Abstract
The cancer-preventive effects of green tea and its main constituent (-)-epigallocatechin gallate [(-)-EGCG] are widely supported by results from epidemiological, cell culture, animal and clinical studies although the molecular target has not been well defined. We previously reported that ester bond-containing tea polyphenols, e. g. (-)-EGCG, and their synthetic analogs potently and specifically inhibited the proteasomal activity. Subsequently, we further demonstrated that methylation on green tea polyphenols under physiological conditions decreased their proteasome-inhibitory activity, contributing to decreased cancer-preventive effects of tea consumption. Since (-)-EGCG is unstable under physiological conditions, we also developed the peracetate-protected or prodrug form of (-)-EGCG, Pro-EGCG (1), and shown that Pro-EGCG (1) increases the bioavailability, stability, and proteasome-inhibitory and anticancer activities of (-)-EGCG in human breast cancer cells and xenografts, suggesting its potential use for cancer prevention and treatment.
Keywords: Tea polyphenols, Proteasome inhibitors, Molecular target, Methylation, Prodrug, Cancer prevention, Chemotherapy
Introduction
Annually, more than 5 million people are diagnosed with cancer and more than 3.5 million people die from cancer worldwide (Ferlay et al., 2001). When analysis of cancer incidence by racial group is performed for many types of cancers, Asian and islander populations have significantly reduced incidence and mortality due to cancer that seems to correlate with dietary intake of green tea (Fujiki, 1999; Gupta et al., 1999; Kazi et al., 2002). The attraction of green tea as a cancer chemopreventative and a chemotherapeutic agent is suggested. Tea consumption is not associated with toxic effects. Populations that practice extensive tea consumption have demonstrated reduced incidence and mortality due to cancer. The principle components of tea exhibit a wide array of cancer preventing activities (Fujiki, 1999; Gupta et al., 1999; Kazi et al., 2002).
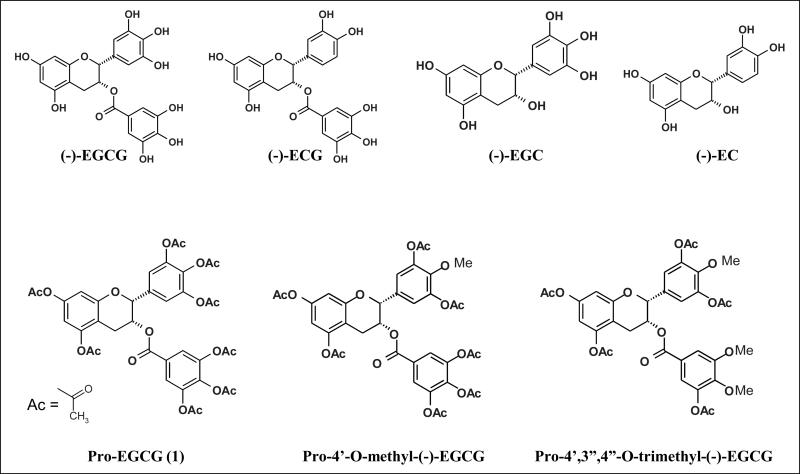
The major polyphenols of green tea include (-)-epigallocatechin-3-gallate [(-)-EGCG], (-)-epigallocatechin [(-)-EGC], (-)-epicatechin-3-gallate [(-)-ECG], and (-)-epicatechin [(-)-EC] (see Fig. 1). Of these (-)-EGCG is the most abundant and has been extensively studied and implicated as a cancer preventative agent (Fujiki, 1999; Gupta et al., 1999; Kazi et al., 2002). In addition, (-)-EGCG, in particular, is known to inhibit telomerase, urokinase, nitric-oxide synthase, tumour necrosis factor alpha, the proteasome (Jankun et al., 1997; Lin and Lin, 1997; Naasani et al., 1998; Okabe et al., 1999; Nam et al., 2001), and other cancer-related targets.
Fig. 1.
Chemical structures of green tea polyphenols and their prodrugs.
