated; SD, standard deviation; SE, standard error of arithmetic mean; x̄, arithmetic mean.
Measured including distended peristomial field.
Approximations because organelles distinctly curved.
Counted in properly orientated morphostatic specimens or oral primordia of middle dividers.
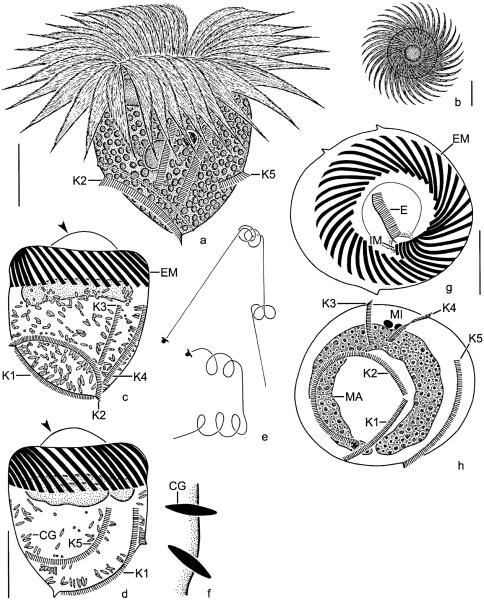
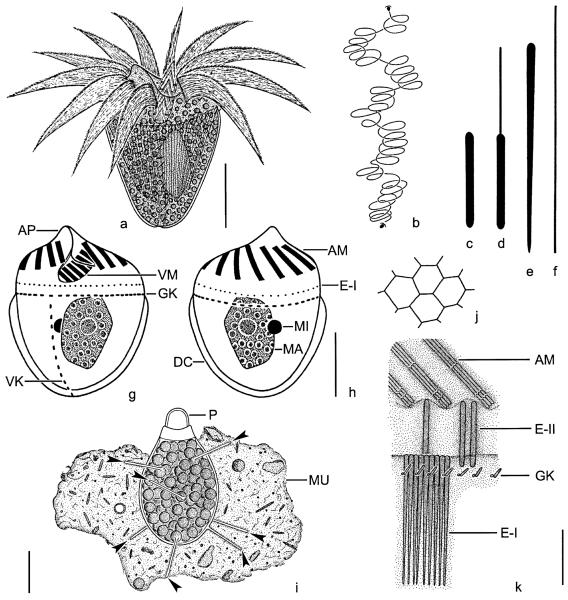
Fig. 1.
Pelagostrobilidium neptuni from life (a, b, e) and after protargol impregnation (c, d, f–h). (a) Dorsolateral view of a representative specimen. (b) Top view. (c, d, g, h) Left lateral, right lateral, top, and posterior polar view. Note the vaulted peristomial field (arrowheads). (e) Swimming traces. (f) Optical section of cell cortex showing ejection stages of cortical granules. CG, cortical granules; E, endoral; EM, external polykinetids; IM, internal polykinetids; K1–5, somatic kineties; MA, macronucleus; MI, micronuclei. Scale bar 20 μm.
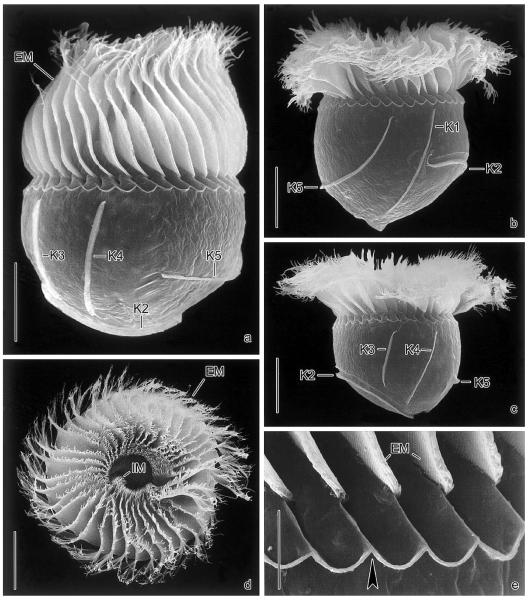
Fig. 2.
Pelagostrobilidium neptuni in the scanning electron microscope. (a–c) Lateral views during ~360° rotation about main cell axis, beginning with the dorsal aspect. (d) Top view showing the conspicuous oral apparatus. (e) Intermembranellar ridges. Arrowhead marks crenated border. EM, external membranelles; IM, internal membranelles; K1–5, somatic kineties. Scale bars 20 μm (a–d) and 5 μm (e).
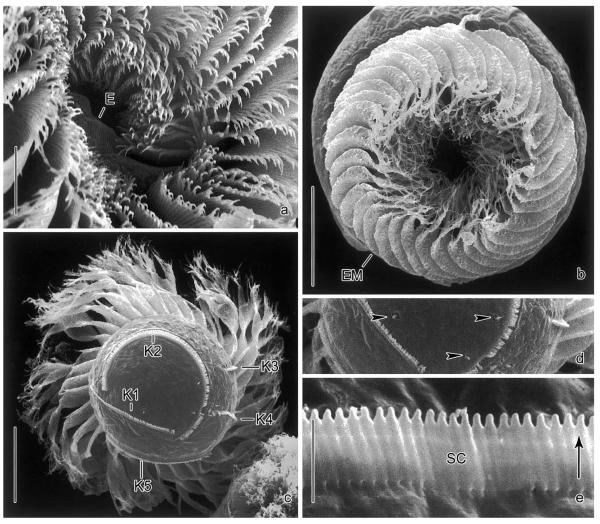
Fig. 3.
Pelagostrobilidium neptuni in the scanning electron microscope. (a) Detail of adoral zone of membranelles and peristomial field. Note the endoral. (b) Top view. In motionless cells, the external membranelles are arranged like an iris diaphragm. (c, d) Posterior polar view and detail of cell surface. The cortical granules are probably extrusive, as they occasionally penetrate the cell surface (arrowheads). (e) Detail of somatic kinety. The ~2 μm long cilia are directed to the left (arrow) due to the kinetal lips covering their proximal portion. E, endoral; EM, external membranelles; K1–5, somatic kineties; SC, somatic cilia. Scale bars 20 μm (b, c), 10 μm (a), and 2 μm (e).
Somatic ciliature as described by Montagnes and Taylor (1994). Somatic kineties commence ~20 μm posterior to anterior cell end, except for kinety 2 usually starting in posterior third of cell (Figs. 1c, d, h, 2a–c, and 3c). Kineties 3 and 4 shorter than kinety 1 and the distinctly curved kineties 2 and 5 (Table 1). A shallow furrow extends above and parallel to kinety 2, bordered by a minute crest anteriorly (Figs. 2b and c). Cilia closely spaced, 1–3 μm long and rod-shaped, usually directed to the left, with kinetal lips covering their proximal portion (Fig. 3e). Kinetid structure uncertain: scanning electron microscopy and protargol preparations reveal closely spaced cilia (basal bodies, possibly monokinetids), while kineties tend to form paired cilia in squashed live cells.
