Abstract
We and others have found that Wnt signaling inhibition is important in mesenchymal stem cell (MSC) self-renewal. Pyrvinium was identified as a potent Wnt inhibitor in a chemical screen for small molecules. In the present study we hypothesized that pyrvinium will enhance MSC self-renewal to improve the clinical efficacy of MSC therapy. Pyrvinium increased MSC proliferation in vitro while inhibiting their osteogenic and chondrogenic lineage commitment by reducing cytoplasmic β-catenin. Although MSCs are a promising target for cell therapy, strategies to enhance their survival and maintain their stemness in the wounded area are essential. Using an in vivo model of granulation tissue formation, we demonstrated that pyrvinium enhanced long-term MSC engraftment. Pyrvinium treated MSC-generated granulation tissue also demonstrated less ectopic differentiation into bone or cartilage. This study highlights the potential of using a therapeutic Wnt inhibitor to enhance MSC-driven regenerative therapy.
Keywords: Mesenchymal stem cells, small molecule Wnt inhibitor, engraftment, proliferation
INTRODUCTION
Mesenchymal stem cells (MSCs), are bone marrow-derived fibroblast-like cells that have the potential to differentiate into osteogenic, chondrogenic, and adipogenic lineages and theoretically give rise to any tissue derived from mesenchyme including bone, muscle, tendon, ligament, and adipose tissue (1, 2). The intrinsic nature of stem cells to self-renew, proliferate, and be able to differentiate to various cell lineages make them a promising candidate for cell therapy. Successful exploitation of MSCs has been reported in several preclinical models (3, 4). These models have demonstrated that MSCs enhance tissue repair either by direct regeneration or by secreting paracrine factors. The therapeutic ability of MSCs has been augmented by genetic modifications, which over-express anti-apoptotic, pro-survival or growth factor genes (5). In clinical trials, MSCs have been able to repair injured myocardium, bone, and soft tissue, although with modest success (6–8). One cannot rule out the possibility of severe, but rare or delayed, side effects that might not have been observed in these clinical trials; however these studies have suggested the safety of both allogeneic and autologous MSCs, although, a good deal of work needs to be done to improve their therapeutic capacity. Low survival and poor engraftment of MSCs greatly limits their therapeutic efficacy, therefore, strategies need to be developed to enhance MSC survival and engraftment. Hence, a major focus in the field is to identify molecular pathways that improve MSC self-renewal (i.e. promote proliferation/survival and inhibit their terminal differentiation).
Wnt signaling has been implicated in the self-renewal and maintenance of pluripotent stem cells and progenitor cells (9, 10). Although cell specific activation of Wnt signaling after myocardial infarction (MI) was observed in vivo in cardiac progenitor, endothelial and leukocyte cells in a study using Wnt (axin2-LacZ) reporter mice, its relationship with wound healing was not evident in the study (11). On the other hand, inhibition of Wnt signaling has been linked with improved outcome in other injury models. Stabilization of cytoplasmic β-catenin is the hallmark of activated Wnt signaling (12). Mice that express stabilized β-catenin in cardiomyocytes demonstrate functional decline after injury (13, 14). In addition, our group has previously demonstrated that MSCs overexpressing sFRP2, a Wnt inhibitor, show enhanced survival and engraftment (3). These studies are promising but do come with their own limitations. These include but are not limited to the short lived nature of the genes transduced and the immune response generated by the gene delivery virus itself.
There is a growing amount of interest in identifying small molecules that target Wnt signaling (15, 16). Identification of small molecule inhibitors of the Wnt pathway would bypass the restrictions of genetic approaches, and would encourage the development of therapeutic reagents. In a chemical screening for small molecules, Thorne et al. identified pyrvinium, an antihelminthic drug previously approved by the FDA, as a Wnt inhibitor (17). Pyrvinium inhibits Wnt signaling by activating casein kinase 1α, which results into stabilization of β-catenin and inhibition of Axin degradation in the cytoplasm and regulation of pygopus stability in the nucleus. Recently, we have also shown that pyrvinium can inhibit Wnt signaling in MSCs in vitro (18). Moreover, our studies identified that pyrvinium induces cellular proliferation in vivo and reduces adverse cardiac remodeling in a myocardial infarction mouse model by directly affecting the host tissue (19). Since Wnt inhibition seems to be an important phenomenon for endogenous healing as well as stem-cell induced healing in multiple injury models described earlier, the rational for our present study is to take a pharmacological approach to enhance the therapeutic efficacy of stem cell function in vivo.
Based on the studies from other researchers and our work, we hypothesize that the efficacy of MSCs in wound tissue engraftment can be improved by utilizing the small molecule Wnt inhibitor, pyrvinium. Therefore, we tested this hypothesis by assessing the effect of pyrvinium on MSC differentiation into multiple lineages and proliferation in vitro. Additionally, long-term engraftment of MSCs was investigated in the wound injury model in vivo.
MATERIALS AND METHODS
Animals/Surgical Interventions
C57Bl6 and NOD/SCID/β-glucuronidase deficient (β-gluc−/−) mice are maintained by PPY. Surgical interventions were carried out as previously described (3). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee (Protocol Number: M/07/236). All experiments were performed using appropriate analgesics and anesthetics, and every effort was made to minimize pain/distress.
Reagents
Pyrvinium and a chemical analog of pyrvinium, compound 211 (which does not effect Wnt signaling pathway) were synthesized by the Vanderbilt Institute of Chemical Biology's Medicinal Chemistry Core and were generously provided by Dr. Ethan Lee.