The ubiquitin-proteasome pathway
In recent years, proteasome inhibition has become increasingly important in cancer and drug resistance research. The vast majority of regulated proteolysis in eukaryotic cells occurs through the actions of the ubiquitin-proteasome pathway (Ciechanover et al., 2000). Although it would seem disastrous to alter the activity of this crucial protein degradation system, proteasome inhibition has been well established as a rational strategy for multiple myeloma (Richardson et al., 2005; Catley et al., 2005), non-Hodgkin lymphoma (Goy et al., 2005) and some other solid tumours (Dou and Goldfarb, 2002). Understanding the mechanisms of action has led to integration into combination regimens using both proteasome inhibitors and standard chemotherapeutics.
The ubiquitin-proteasome pathway involves two successive steps: conjugation of multiple ubiquitin molecules to the protein substrate, and degradation of the tagged protein by the 26S proteasome (Fig. 2, left). Ubiquitin is a highly conserved 76-amino acid protein that becomes covalently ligated to a target protein by a multi-enzymatic system consisting of Ub-activating (E1), Ub-conjugating (E2), and the Ub-ligating (E3) enzymes, which act in a sequential manner. This is a three-stage process that starts with activation of ubiquitin by the E1 enzyme in an ATP-requiring reaction that generates a high-energy thiol ester intermediate, E1-S~ubiquitin. Activated ubiquitin is then transferred from E1, by one of several ubiquitin-conjugating enzymes, E2, via an additional high-energy thiol-ester intermediate, E2-S~ubiquitin. From E2 to the E3-bound substrate, the activated ubiquitin can be then transferred directly or via a third high-energy thiol ester intermediate, E3-S~ubiquitin (Ciechanover et al., 2000).
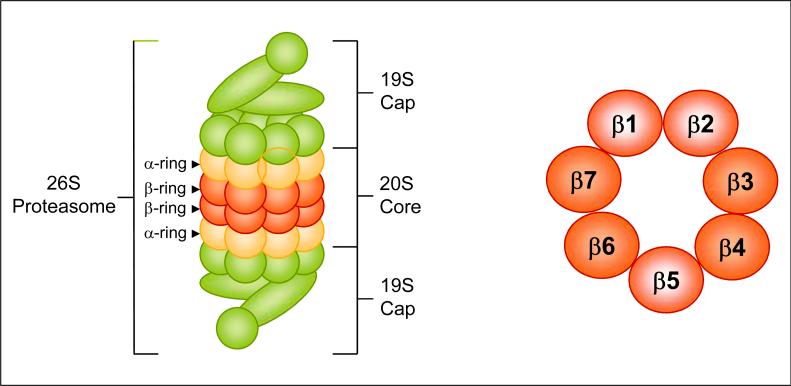
Fig. 2.
(Left) Structure of 26S proteasome. The 26S protea-some is a large multi-subunit protease complex containing the proteolytic core, the 20S proteasome, and two 19S caps. The 20S proteasome is composed of 28 subunits arranged in four heptameric, tightly stacked rings (α7, β7, β7, α7) to form a cylindrical structure. (Right) The proteasomal β-ring. Two β-rings comprise the catalytic core of the proteasome (cross section of one is shown). Each ring contains at least three protease activities, chymotrypsin-like (associated with β5), trypsin-like (associated with β2), and PGPH-like (associated with β1).
Ubiquitinated proteins are recognized by the 26S protea-some, a large multi-subunit protease complex that is localized in the nucleus and cytosol and selectively degrades intracellular proteins. In almost all of the cases, only proteins containing polyubiquitin chains on sequential lysine residues are recognized and degraded by the proteasome and the ubiquitin is released and recycled. The proteolytic core of this complex, the 20S proteasome, contains multiple peptidase activities and functions as the catalytic machine. This core is composed of 28 subunits arranged in four heptameric, tightly stacked rings (α7, β7, β7, α7) to form a cylindrical structure (Groll et al., 1999). The α-subunits make up the two outer, and the β-subunits the two inner, rings of the stack (Fig. 2, right). The entrance of substrate proteins to the active site of the complex is guarded by the α-subunits that allow access only to unfolded and extended polypeptides. The proteolytic activities are confined to the β-subunits conferring the unique and distinguishing proteasome feature of multiple peptidase activities that include chymotrypsin-like (cleavage after hydrophobic side chains, mediated by the β5 subunit), peptidylglutamyl peptide hydrolyzing-like or PGPH-like (cleavage after acidic side chains, mediated by the β1 subunit), and trypsin-like (cleavage after basic side chains, mediated by the β2 subunit) activities (Groll et al., 1999) (Fig. 2).