Oral apparatus occupies anterior cell end (Figs. 1a, b, g, 2d, 3a, and b). Adoral zone of membranelles closed, comprises ~35 external membranelles, of which nine or ten are gradually elongated and extend into the eccentric oral cavity; usually two minute, gradually shortened internal membranelles (Table 1). Individual external membranelles clockwise inclined across peristomial rim, distally frayed, with cilia up to ~30 μm long, polykinetids 16–25 μm long. Intermembranellar ridges conspicuous, distally bounded by a crenated, slightly argyrophilic ridge (Figs. 2a–c and e). Endoral slightly sigmoidal or straight, extends across peristomial field and right inner wall of oral cavity, composed of a single row of basal bodies (probably monostichomonad), cilia not recognized in vivo and only very rarely in protargol preparations and scanning electron micrographs, probably because usually covered by perilemma (Figs. 1g and 3a). Oral funnel eccentric; distance between anterior end of cell and buccal vertex highly variable in protargol preparations, namely, 11–21 μm, due to variation in distension of peristomial field.
Gene sequence analyses
The PCR product of the complete SSrRNA gene of P. neptuni is 1683 nucleotides in length and has a GC content of 46%. The sequence is available under the GenBank accession number AY541683.
Ontogenesis
Cell division of P. neptuni can be divided into three main stages. While the observations of early, middle, and late dividers are based on several specimens each, very late dividers were rare and inadequately impregnated.
Early dividers
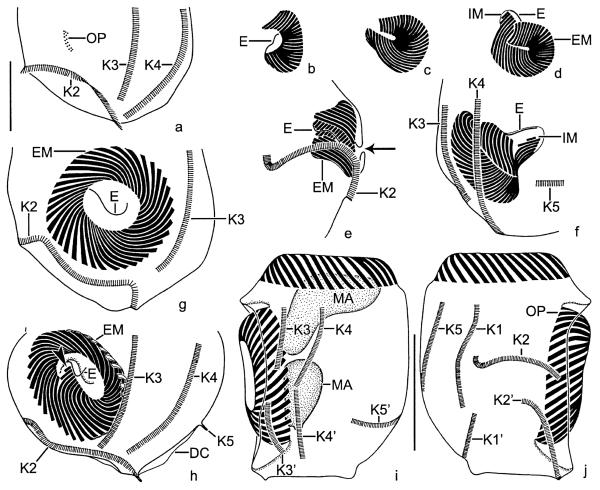
Cell length of early dividers increases by ~27%. Stomatogenesis commences with the apokinetal development of a slightly cuneate, longitudinally orientated anarchic field of basal bodies in a shallow depression on the left cell side anterior to kinety 2, i.e., between kineties 1 and 3 (Fig. 4a). The oral primordium sinks into a bowl-shaped subsurface pouch, enlarges, and becomes inverted C-shaped, while the polykinetids differentiate (Fig. 4b). The endoral originates de novo. When the final number of polykinetids has been generated, the posterior portion of the oral primordium performs a distinct clockwise rotation, causing the clockwise curvature of the polykinetids observed in the morphostatic specimens (Figs. 4b–d). Next, the proximal portion of the new zone with the internal membranelles and the elongated external ones curves below the distal end of the zone (Fig. 4d). Then, the membranellar zone orientates perpendicularly to the cell surface, forming a funnel (Figs. 4e and f). Since the macronucleus is usually covered by the darkly impregnated external membranelles, the migration of the replication bands could not be followed; however, a dorsal bulge, from which the replication bands originate in Strobilidium, according to Deroux (1974), is not recognizable. Likewise, the micronuclei impregnated too faintly to describe their ontogenetic behaviour.
Fig. 4.
Pelagostrobilidium neptuni, early (a–f), early middle (g, h), and late (i, j) dividers after protargol impregnation. (a) Left lateral view showing the slightly cuneate oral primordium anterior to somatic kinety 2. (b–d) The polykinetids are differentiated and the posterior portion of the oral primordium performs a distinct clockwise rotation. The endoral originates de novo. Finally, the internal polykinetids and the elongated external ones extend below the distal end of the zone. (e, f) Lateral views of the new oral apparatus. First, the adoral zone of membranelles forms a funnel (e). Then, the internal polykinetids and the elongated external ones sink deeper into the forming oral cavity (f). Note the minute opening of the subsurface pouch (e; arrow). (g, h) Left lateral views showing the oral primordium with spread polykinetids and endoral. Arrowhead marks the two minute internal polykinetids (h). (i, j) Dorsal and ventral views of the same specimen. The macronucleus and the somatic kineties split. The daughter's oral apparatus evaginated and shaped. DC, distended cell surface; E, endoral; EM, external polykinetids; IM, internal polykinetids; K1–5, (proter's) somatic kineties; K1′–5′, opisthe's somatic kineties; MA, macronucleus; OP, oral primordium. Scale bars 20 μm (a–h) and 40 μm (i, j).
Middle dividers
Middle dividers are larger than early dividers by ~6%. The rotation of the oral primordium has finished and the opisthe's right-side faces the proter's left. The diameter of the membranellar zone increases by spreading of the polykinetids (Figs. 4g and h). The cilia grow out and the membranelles arrange like an iris diaphragm within the pouch (Figs. 5a and b). The new oral apparatus evaginates, commencing with the anterior portion (right side of the opisthe). Simultaneously, the peristomial rim and the peristomial field are shaped. The somatic kineties lengthen by intrakinetal proliferation of basal bodies. The macronucleus condenses to a longitudinally orientated ellipsoidal mass.
Fig. 5.
Pelagostrobilidium neptuni, dividers in the scanning electron microscope. (a, b) Middle dividers showing the opisthe's oral apparatus in a subsurface pouch on the left cell side anterior to kinety 2. The daughter's external membranelles are arranged like an iris diaphragm. (c) Oblique top view of a late divider showing the evaginated new adoral zone of membranelles. The proter's and opisthe's oral apparatus form a right angle, i.e., the opisthe's right-side faces the proter's left. K1–5, somatic kineties; OP, oral primordium. Scale bar 20 μm.
Late dividers
The late division stages show a further increase in size by ~6%; the main enlargement, however, takes place in very late dividers or postdividers. The formation of the new oral apparatus has finished (Figs. 4i, j and 5c). The somatic kineties split slightly below mid-body. Somatic kinety 2 delimits the evagination opening posteriorly. The final arrangement of the somatic kineties takes place in later division stages and possibly roughly follows the pattern described in another species with several rows of somatic basal bodies, i.e., in Halteria grandinella (Fauré-Fremiet 1953). The macronucleus has divided into two ellipsoidal nodules; the opisthe's nodule is already underneath its membranellar zone, while the proter's migrates to its final position (Fig. 4i). The elongation and shaping of the macronuclei occurs only in very late dividers.
