Abstract
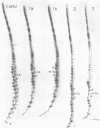
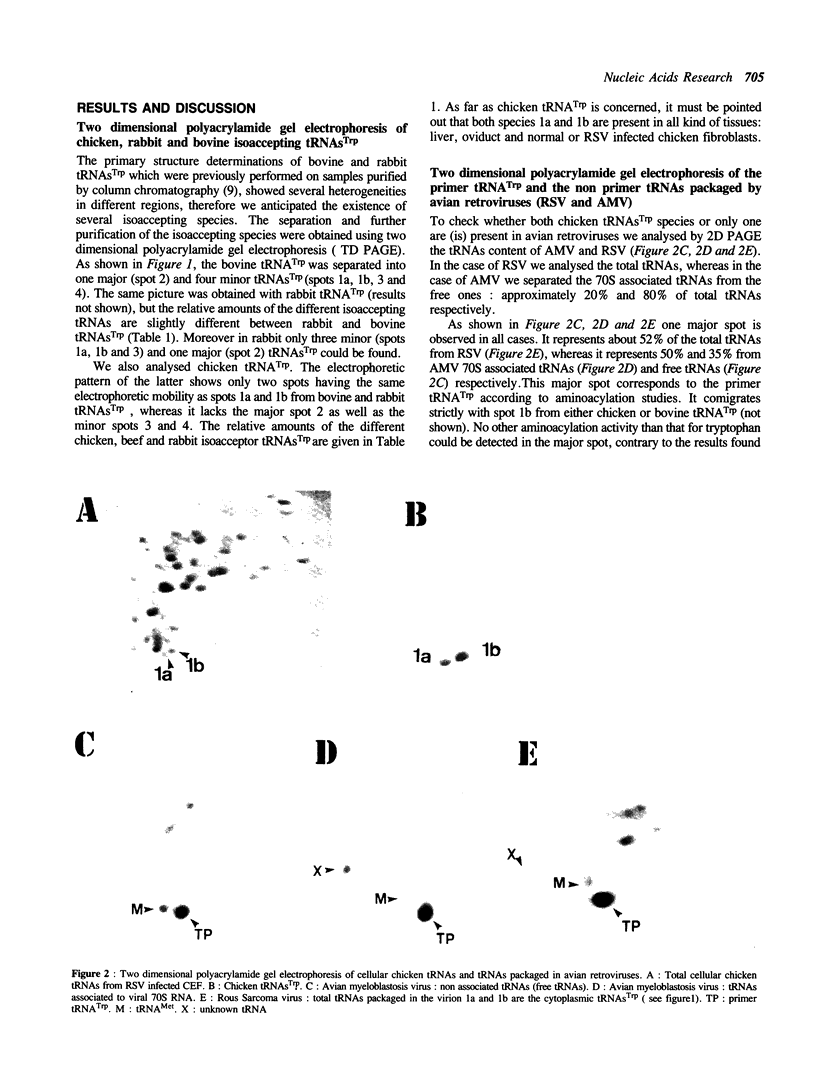
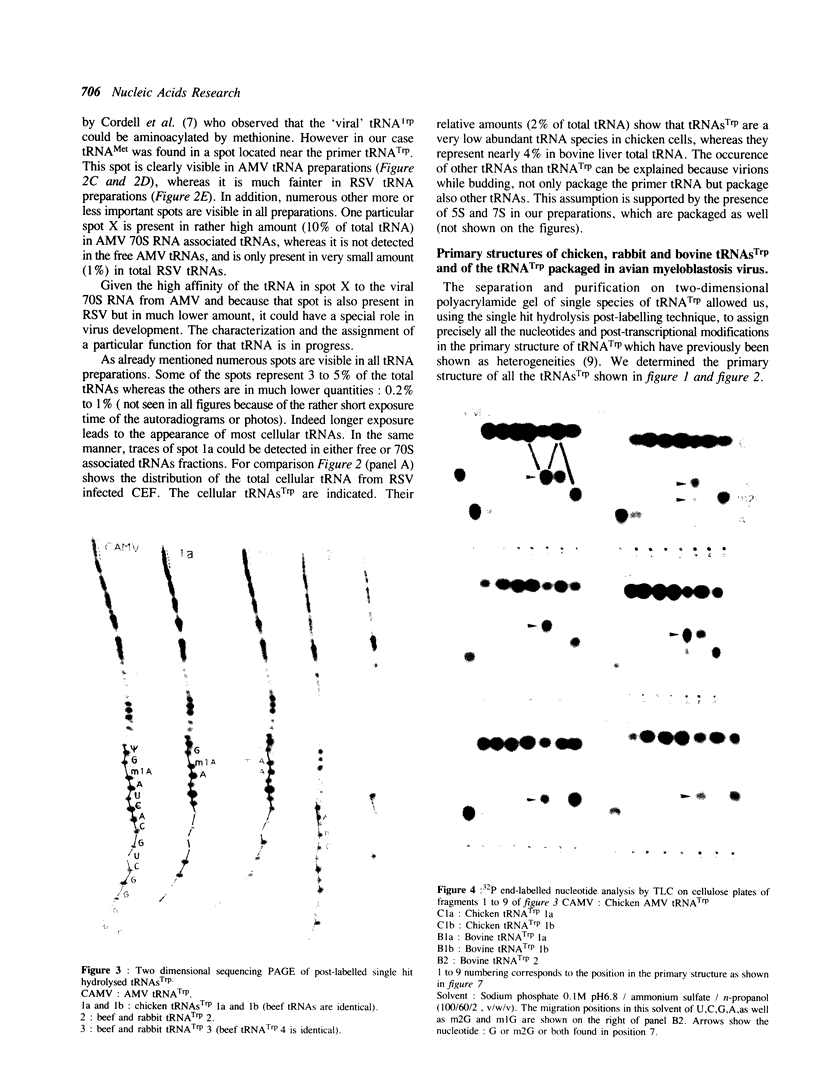
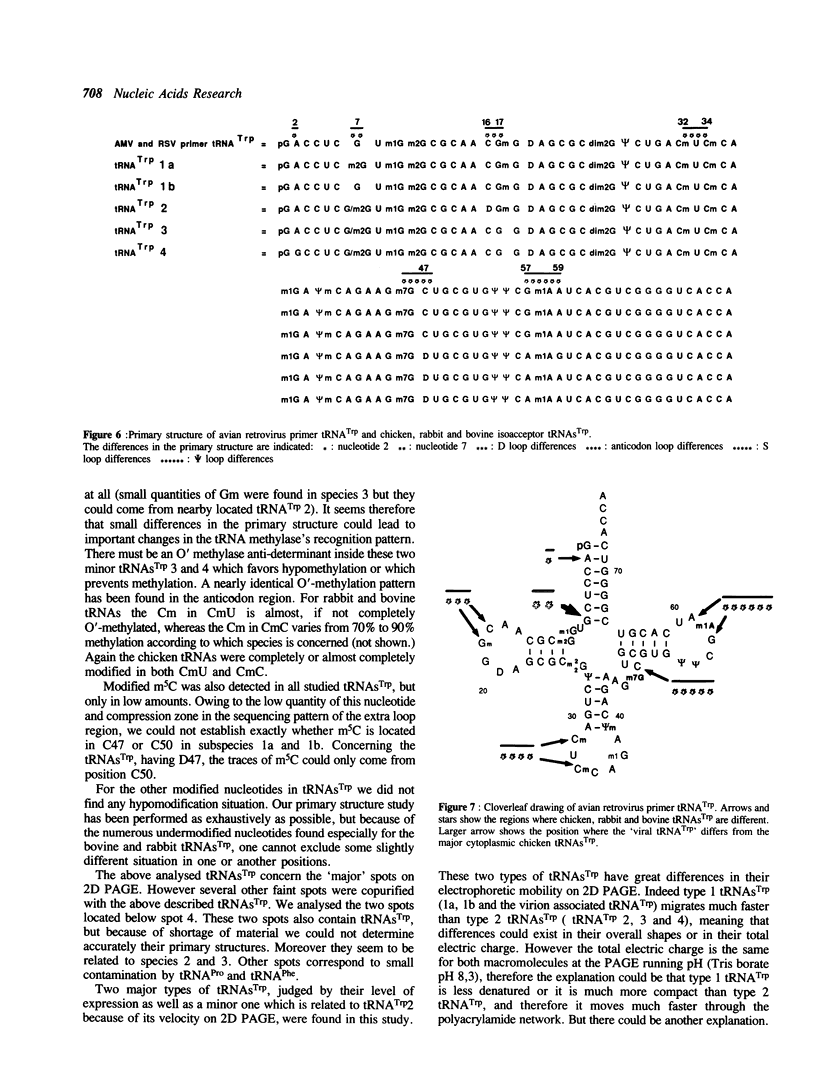
Bovine, rabbit and chicken tRNA(Trp) species and tRNA(Trp) packaged in avian myeloblastosis virus were separated and purified using two-dimensional gel electrophoresis and their primary structures were determined. Two major tRNA(Trp) species (1 and 2) were identified in beef and rabbit, two minor ones (3 and 4) in beef and only one minor in rabbit. Their structures differ by 4 nucleotide substitutions located in the D, S and T loops (positions 16, 47, 57 and 59). Species 3 and 4 differ from one another by only one nucleotide at position 2. Differences between tRNA(Trp) species were also observed in the extent of methylation of some nucleotides. Chicken tRNA(Trp) presents only one species similar to the mammalian type 1 tRNA(Trp). In the case of the three studies animals this tRNA could be separated into two subspecies, which differ by a post-transcriptional modification of nucleotide 7 in the acceptor stem: G or m2G. However only the nonmethylated species is used as the primer of DNA-RNA directed retrotranscription since it is only that form which was found in avian retroviruses. The methylation of G to m2G at position 7 could thus prevent the recognition of tRNA(Trp) by retroviral protein(s) responsible for the selective packaging of the primer tRNA(Trp).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya A., Sarih L., Litvak S. Reverse transcriptase mediated binding of primer tRNA to the viral genome. Nucleic Acids Res. 1979 Aug 24;6(12):3831–3843. doi: 10.1093/nar/6.