The ubiquitin-proteasome pathway is vital in the degradation of proteins involved in cell cycle progression, proliferation, apoptosis and a vast majority of abnormal proteins that result from oxidative damage and mutations. The proteasome could therefore contribute to the pathological state of several human diseases including cancer, in which some regulatory proteins are either stabilized due to decreased degradation or lost due to accelerated degradation (Ciechanover, 1998). Many important target proteins of the proteasome have been identified, including cyclins A, B, D and E, tumour suppressor protein p53, pro-apoptotic protein Bax (Li and Dou, 2000), cyclin-dependent kinase inhibitor p27 (Pagano et al., 1995; Sun et al., 2001), and the NFκB inhibitor, IκB-α (Perkins, 2000). Since inhibition of the ubiquitin-proteasome pathway in tumour cells results in accumulation of tumour suppressor and pro-apoptotic proteins, the possibility of targeting this pathway in cancer therapy is a viable option.
Green tea and (-)-EGCG inhibit the proteasome activity in tumour cells
It has been suggested that proteasomal activity is essential for tumour cell proliferation and development of drug resistance (Hideshima et al., 2001). Therefore, the proteasome-mediated degradation pathway has been considered to be an important target for cancer therapy and prevention. We and others have reported that inhibition of the proteasomal chymotrypsin-like activity is associated with induction of apoptosis in tumour cells (An et al., 1998, Lopes et al., 1997). The proteasome inhibitor Bortezomib (Velcade, PS-341) has been used in clinical trials and its antitumour activity has been reported in a variety of tumour models (Adams, 2002, Dou et al., 2002, Kane et al., 2006).
We have also shown that ester bond-containing tea polyphenols, e. g. (-)-EGCG, potently and specifically inhibit the proteasomal chymotrypsin-like (β5) and PGPH-like (β1), but not trypsin-like (β2), activities of the proteasome (Nam et al., 2001). Using an in silico docking method, we have also shown that inhibition of the chymotrypsin activity of the 20S proteasome may be due to acylation of the β5-subunit's catalytic N-terminal threonine (Thr 1) (Smith et al., 2004). Furthermore, EGCG appears to bind the chymotrypsin site in an orientation and conformation that is suitable for a nucleophilic attack by Thr 1. Our in silico model has been corroborated by comparing the predicted and actual activities of several EGCG analogs. In the biological setting, EGCG exhibits strong inhibitory activity against a purified 20S proteasome (IC50 = 86–194 nM), and 26S proteasome in intact tumour cells (1–10 μM). These inhibitory concentrations are similar to those found in the serum of green tea drinkers. EGCG is also able to induce proteasome inhibition in whole cells, resulting in accumulation of the natural proteasome substrates p27 and IκB-α and induction of arrest of tumour cells in theG1 phase. In sharp contrast, EGCG has little to no effect on normal, non-transformed cells (Nam et al., 2001; Kuhn et al., 2005; Landis-Piwowar et al., 2005). These studies strongly suggest that the cancer-preventative properties of green tea could be attributed, at least in part, to its ability to inhibit proteasomal activity and the low toxicity of EGCG, pointing to its potential use as an adjuvant to current anticancer drugs.
We also found that synthetic (-)-EGCG amides and (-)-EGCG analogs with modifications in the A-ring, C-ring or ester bond inhibited the chymotrypsin-like activity of purified 20S proteasome with altered potencies, induced growth arrest in the G1 phase of the cell cycle in leukemia Jurkat T cells, and suppressed colony formation of human prostate cancer LNCaP cells (Kazi et al., 2004).
While (-)-EGCG remains to be the most potent polyphenol in green tea, it is unstable under physiologic conditions. In an effort to discover more stable polyphenol proteasome inhibitors, we synthesized several novel (-)-EGCG analogs with -OH groups eliminated from the B- and/or D-rings. In addition, we also synthesized their putative prodrugs with -OH groups protected by acetate that can be removed by cellular cytosolic esterases. We first examined the structure-activity relationship of these unprotected and protected com-pounds with respect to their proteasome inhibitory potentials. We found that decreasing the number of -OH groups from either the B- or D-ring leads to diminished proteasome inhibitory activity in vitro. However, in cultured tumour cells, the protected analogs were capable of potently inhibiting the proteasomal chymotrypsin-like activity by as much as 97 % (Landis-Piwowar et al., 2005). Furthermore, we found that, compared to (-)-EGCG, protected analogs exhibited greater potency to inhibit proliferation and induce apoptosis in human leukemic, prostate, breast, and simian virus 40-transformed cells (Kuhn et al., 2005). The protected analogs were non-toxic to human normal and non-transformed cells (Kuhn et al., 2005).