During ontogenesis, the parental oral ciliature does not reveal any signs of reorganization.
Occurrence and ecology
Montagnes and Taylor (1994) discovered P. neptuni in subsurface waters off the Canadian west coast (British Columbia) in March. The species was also found in the spring plankton of the Weddell Sea, Antarctica (Petz et al. 1995); near the Island of Sylt, North Sea, from September to December (S.A.); in the Irish Sea off the Isle of Man in May (S.A.); and the Mediterranean Sea almost all the year round (this study). All records are substantiated by morphometric data and/or illustrations. According to literature and our data, P. neptuni tolerates a wide range of temperatures (−1.2–20 °C) and salinities (16–37%). Pelagostrobilidium neptuni grows on Isochrysis galbana, Chroomonas salina, and Rhodomonas lens (Montagnes and Taylor 1994) or the algal mixture used in this study; however, field specimens often contain centric and pennate diatoms (Montagnes and Taylor 1994; Petz et al. 1995), and growth on small ciliates was observed in raw cultures (A.B.). Montagnes (1996) observed a maximum growth rate of 1.5 d−1 in laboratory experiments.
Ontogenetic comparison
Detailed ontogenetic investigations are available for a few choreotrichid ciliates. The oral primordium originates on the left cell side between kineties 1 and 3 and anterior to kinety 2 not only in P. neptuni, but also in the Pelagostrobilidium populations with six somatic kineties (S.A.; Ota and Taniguchi 2003). Likewise, the new oral apparatus develops left laterally in Rimostrombidium lacustris (as estimated from micrographs in Foissner et al. 1999) and the Strobilidium populations studied by Fauré-Fremiet (1924) and Deroux (1974). In contrast, the oral primordium forms dorsally in Strobilidium caudatum (Kormos and Kormos 1958; Petz and Foissner 1992), R. caudatum, R. conicum, R. veniliae (Agatha and Riedel-Lorjé 1998), and Strombidinopsis species (Agatha 2003b; Dale and Lynn 1998); R. orientale develops the oral primordium on the ventral side (Song and Bradbury 1998). Although the location of the oral primordium relative to the oral cavity is different in the strobilidiid species, its position relative to the kineties is usually similar, i.e., the area anterior to kinety 2 in Pelagostrobilidium probably corresponds to the region between kineties 2 and 3 in R. caudatum and R. conicum. Interestingly, the oral primordium even forms between these two kineties in the Strobilidium population studied by Deroux (1974), when numbering is slightly changed, viz., kinety 1 becomes kinety 2 and so on. According to Krainer (1995), R. brachykinetum generates the daughter's membranellar zone between kineties 4 and 5; however, the illustrations provided combine different focal planes, like those of R. humile (Krainer 1995), and are thus confusing. Assuming that most strobilidiids develop their oral primordium between kineties 2 and 3, the position of the new membranellar zone in the cell may only depend on the shape and length of kinety 2. Further comparative studies should test this hypothesis.
Comparison with related species
Pelagostrobilidium neptuni differs from the Chinese Pelagostrobilidium sp. mainly in the number of somatic kineties (5 vs. 6) and the course of kinety 5 (inverted L-shaped vs. longitudinal; Fig. 17 in Ota and Taniguchi 2003). In P. simile, somatic kinety 2 does not abut on the end of kinety 1 but surrounds it (Song and Bradbury 1998); therefore, the two kineties do not enclose a semicircular area in the left posterior cell portion, as in P. neptuni. In contrast to P. neptuni and the other congeners, the somatic kinety 2 of P. epacrum commences not in the posterior cell half, but at the same level as the other somatic kineties (Lynn and Montagnes 1988).
According to an ultrastructural study, Rimostrombidium velox has apparently monokinetidal somatic kineties (Grim 1987). However, drawings of live and protargol-impregnated specimens of R. brachykinetum Krainer, 1995 and micrographs of protargol-impregnated cells of R. humile (Penard, 1922) Petz and Foissner, 1992 indicate a dikinetidal structure (Foissner et al. 1999). Likewise, the somatic kineties tend to form paired cilia in squashed cells of P. neptuni.
Since P. neptuni is the sole species of the genus that has been studied in the scanning electron microscope, the transverse ridge posterior to the membranellar zone cannot be verified in its congeners (Figs. 2a–c and e). However, other ultrastructurally investigated strobilidiids lack such a structure (Foissner et al. 1988, 1991, 1999; Grim 1987; Montagnes and Taylor 1994). On the other hand, P. neptuni shows pores at the proximal end of the external membranelles (Fig. 2b in Montagnes and Taylor 1994) similar to those described in R. lacustris by Foissner et al. (1999).
Argyrophilic cortical granules, probably extrusomes, occur in P. neptuni, P. spirale, R. humile, and R. veniliae, where they have a similar size and shape (Agatha and Riedel-Lorjé 1998; Foissner et al. 1991; Krainer 1995; Lynn and Montagnes 1988).
The cilia of the endoral are usually covered by perilemma in strobilidiids and are only rarely recognizable in scanning electron micrographs (Fig. 2a; Montagnes and Taylor 1994). This seems also to be so for at least some strombidiids and strombidinopsids (Agatha 2003a, b; Agatha et al. 2004; Foissner et al. 1999; Modeo et al. 2003; Montagnes et al. 2002).
Comparison of populations
The specimens from the Weddell Sea (Petz et al. 1995) and Italy (this study) match the type population (Montagnes and Taylor 1994), except for a slightly lower number of external membranelles in the Italian specimens (36–40 vs. 33–37) and the absence of the argyrophilic cortical granules in the Antarctic cells. However, the latter feature depends highly on the preparation procedures and thus should not be over-interpreted. While the Antarctic and Italian specimens have one or two internal membranelles, Montagnes and Taylor (1994) mentioned 9–20 membranelles. This difference probably results from the fact that the accurate number of internal membranelles is difficult to discern in morphostatic specimens due to the vaulted peristomial rim, while it is easily ascertainable in the oral primordium of late early dividers.
Order: Oligotrichida Bütschli, 1889.
Family: Strombidiidae Fauré-Fremiet, 1970.
Strombidium biarmatum nov. spec.
Diagnosis
Size ~25 × 20 μm in vivo, ~21 × 15 μm after protargol impregnation; broadly obovoidal with apical protrusion at right anterior end. Macronucleus broadly ellipsoidal, micronucleus globular. Two types of extrusomes: type I needle-shaped, ~12 × 0.5 μm, attached anterior to girdle kinety; type II rod-shaped, ~6 × 0.5 μm, attached to distal end of intermembranellar ridges. Girdle kinety pre-equatorial, ostensibly continuous, composed of ~50 dikinetids. Ventral kinety extends meridionally between girdle kinety and posterior cell end, composed of ~10 dikinetids. Adoral zone of membranelles almost closed, anterior portion with ~13 membranelles distinctly separate from ventral portion with ~6 membranelles.