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroudy B. M., Fournier M., Labouesse J., Papas T. S., Chirikjian J. G. tRNATrp (bovine) binding to the reverse transcriptase of avian myeloblastosis virus and function as a heterologous primer. Proc Natl Acad Sci U S A. 1977 May;74(5):1889–1893. doi: 10.1073/pnas.74.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittanova N., Petrissant G. Purification of mammalian tryptophan tRNA. Fluorescence properties of the free and the enzymatically acylated tryptophan tRNA. Biochim Biophys Acta. 1972 Mar 24;262(3):308–313. doi: 10.1016/0005-2787(72)90267-5. [DOI] [PubMed] [Google Scholar]
- Cordell B., DeNoto F. M., Atkins J. F., Gesteland R. F., Bishop J. M., Goodman H. M. The forms of tRNATrp found in avian sarcoma virus and uninfected chicken cells have structural identity but functional distinctions. J Biol Chem. 1980 Oct 10;255(19):9358–9368. [PubMed] [Google Scholar]
- Fournier M., Dorizzi M., Sarger C., Labouresse J. Purification of tRNATrp, tRNAVal, and partial purification of tRNAIle and tRNAMfet from beef liver. Biochimie. 1976;58(10):1159–1165. doi: 10.1016/s0300-9084(76)80114-9. [DOI] [PubMed] [Google Scholar]
- Fournier M., Labouesse J., Dirheimer G., Fix C., Keith G. Primary structure of bovine liver tRNATrp. Biochim Biophys Acta. 1978 Nov 21;521(1):198–208. doi: 10.1016/0005-2787(78)90262-9. [DOI] [PubMed] [Google Scholar]
- Henckes G., Panayotakis O., Heyman T. Isoaccepting species of serine tRNA coded by bacteriophage T5sto. J Virol. 1976 Feb;17(2):316–325. doi: 10.1128/jvi.17.2.316-325.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith G., Pixa G., Fix C., Dirheimer G. Primary structure of three tRNAs from brewer's yeast: tRNAPro2, tRNAHis1 and tRNAHis2. Biochimie. 1983 Nov-Dec;65(11-12):661–672. doi: 10.1016/s0300-9084(84)80030-9. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Wimmer E., Tener G. M. The isolation of yeast tyrosine and tryptophan transfer ribonucleic acids. Biochemistry. 1968 Jul;7(7):2629–2634. doi: 10.1021/bi00847a027. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Vogt P. K. Effects of genetic cellular resistance on cell transformation and virus replication in chicken hematopoietic cell cultures infected with avian myeloblastosis virus (BAI-A). Virology. 1968 Aug;35(4):487–497. doi: 10.1016/0042-6822(68)90278-x. [DOI] [PubMed] [Google Scholar]
- Ouenzar B., Agoutin B., Reinisch F., Weill D., Perin F., Keith G., Heyman T. Distribution of isoaccepting tRNAs and codons for proline and glycine in collagenous and noncollagenous chicken tissues. Biochem Biophys Res Commun. 1988 Jan 15;150(1):148–155. doi: 10.1016/0006-291x(88)90498-6. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Hu J. Reverse transcriptase as the major determinant for selective packaging of tRNA's into Avian sarcoma virus particles. J Virol. 1980 Dec;36(3):692–700. doi: 10.1128/jvi.36.3.692-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Sarih L., Gabus C., Litvak S., Keith G., Darlix J. L. Small finger protein of avian and murine retroviruses has nucleic acid annealing activity and positions the replication primer tRNA onto genomic RNA. EMBO J. 1988 Jun;7(6):1777–1783. doi: 10.1002/j.1460-2075.1988.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch F., Heyman T. Polyacrylamide gel mapping of chicken tRNA: comparison of polysome-bound and whole-cell tRNA from normal and avian sarcoma virus-infected chicken embryo fibroblasts. Mol Cell Biol. 1982 Oct;2(10):1247–1257. doi: 10.1128/mcb.2.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]