We have also provided evidence that when cultured human breast cancer MDA-MB-231 cells were treated with the prodrug of (-)-EGCG, Pro-EGCG (1) (see Fig. 1) (-)-EGCG not only had been converted but also accumulated, accompanied by enhanced levels of proteasome inhibition, growth suppression and apoptosis induction, compared to cells treated with natural (-)-EGCG. To investigate the potential use of Pro-EGCG (1) as a novel pro-drug that converts to a cellular proteasome inhibitor and anticancer agent in vivo, MDAMB-231 tumours were induced in nude mice, followed by treatment with Pro-EGCG (1) or (-)-EGCG for 31 days. Results of this in vivo study demonstrated a significant inhibition of breast tumour growth by Pro-EGCG (1), compared to (-)-EGCG, associated with increased proteasome inhibition and apoptosis induction in tumour tissues (Landis-Piwowar et al., 2007a). In summary, we have shown that Pro-EGCG (1) increases the bioavailability, stability, and proteasome-inhibitory and anticancer activities of (-)-EGCG in human breast cancer cells and tumours, suggesting its potential use for cancer prevention and treatment.
Under physiological conditions, biotransformation reactions, such as methylation, can modify green tea polyphenols and therefore limit their in vivo cancer-preventive activity. Although a recent case-control study suggested that methylated polyphenols are less cancer-protective, the molecular basis for this observation is unknown. We hypothesize that methylated green tea polyphenols have decreased protea-some-inhibitory abilities. To test this hypothesis, methylated (-)-EGCG and (-)-ECG analogs that can be found in vivo were synthesized and studied for their structure-activity relationships (SARs) using a purified 20S proteasome. The addition of a single methyl group on (-)-EGCG or (-)-ECG led to decreased proteasome inhibition and, as the number of methyl groups increased, the inhibitory potencies further decreased. These SARs were supported by our findings from in silico docking analysis published recently. Previously, we synthesized a peracetate-protected (-)-EGCG molecule, Pro-EGCG (1) (see Fig. 1), to enhance its cellular permeability and stability, and current HPLC analysis confirms conversion of Pro-EGCG (1) to (-)-EGCG in cultured human leukemic Jurkat T cells. Furthermore, in this study, peracetate-protected forms of methylated green tea polyphenols were added in intact Jurkat T cells to observe the intracellular effects of methylation. Peracetate-protected, monomethylated (-)-EGCG (see Fig. 1) induced greater cellular protea-some inhibition and apoptosis than did peracetate-protected, trimethylated (-)-EGCG (see Fig. 1), consistent with the potencies of the parent methylated analogs against a purified 20S proteasome (Landis-Piwowar et al., 2007b). Therefore, methylation on green tea polyphenols, under physiological conditions, could decrease their proteasome-inhibitory activity, contributing to decreased cancer-preventive effects of tea consumption.
Conclusions
Although tea has been consumed for centuries, it has only recently been studied extensively as a health-promoting beverage that may act to prevent a number of chronic diseases and cancers. The cancer-preventive effects of green tea are widely supported by results from epidemiological, cell culture, animal and clinical studies. Studies showed that tea polyphenols potently induce apoptotic cell death and cell cycle arrest in tumour cells but not in their normal cell counterparts and that green tea polyphenols affect several biological pathways. Various animal studies have revealed that treatment with green tea inhibits tumour incidence and multiplicity in different organ sites such as skin, lung, liver, stomach, mammary gland and colon, and recently, phase I and II clinical trials have been conducted to explore the anticancer effects of green tea in humans. Studies focusing on the purified tea polyphenol compound (-)-EGCG should continue to provide researchers an improved understanding of tea polyphenol absorption, distribution, role in anti-cancer reactions, metabolism and anti-cancer mechanisms. Work should continue on synthesizing and evaluating more analogs of green tea polyphenols to find more potent, stable and specific polyphenol proteasome inhibitors as novel anti-cancer agents. A major challenge of cancer prevention is to integrate new molecular findings into clinical practice. Identification of more molecular targets or biomarkers for tea polyphenols is paramount to cancer prevention and treatment by green tea and will greatly assist in a better understanding of its anti-cancer mechanisms.