Type location
Pelagial, Gulf of Trieste, Northern Adriatic, Mediterranean Sea, ~45°42′N, 13°42′E.
Etymology
Composite of the Latin prefix bi (twice) and the adjective armatus (armed), referring to the two types of extrusomes.
Description
Size in vivo 20–40 × 15–30 μm, usually ~25 × 20 μm; after protargol impregnation 18–24 × 13–16 μm, usually ~21 × 15 μm (Table 2). Shape broadly obovoidal or obconical, posterior end rounded, anterior slightly domed or transversely truncate with hyaline, ~3 μm high apical protrusion at right side of peristome recognized both in vivo and protargol preparations (Figs. 6a and g). Macronucleus left of mid-line and ~40% posterior to anterior cell end, broadly ellipsoidal, contains some small nucleoli ~1 μm across and often one large nucleolus ~3 μm across. Micronucleus usually in lateral indentation of macronucleus, ~2 μm across, rarely recognizable because faintly impregnated (Fig. 6h). Neither a contractile vacuole nor a cytopyge recognized. Hemitheca posterior to girdle kinety, composed of hexagonal or polygonal platelets ~3 μm across (Fig. 6j). Cell surface distinctly distended posterior to girdle kinety in prepared cells, except for region around ventral kinety. Two types of extrusomes: type I needle-shaped, ~12 × 0.5 μm, with rounded anterior and pointed posterior end, attached in probably single row to shallow bulge anterior to girdle kinety; type II rod-shaped, ~6 × 0.5 μm, attached singly or in pairs to distal end of intermembranellar ridges (Figs. 6c, e, k, and 7a–d). Extrusomes recognizable in vivo and, rarely, in strongly over-bleached, protargol-impregnated specimens. Ejected type I extrusomes thread-like and ~60 × 0.5 μm; possibly incompletely exploded type II extrusomes with an ~6 μm long, filiform anterior portion and a rod-shaped, ~6 × 0.5 μm sized posterior portion (Figs. 6d and f). Cytoplasm colourless, contains green inclusions ~1–2 μm across, possibly sequestered plastids (Figs. 7a and c). Swims ~500 μm s−1 by rotation about main cell axis, performing spirals ~100 μm across interrupted by sudden changes in direction (Fig. 6b). Easily bursts when water temperature increases or when contacting the water surface.
Table 2.
Morphometric data on Strombidium biarmatum
| Characteristicsa | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|
| Cell, lengthb | 20.7 | 20.0 | 1.8 | 0.4 | 8.6 | 18.0 | 24.0 | 25 |
| Cell, widthb | 14.8 | 15.0 | 1.0 | 0.2 | 6.9 | 13.0 | 16.0 | 25 |
| Cell, length:width ratiob | 1.4 | 1.4 | 0.1 | 0.0 | 8.6 | 1.2 | 1.6 | 25 |
| Anterior cell end to macronucleus, distance | 8.4 | 9.0 | 1.4 | 0.3 | 16.5 | 6.0 | 10.0 | 25 |
| Anterior cell end to buccal vertex, distance | 7.4 | 8.0 | 1.0 | 0.2 | 14.0 | 5.0 | 10.0 | 25 |
| Anterior cell end to girdle kinety, distance | 8.2 | 8.0 | 1.2 | 0.2 | 14.4 | 6.0 | 11.0 | 25 |
| Adoral zone of membranelles, outer diameter | 12.8 | 13.0 | 1.0 | 0.2 | 7.9 | 11.0 | 15.0 | 25 |
| Macronucleus, length | 8.4 | 9.0 | 1.2 | 0.2 | 13.7 | 6.0 | 10.0 | 25 |
| Macronucleus, width | 5.2 | 5.0 | 0.7 | 0.1 | 13.6 | 4.0 | 6.0 | 25 |
| Micronucleus, length | 2.2 | 2.0 | 0.4 | 0.1 | 19.5 | 2.0 | 3.0 | 21 |
| Micronucleus, width | 2.2 | 2.0 | 0.4 | 0.1 | 19.5 | 2.0 | 3.0 | 21 |
| Macronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 25 |
| Micronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| Anterior membranelles, number | 13.4 | 13.0 | 0.9 | 0.2 | 6.4 | 12.0 | 15.0 | 25 |
| Ventral membranelles, number | 6.4 | 6.0 | 0.5 | 0.1 | 7.8 | 6.0 | 7.0 | 25 |
| Girdle kinety, number of dikinetidsc | 48.6 | 48.0 | 4.0 | 0.9 | 8.2 | 42.0 | 56.0 | 18 |
| Ventral kinety, number of dikinetids | 9.8 | 10.0 | 1.2 | 0.3 | 12.7 | 7.0 | 12.0 | 18 |
| Resting cyst, lengthin vivo | 33.4 | 33.0 | 2.5 | 0.8 | 7.6 | 30.0 | 36.0 | 11 |
| Resting cyst, widthin vivo | 19.5 | 20.0 | 0.5 | 0.2 | 2.7 | 19.0 | 20.0 | 11 |
| Resting cyst, length:width ratio in vivo | 1.7 | 1.7 | 0.1 | 0.0 | 8.5 | 1.5 | 1.9 | 11 |
Data are based, if not stated otherwise, on protargol-impregnated, mounted, and randomly selected specimens from clonal cultures. Measurements in micron. CV, coefficient of variation in %; M, median; Max, maximum; Min, minimum; n, number of individuals investigated; SD, standard deviation; SE, standard error of arithmetic mean; x̄, arithmetic mean.
Measured without distended cell surface.
Approximation because usually discernible on only one cell side.
Fig. 6.
Strombidium biarmatum from life (a–f, i–k) and after protargol impregnation (g, h). (a) Ventral view of a representative specimen. (b) Swimming trace. (c, d) Resting and possibly incompletely exploded type II extrusome. (e, f) Resting and exploded type I extrusome. (g, h) Ventral and dorsal view. (i) Resting cyst with some strong spines (arrowheads). (j) Reticulation of posterior cell surface. (k) Scheme showing the arrangement of the extrusomes: type I is attached in a single row anterior to the girdle kinety, while type II inserts at the distal end of the intermembranellar ridges. Note that type I extrusomes are removed in the right half of the scheme to show type II more clearly. AM, anterior membranelles/polykinetids; AP, apical protrusion; DC, distended cell surface; E-I, type I extrusomes; E-II, type II extrusomes; GK, girdle kinety; MA, macronucleus; MI, micronucleus; MU, mucus; P, plug; VK, ventral kinety; VM, ventral polykinetids. Scale bars 10 μm (a, g–i) and 5 μm (k).
Fig. 7.
Strombidium biarmatum from life (a–d, j) and in the SEM (e–i). (a, c, d) Lateral views. The type I extrusomes form a stripe near mid-body, while some type II extrusomes (arrowheads) insert at the distal ends of the intermembranellar ridges. (b) Type II extrusome, 6 × 0.5 μm. (e) Lateral view. (f) Posterior ventral half of cell. (g–j) Resting cysts with some strong spines (arrowheads). AM, anterior membranelles; E-I, type I extrusomes; GK, girdle kinety; P, plug; VK, ventral kinety; VM, ventral membranelles. Scale bars 10 μm (a, c, d, e, f, h), 20 μm (g, j), and 5 μm (i).
Somatic cilia ~1–2 μm long and rod-shaped, arranged in a girdle and a ventral kinety (Figs. 6g, h, k, 7e, and f). Girdle kinety pre-equatorial, viz., on average 40% posterior to anterior cell end, ostensibly continuous, composed of ~50 horizontally orientated dikinetids, each having a cilium associated only with the left basal body. No associated fibres recognizable in protargol impregnations. Ventral kinety extends meridionally in a furrow between girdle kinety and posterior end of ventral side, composed of ~10 meridionally orientated dikinetids, each having a cilium associated only with the anterior basal body.
Oral apparatus occupies anterior end of cell (Figs. 6a, g, and 7e). Adoral zone of membranelles almost closed, surrounds apical protrusion, terminates ~36% posterior to anterior cell end in buccal cavity, divided into an anterior portion with ~13 membranelles and a ventral portion with ~6 membranelles. Anterior membranelles distinctly separate from ventral ones, composed of three rows of basal bodies with cilia up to 15–17 μm long; orientated perpendicularly to main cell axis in swimming specimens. Ventral membranelles with cilia up to ~6 μm long, structure not recognizable. Ridges between anterior membranelles low and with indistinct bulge where type II extrusomes insert. Endoral on inner wall of buccal lip on right side of oral cavity, rarely recognizable in protargol preparations and in vivo, probably usually covered by perilemma, composed of a single row of about ten basal bodies, probably monostichomonad. Pharyngeal fibres not observed.
Resting cysts 30–36 × 19–20 μm in vivo, flask-shaped; length:width ratio 1.5–1.9:1, usually 1.7:1 (Figs. 6i and 7g–j). Cyst wall colourless, composed of a single ~0.5 μm thick layer, smooth, except for several (~12–24) strong, rod-shaped spines ~8–10 × 0.5 μm, between which mucus and organic debris accumulate. Hyaline plug (“papulla”) closing emergence pore composed of a ~5 μm high and apparently hollow hemispherical distal portion and a ~2–8 μm high and possibly solid cylindroidal proximal portion, could not be removed by pressure. Protargol impregnates wall but not plug, indicating that they likely differ in their chemical composition. Cytoplasm of 1 or 2 days old cysts filled with green inclusions (food remnants or sequestered plastids).
Gene sequence analyses
The partial SSrRNA gene sequence of S. biarmatum is 1376 nucleotides in length, has a GC content of 49%, and is available under the GenBank accession number AY541684.
Comparison with related species
At first glance, S. biarmatum looks like an ordinary Strombidium species due to the needle-shaped extrusomes attached anterior to the horizontal girdle kinety. Live observations, however, reveal a distinct difference, i.e., a second row of extrusomes attached to the distal end of the intermembranellar ridges. Only one congener is known to share this feature, viz., Strombidium rehwaldi Petz and Foissner, 1992. This species differs from S. biarmatum by its larger size (33–46 × 26–36 μm vs. 18–24 × 13–16 μm after protargol impregnation), the shape of the adoral zone of membranelles (widely open vs. almost closed), the girdle kinety (distinctly discontinuous vs. ostensibly continuous), and the habitat (freshwater vs. marine waters). In Strombidium faurei, S. macronucleatum, S. fourneleti (Dragesco 1960), and S. latum (Fauré-Fremiet 1950), some scattered extrusomes are close to the membranellar zone; these species differ from S. biarmatum in the cell size (>50 μm) and shape and a widely open membranellar zone. Strombidium biarmatum is the only known species with two morphologically different types of extrusomes; a displacement of extrusomes from the girdle kinety to the distal end of the intermembranellar ridges can thus be excluded. However, there may be further species with two sets of extrusomes. Unfortunately, many descriptions lack the indispensable live observations and are merely based on preserved material, where the arrangement, size, and shape of the extrusomes hardly can be seen (e.g., Lynn et al. 1988; Montagnes et al. 1988); furthermore, nobody expects extrusomes underneath the intermembranellar ridges in otherwise typical Strombidium cells. Nevertheless, there are only two other Strombidium species sharing a pre-equatorial and ostensibly continuous girdle kinety, an apical protrusion, and an almost closed adoral zone of membranelles with the anterior portion separated from the ventral: S. vestitum (Leegaard, 1915) Kahl, 1932, as redescribed by Agatha and Riedel-Lorjé (1997; extrusome attachment sites in oblique rows, forming an ~1–2 μm wide stripe anterior to the girdle kinety; no further extrusomes recognized in vivo or in the neotype slides re-investigated with interference contrast) and S. tressum Lynn et al., 1988 (anterior membranelles extremely long, i.e., ~1.5 times cell length; Fig. 4f in Lynn et al. 1988).
Resting cysts
In contrast to tintinnid cysts, which are formed within the lorica (see Reid and John 1978), cysts of oligotrichids and aloricate choreotrichids are difficult to detect and identify. Thus, the resting cysts of only a few species are known: S. biarmatum (this study); S. oculatum Gruber, 1884; S. conicum (Lohmann, 1908) Wulff, 1919; S. crassulum (Leegaard, 1915) Kahl, 1932 (probably confused with an aloricate choreotrichid); Limnostrombidium viride (Stein, 1867) Krainer, 1995; Pelagostrombidium fallax (Zacharias, 1895) Krainer, 1991; and Cyrtostrombidium boreale Kim, Suzuki, and Taniguchi, 2002. The cysts of Strombidium, Limnostrombidium, and Pelagostrombidium are affiliated with the flask-shaped Sphaeropsis-type established by Meunier (1910), but differ in the structure of the cyst wall: without spines in S. oculatum (Fauré-Fremiet 1948a, b; Jonsson 1994; Montagnes et al. 2002), S. conicum (Kim and Taniguchi 1995), and L. viride (Müller and Wünsch 1999); with many fine spines in S. crassulum (Reid 1987) and P. fallax (Müller 1996, 2002; Müller and Wünsch 1999); and with few strong spines in S. biarmatum (this study). The emergence pore of the cyst is closed by a plug (“papulla”), which is hemispherical and apparently hollow in S. biarmatum, S. conicum, S. crassulum, and P. fallax, while slightly frothy in L. viride and distinctly frothy in S. oculatum.