Acknowledgements
This work is supported in part by research grants from the National Cancer Institute-National Institutes of Health (to Q. P. D.; 1R01CA120009; 5R03CA112625) and the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Administrative Region, China (Project No. AoE/P-10/01, to T. H. C.) and NSERC of Canada (to T.H.C).
References
- Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–75. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- Catley L, Tai YT, Chauhan D, Anderson KC. Perspectives for combination therapy to overcome drug-resistant multiple myeloma. Drug Resist. Updates. 2005;8:205–18. doi: 10.1016/j.drup.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–60. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–51. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals). IDrugs. 2002;5:828–34. [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM, GLOBOCAN . IARC CancerBase. Version 1.0. IARCPress; Lyon, France: 2000. Cancer Incidence, Mortality and Prevalence Worldwide. (2001) [Google Scholar]
- Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125:589–97. doi: 10.1007/s004320050321. [DOI] [PubMed] [Google Scholar]
- Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hage-meister F, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 2005;23:667–75. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc. Natl. Acad. Sci. (USA) 1999;96:10976–83. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Ahmad N, Mohan RR, Husain MM, Mukhtar H. Prostate cancer chemoprevention by green tea: in vitro and in vivo inhibition of testosterone-mediated induction of ornithine decarboxylase. Cancer Res. 1999;59:2115–20. [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6. [PubMed] [Google Scholar]
- Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Sridhara R, Pazdur R. United States food and drug administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–60. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- Kazi A, Smith DM, Daniel K, Zhong S, Gupta P, Bosley ME, Dou QP. Potential molecular targets of tea polyphenols in human tumor cells: significance in cancer prevention. In Vivo. 2002;16:397–403. [PubMed] [Google Scholar]
- Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure-activity relationships of synthetic analogs of (-)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24:943–54. [PubMed] [Google Scholar]
- Kuhn DJ, Lam WH, Kazi A, Daniel KG, Song S, Chow LM, Chan TH, Dou QP. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front Biosci. 2005;10:1010–23. doi: 10.2741/1595. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Kuhn DJ, Wan SB, Chen D, Chan TH, Dou QP. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (-)-EGCG analogs and their prodrugs. Int J Mol Med. 2005;15:735–42. [PubMed] [Google Scholar]
- Landis-Piwowar KR, Huo CD, Chen D, Cui QC, Minic V, Shi GQ, et al. A Novel Pro-drug of the Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate as a Potential Anti-Cancer Agent. Cancer Res. 2007a;67:4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn D,J, Chan TH, Dou QP. Methylation Suppresses the Protea-some-Inhibitory Function of Green Tea Polyphenols. J Cell. Physiol. 2007b;213:252–60. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc. Natl. Acad. Sci. (USA) 2000;97:3850–5. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Lin JK. (-)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol. 1997;52:465–72. [PubMed] [Google Scholar]
- Lopes UG, Erhardt P, Yao R, Cooper GM. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–6. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- Naasani I, Seimiya H, Tsuruo T. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem Biophys Res Commun. 1998;249:391–6. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Okabe S, Ochiai Y, Aida M, Park K, Kim SJ, Nomura T, et al. Mechanistic aspects of green tea as a cancer preventive: effect of components on human stomach cancer cell lines. Jpn J Cancer Res. 1999;90:733–9. doi: 10.1111/j.1349-7006.1999.tb00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Perkins ND. The Rel/NF-kappa B family: friend and foe. Trends Biochem. Sci. 2000;25:434–40. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Smith DM, Daniel KG, Wang Z, Guida WC, Chan T-H, Dou QP. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins: Structure, Function, and Bioinformatics. 2004;54:58–70. doi: 10.1002/prot.10504. [DOI] [PubMed] [Google Scholar]
- Sun J, Nam S, Lee CS, Li B, Coppola D, Hamilton AD, et al. CEP1612, a dipeptidyl proteasome inhibitor, induces p21WAF1 and p27KIP1 expression and apoptosis and inhibits the growth of the human lung adenocarcinoma A-549 in nude mice. Cancer Res. 2001;61:1280–4. [PubMed] [Google Scholar]