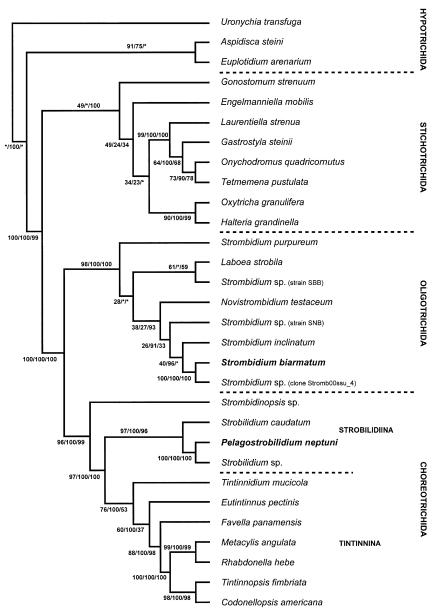
Phylogeny
The three phylogenetic methods (MP, ML, NJ) resulted in trees with congruent topologies, except for the branching within the order Oligotrichida (see below). Only the most parsimonious tree is shown, including all support values for the internal nodes (Fig. 8). Pelagostrobilidium neptuni always groups within a clade corresponding to the family Strobilidiidae in the order Choreotrichida. This placement is highly supported (100% MP, ML, NJ). Strombidium biarmatum clusters within the order Oligotrichida (98% MP, 100% ML, NJ) represented by the genera Laboea, Novistrombidium, and Strombidium. Morphologically, P. neptuni belongs to the choreotrichid family Strobilidiidae because (i) the adoral zone of membranelles is closed, (ii) the oral primordium originates hypoapokinetally within a pouch, and (iii) the somatic kineties consist of closely spaced cilia covered by a kinetal lip. Strombidium biarmatum is classified in the Oligotrichida because it has a C-shaped adoral zone of membranelles, a ventral and a girdle kinety composed of dikinetids, extrusomes (“trichites”), and a hemitheca. The molecular placement of the two species within the orders Choreotrichida and Oligotrichida thus matches the morphologic, ontogenetic, and ultrastructural data (Agatha 2004b; Lynn and Small 2002).
Fig. 8.
The most parsimonious tree inferred from small subunit rRNA gene sequences of spirotrich ciliates and computed with PAUP* ver. 4.0b10 (Swofford 2002). The numbers on the branches are support values for the internal nodes: The first value represents the bootstrap support for the MP analysis (percentage out of 1000 replicates); the second value gives the posterior probability out of 15,001 trees from an ML analysis performed with MrBayes ver. 2.01, employing the Bayesian Inference (Huelsenbeck and Ronquist 2001). The third number is the bootstrap value for the NJ tree (Saitou and Nei 1987), implemented in Phylip ver. 3.6a2 (Felsenstein 1993). Asterisks mark nodes with support values below 12%.
Pelagostrobilidium neptuni forms a well-supported branch with an undetermined strobilidiid species (Strobilidium sp.; Snoeyenbos-West et al. 2002) and Strobilidium caudatum (Strüder-Kypke and Lynn 2003). Since the gene sequences of P. neptuni and Strobilidium sp. are almost identical, we assume that the unidentified Strobilidium sp. of Snoeyenbos-West et al. (2002) represents a population of P. neptuni. Probably, the sequence divergence of d = 0.0018 between the two clones is merely a result of partial sequencing. The considerable sequence divergence between the Pelagostrobilidium species (P. neptuni and Strobilidium sp.) and Strobilidium caudatum (genetic distance d = 0.074) supports the maintenance of the former genus (see above), as this distance is comparable to other intergeneric distances within the order Choreotrichida.
In all analyses, S. biarmatum is a sister species to an undetermined Strombidium species (clone Stromb00ssu_4; 100% MP, ML, NJ) and groups closely with S. inclinatum. Due to the large genetic diversity within the Strombidiidae and the unequal sampling, the topology remains unresolved in our trees, as in previous studies (Agatha et al. 2004; Modeo et al. 2003; Snoeyenbos-West et al. 2002; Strüder-Kypke and Lynn 2003). Not only the relationships between Strombidium species, but also among the strombidiid genera Laboea, Strombidium, and Novistrombidium are unstable due to the very low support values for all branches and the different topology in different analyses. Furthermore, Laboea and Novistrombidium group within the genus Strombidium. This and the great genetic distances support a splitting of the genus Strombidium into several morphologically and ontogenetically defined genera, as suggested by Agatha (2004a). Future molecular genetic analyses of further species, especially, of the recently established genera, will provide a test of the robustness of 2these generic conceptions.
Acknowledgements
The taxonomic investigations by S. Agatha in the Gulf of Trieste and the Irish Sea were supported by the Austrian Science Foundation (FWF; Project T 116), those on the Island of Sylt by a grant of the Biologische Anstalt Helgoland. The phylogenetic work of M.C. Strüder-Kypke was supported by a grant from the Natural Science and Engineering Research Council (NSERC) awarded to D.H. Lynn. Many thanks are given to W. Foissner (Organismal Biology, University of Salzburg, Austria) and David Montagnes (School of Biological Sciences, University of Liverpool, UK) for their constructive criticism. Thanks go also to David Montagnes, Serena Fond Umani (Laboratorio di Biologia Marina, S. Croce/Trieste, Italy), Malte Elbrächter (Biologische Anstalt Helgoland, Island of Sylt, Germany), and Jeanette Cornelie Riedel-Lorjé (Institut für Frischwasser- und Abwasserbiologie, Hamburg, Germany), for placing their laboratories at the senior author's disposal.
References
- Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa, Ciliophora): a comparative light microscopical and SEM study. Eur. J. Protistol. 2003a;39:245–266. [Google Scholar]
- Agatha S. Redescription of Strombidinopsis minima (Gruber, 1884) Lynn et al., 1991 (Protozoa, Ciliophora), with notes on its ontogenesis and distribution. Eur. J. Protistol. 2003b;39:233–244. [Google Scholar]
- Agatha S. Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications. Zoology. 2004a;107:153–168. doi: 10.1016/j.zool.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatha S. A cladistic approach for the classification of oligotrichid ciliates (Ciliophora: Spirotricha) Acta Protozool. 2004b;43:201–217. [PMC free article] [PubMed] [Google Scholar]
- Agatha S. New observations on the tontoniid ciliate Spirotontonia grandis (Suzuki and Han, 2000) Agatha, 2004 (Ciliophora, Oligotrichida, Tontoniidae); comparison with the similar Laboea strobila. Eur. J. Protistol. 2004c;40:295–301. [Google Scholar]
- Agatha S, Riedel-Lorjé JC. Morphology, infraciliature, and ecology of halteriids and strombidiids (Ciliophora, Oligotrichea) from coastal brackish water basins. Arch. Protistenkd. 1997;148:445–459. [Google Scholar]
- Agatha S, Riedel-Lorjé JC. Morphology, infraciliature, and ecology of some strobilidiine ciliates (Ciliophora, Oligotrichea) from coastal brackish water basins of Germany. Eur. J. Protistol. 1998;34:10–17. [Google Scholar]
- Agatha S, Strüder-Kypke MC, Beran A. Morphologic and genetic variability in the marine planktonic ciliate Laboea strobila Lohmann, 1908 (Ciliophora, Oligotrichia), with notes on its ontogenesis. J. Euk. Microbiol. 2004;51:267–281. doi: 10.1111/j.1550-7408.2004.tb00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard D, Stechmann A, Foissner W, Ammermann D, Hehn M, Schlegel M. Phylogenetic relationships within the class Spirotrichea (Ciliophora) inferred from small subunit rRNA gene sequences. Mol. Phylogenet. Evol. 2001;21:86–92. doi: 10.1006/mpev.2001.0997. [DOI] [PubMed] [Google Scholar]
- Chen Z, Song W. Phylogenetic positions of Uronychia transfuga and Diophrys appendiculata (Euplotida, Hypotrichia, Ciliophora) within hypotrichous ciliates inferred from the small subunit ribosomal RNA gene sequences. Eur. J. Protistol. 2001;37:291–301. [Google Scholar]
- Chen Z, Song W. Phylogenetic positions of Aspidisca steini and Euplotes vannus within the order Euplotida (Hypotrichia: Ciliophora) inferred from complete small subunit ribosomal RNA gene sequences. Acta Protozool. 2002;41:1–9. [Google Scholar]
- Dale T, Lynn DH. Stomatogenesis of the ciliate genus Strombidinopsis with an improved description of S. spiniferum and S. acuminatum. J. Euk. Microbiol. 1998;45:210–217. [Google Scholar]
- De Rijk P, De Wachter R. DCSE, an interactive tool for sequence alignment and secondary structure research. CABIOS. 1993;9:735–740. doi: 10.1093/bioinformatics/9.6.735. [DOI] [PubMed] [Google Scholar]
- Deroux G. Quelques précisions sur Strobilidium gyrans Schewiakoff. Cah. Biol. Mar. 1974;15:571–588. [Google Scholar]
- Dragesco J. Ciliés mésopsammiques littoraux. Systématique, morphologie, écologie. Trav. Stn. Biol. Roscoff (N.S.) 1960;12:1–356. [Google Scholar]
- Elwood HJ, Olsen GJ, Sogin ML. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol. Biol. Evol. 1985;2:399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Fauré-Fremiet E. Contribution a la connaissance des infusoires planktoniques. Bull. Biol. Fr. Belg. 1924;(Suppl. 6):1–171. [Google Scholar]
- Fauré-Fremiet E. Le rythme de marée du Strombidium oculatum Gruber. Bull. Biol. Fr. Belg. 1948a;82:3–23. [PubMed] [Google Scholar]
- Fauré-Fremiet E. The ecology of some infusorian communities of intertidal pools. J. Anim. Ecol. 1948b;17:127–130. [Google Scholar]
- Fauré-Fremiet E. Écologie des ciliés psammophiles littoraux. Bull. Biol. Fr. Belg. 1950;84:35–75. [PubMed] [Google Scholar]
- Fauré-Fremiet E. La bipartition énantiotrope chez les ciliés oligotriches. Arch. Anat. Microsc. Morph. Exp. 1953;42:209–225. [Google Scholar]
- Fauré-Fremiet E, Ganier M.-Cl. Structure fine du Strombidium sulcatum Cl. et L. (Ciliata Oligotrichida) Protistologica. 1970;6:207–223. [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.6a2, distributed by the author. Department of Genetics, University of Washington; Seattle: 1993. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W, Skogstad A, Pratt JR. Morphology and infraciliature of Trochiliopsis australis n. sp., Pelagohalteria viridis (Fromentel, 1876) n. g., n. comb., and Strobilidium lacustris n. sp. (Protozoa, Ciliophora) J. Protozool. 1988;35:489–497. [Google Scholar]
- Foissner W, Blatterer H, Berger H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems. Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea. Informationsberichte des Bayer; Landesamtes für Wasserwirtschaft: 1991. 1/91. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer; Landesamtes für Wasserwirtschaft: 1999. 3/99. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Part I: Text and line drawings. Part II: Photographs. Denisia. 2002;5:1–1459. [Google Scholar]
- Fonda Umani S, Beran A. Seasonal variations in the dynamics of microbial plankton communities: first estimates from experiments in the Gulf of Trieste, Northern Adriatic Sea. Mar. Ecol. Prog. Ser. 2003;247:1–16. [Google Scholar]
- Grim JN. The kinetid structures of the choreotrichous ciliate Strobilidium velox and an assessment of its evolutionary lineage. J. Protozool. 1987;34:117–123. [Google Scholar]
- Hewitt EA, Müller KM, Cannone J, Hogan DJ, Gutell R, Prescott DM. Phylogenetic relationships among 28 spirotrichous ciliates documented by rDNA. Mol. Phylogenet. Evol. 2003;29:258–267. doi: 10.1016/s1055-7903(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist FR. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jerome CA, Lynn DH. Identifying and distinguishing sibling species in the Tetrahymena pyriformis complex (Ciliophora, Oligohymenophorea) using PCR/RFLP analysis of nuclear ribosomal DNA. J. Euk. Microbiol. 1996;43:492–497. doi: 10.1111/j.1550-7408.1996.tb04509.x. [DOI] [PubMed] [Google Scholar]
- Jonsson PR. Tidal rhythm of cyst formation in the rock pool ciliate Strombidium oculatum Gruber (Ciliophora, Oligotrichida): a description of the functional biology and an analysis of the tidal synchronization of encystment. J. Exp. Mar. Biol. Ecol. 1994;175:77–103. [Google Scholar]
- Kim Y-O, Taniguchi A. Excystment of the oligotrich ciliate Strombidium conicum. Aquat. Microb. Ecol. 1995;9:149–156. [Google Scholar]
- Kormos J, Kormos K. Die Zellteilungstypen der Protozoen. Acta Biol. Hung. 1958;8:127–148. [Google Scholar]
- Krainer K-H. Taxonomische Untersuchungen an neuen und wenig bekannten planktischen Ciliaten (Protozoa: Ciliophora) aus Baggerseen in Österreich. Lauterbornia. 1995;21:39–68. [Google Scholar]
- Laval-Peuto M, Grain J, Deroux G. Classe des Oligotrichea Bütschli, 1887. Ordres des Oligotrichida Bütschli, 1887 et des Choreotrichida Small & Lynn, 1985. In: Puytorac P. de., editor. Traité de Zoologie, Anatomie, Systématique, Biologie T. II Fasc. 2. Masson; Paris, Milan, Barcelona: 1994. pp. 153–179. [Google Scholar]
- Lynn DH, Montagnes DJS. Taxonomic descriptions of some conspicuous species of strobilidiine ciliates (Ciliophora: Choreotrichida) from the Isles of Shoals, Gulf of Maine. J. Mar. Biol. Assoc. UK. 1988;68:639–658. [Google Scholar]
- Lynn DH, Small EB. Phylum Ciliophora Doflein, 1901. In: Lee JJ, Leedale GF, Bradbury P, editors. An Illustrated Guide to the Protozoa. Organisms Traditionally Referred to as Protozoa, or Newly Discovered Groups. second ed. Society of Protozoologists, Allen Press, Lawrence; 2002. pp. 371–656. [Google Scholar]
- Lynn DH, Montagnes DJS, Small EB. Taxonomic descriptions of some conspicuous species in the family Strombidiidae (Ciliophora: Oligotrichida) from the Isles of Shoals, Gulf of Maine. J. Mar. Biol. Assoc. UK. 1988;68:259–276. [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Meunier A. Microplankton des Mers de Barents et de Kara. In: Bulens C, editor. Campagne Arctique de 1907. Bruxelles: 1910. [Google Scholar]
- Modeo L, Petroni G, Rosati G, Montagnes DJS. A multidisciplinary approach to describe protists: redescriptions of Novistrombidium testaceum Anigstein 1914 and Strombidium inclinatum Montagnes, Taylor, and Lynn 1990 (Ciliophora, Oligotrichia) J. Euk. Microbiol. 2003;50:175–189. doi: 10.1111/j.1550-7408.2003.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Montagnes DJS. Growth responses of planktonic ciliates in the genera Strobilidium and Strombidium. Mar. Ecol. Prog. Ser. 1996;130:241–254. [Google Scholar]
- Montagnes DJS, Taylor FJR. The salient features of five marine ciliates in the class Spirotrichea (Oligotrichia), with notes on their culturing and behaviour. J. Euk. Microbiol. 1994;41:569–586. [Google Scholar]
- Montagnes DJS, Lynn DH, Stoecker DK, Small EB. Taxonomic descriptions of one new species and redescription of four species in the family Strombidiidae (Ciliophora, Oligotrichida) J. Protozool. 1988;35:189–197. [Google Scholar]
- Montagnes DJS, Lowe CD, Poulton A, Jonsson PR. Redescription of Strombidium oculatum Gruber 1884 (Ciliophora, Oligotrichia) J. Euk. Microbiol. 2002;49:329–337. doi: 10.1111/j.1550-7408.2002.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Müller H. Encystment of the freshwater ciliate Pelagostrombidium fallax (Ciliophora, Oligotrichida) in laboratory culture. Aquat. Microb. Ecol. 1996;11:289–295. [Google Scholar]
- Müller H. Laboratory study of the life cycle of a freshwater strombidiid ciliate. Aquat. Microb. Ecol. 2002;29:189–197. [Google Scholar]
- Müller H, Wünsch C. Seasonal dynamics of cyst formation of pelagic strombidiid ciliates in a deep prealpine lake. Aquat. Microb. Ecol. 1999;17:37–47. [Google Scholar]
- Ota T, Taniguchi A. Conjugation in the marine aloricate oligotrich Pelagostrobilidium (Ciliophora: Oligotrichia) Eur. J. Protistol. 2003;39:149–160. [Google Scholar]
- Petroni G, Spring S, Schleifer K-H, Verni F, Rosati G. Defensive extrusive ectosymbionts of Euplotidium (Ciliophora) that contain microtubule-like structures are bacteria related to Verrucomicrobia. Proc. Natl. Acad. Sci. USA. 2000;97:1813–1817. doi: 10.1073/pnas.030438197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petz W, Foissner W. Morphology and morphogenesis of Strobilidium caudatum (Fromentel), Meseres corlissi n. sp., Halteria grandinella (Müller), and Strombidium rehwaldi n. sp., and a proposed phylogenetic system for oligotrich ciliates (Protozoa, Ciliophora) J. Protozool. 1992;39:159–176. [Google Scholar]
- Petz W, Song W, Wilbert N. Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the endopagial and pelagial of the Weddell Sea, Antarctica. Stapfia. 1995;40:1–223. [Google Scholar]
- Pierce RW, Turner JT. Ecology of planktonic ciliates in marine food webs. Rev. Aquat. Sci. 1992;6:139–181. [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Reid PC. Mass encystment of a planktonic oligotrich ciliate. Mar. Biol. 1987;95:221–230. [Google Scholar]
- Reid PC, John AWG. Tintinnid cysts. J. Mar. Biol. Assoc. UK. 1978;58:551–557. +Plate 1. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schlegel M, Elwood HJ, Sogin ML. Molecular evolution in hypotrichous ciliates: sequence of the small subunit ribosomal RNA genes from Onychodromus quadricornutus and Oxytricha granulifera (Oxytrichidae, Hypotrichida, Ciliophora) J. Mol. Evol. 1991;32:64–69. doi: 10.1007/BF02099930. [DOI] [PubMed] [Google Scholar]
- Shin MK, Hwang UW, Kim W, Wright A-DG, Krawczyk C, Lynn DH. Phylogenetic position of the ciliates Phacodinium (order Phacodiniida) and Protocruzia (subclass Protocruziidia) and systematics of the spirotrich ciliates examined by small subunit ribosomal RNA gene sequences. Eur. J. Protistol. 2000;36:293–302. [Google Scholar]
- Snoeyenbos-West OLO, Salcedo T, McManus GB, Katz LA. Insights into the diversity of choreotrich and oligotrich ciliates (Class: Spirotrichea) based on genealogical analyses of multiple loci. Int. J. Syst. Evol. Microbiol. 2002;52:1901–1913. doi: 10.1099/00207713-52-5-1901. [DOI] [PubMed] [Google Scholar]
- Song W, Bradbury PC. Studies on some new and rare reported marine planktonic ciliates (Ciliophora: Oligotrichia) from coastal waters in North China. J. Mar. Biol. Assoc. UK. 1998;78:767–794. [Google Scholar]
- Song W, Wilbert N. Benthische Ciliaten des Süßwassers. In: Röttger R, editor. Praktikum der Protozoologie. G. Fischer; Stuttgart: 1995. pp. 156–168. [Google Scholar]
- Strüder-Kypke MC, Lynn DH. Sequence analyses of the small subunit rRNA gene confirm the paraphyly of oligotrich ciliates sensu lato and support the monophyly of the subclasses Oligotrichia and Choreotrichia (Ciliophora, Spirotrichea) J. Zool. 2003;260:87–97. [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates; Sunderland: 2002. [Google Scholar]
- Valbonesi A, Luporini P. Description of two new species of Euplotes and Euplotes rariseta from Antarctica. Polar Biol. 1990;11:47–53. [Google Scholar]
- Walsh PS, Metzger DA, Higuchi R. Chelex® 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10:506–513. [PubMed] [Google Scholar]