Cells
Primary mouse MSC were obtained from the pooled bone marrow of C57Bl6 mice (n= 3) as described previously (2) and were maintained in DMEM-1g/L glucose (Mediatech Cellgro, Manassas, VA), 10% defined HyClone FBS (Thermo-Scientific, Waltham, MA), antibiotics (penicillin and streptomycin), fungizone (Amphotericin B, Sigma-Aldrich, St. Louis, MO) and 20 μg/ml human platelet-derived growth factor (PDGF, R & D systems, Minneapolis, MN). Adult, human donor, bone marrow aspirates were kindly provided by Orthopedics and Rehabilitation, Vanderbilt Orthopedic Institute, Vanderbilt University Medical Center. Human MSCs were isolated from the bone marrow aspirates as described earlier (20). Briefly, bone marrow cell suspension was diluted in Hank’s buffered salt solution (HBSS; Thermo-Scientific Hyclone, Waltham, MA), filtered through a cell strainer and then layered on Histopaque (Sigma-Aldrich). Following centrifugation at 2500 × rpm, the mononuclear cells were collected from the interface and washed with HBBS three times. The cell pellet was resuspended, cultured, and maintained in human MSC media MEM-α (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 20% Premium Select FCS (Atlanta Biologicals, Lawrenceville, GA).
BrdU Proliferation Assay
The proliferation of MSCs was assessed and quantified by 5-bromo- 2'-deoxyuridine (BrdU) Cell Proliferation Assay (Calbiochem, Gibbstown, NJ). Briefly, 0.5 × 103 cells were seeded on 96 well plates and cultured in full media for the 24 hours before the addition of BrdU for 16 hours. BrdU incorporation in the cells was assessed by ELISA and read at a dual wavelength of 450/595 using the SOFTMax Version 2.35 software.
Repair/Granulation Tissue Stimulation
NOD/SCID mice that contained an inactivating mutation in the β-glucuronidase (β-gluc) gene were used to study granulation tissue deposition and to quantify exogenous MSC engraftment (21). β-gluc, a lysosomal enzyme, is ubiquitously present in all normal cell types. Exogenously transplanted normal murine MSCs into a β-gluc-negative host can be identified by their β-gluc expression through quantitative biochemical analysis (21). Each mouse was implanted with multiple PVA sponges (soaked with 0.75 × 106 MSCs pretreated with 100 nM compound 211 (compd 211), or 100 nM pyrvinium) to assess the effect of the treatments on MSC-mediated, granulation tissue formation in sponges within the same animal. Additionally, sponges were injected with compd 211 or pyrvinium every other day. Animals were taken down on day 30 after sponge implantation.
β-Glucuronidase Biochemical Assay
β-gluc enzyme specific activity of sponge homogenates was measured as described earlier (21). β-gluc is expressed at a constitutive level in all cells, including MSCs (3). Hence, measurement of β-gluc enzyme activity in tissue has been extensively utilized to quantify and track implanted cells in tissues of host mice genetically deficient for β-gluc (21–23). Each sponge was homogenized with a motorized pestle in homogenization buffer (10 mM Tris (pH 7.5), 0.2% Triton X-100, and 1 mM dithiothreitol). Cell debris was removed from the sponge homogenates by centrifugation at 10,000 × g for 5 minutes. An aliquot of the sponge homogenate supernatant was incubated with 5mM of β-gluc substrate (4-Methyl Umbelliferyl β-D-glucuronide, Sigma) in 0.1M sodium acetate buffer at 37°C for one hour. The reaction was stopped with a stopper reagent (0.2M sodium carbonate and 0.32M glycine, pH 10.5) and β-gluc activity was measured fluorometrically at excitation of 365 nm and emission of 448 nm on a SPECTRAmax GEMINI XS ROM fluorometer (Molecular devices, Sunnyvale, CA). The data were normalized to DNA content using the FluoReporter Blue Fluorometric dsDNA Quantitation Kit (Molecular Probes, Carlsbad, CA) according to manufacturer’s instructions.
Phenotyping
Human MSCs were phenotyped using Human MSC Phenotyping Kit (Macs Miltenyi Biotec, Auburn, CA,). Cells were immunofluorescently stained for CD14, CD20, CD34, CD45, CD73, CD90, and CD105 and then analyzed with a 5-laser flow cytometer (355nm, 408nm, 488nm, 532nm, 635nm). The cells were negative for CD14, CD20, CD34, and CD45 and positive for CD73, CD90 and CD105, which follows the definition of human MSC set by the International Society for Cellular Therapy (ISCT).
Differentiation
Mouse MSCs were plated at a minimal density of about 6.0 x 103 cells/well in 6 well tissue culture plates and tissue culture slides (BD Falcon, Bedford, MA) with freshly made differentiation media (2). Fresh media was given to the cultures every 3 days. Cells undergoing chondrogenic (100 μM dexamethasone, 0.01 μg/ml TGF-β1 (Transforming growth factor- β1) and osteogenic (100 μM dexamethasone, 0.1 mM ascorbic acid, 10 nM β-glycerophosphate) differentiation were cultured for 8 days at 37°C in the presence or absence of treatments. MSC treatment included 100 nM pyrvinium in the presence or absence of Wnt3a (R&D). Wnt3a (50ng/ml) and compd 211 (100 nM) alone were used as positive and negative controls, respectively. Pyrvinium and compd 211 were added every 3rd day and Wnt3a was added every day for 8 days.
Alizarin Red and Toluidine Blue Staining
To identify osteogenic lineage commitment, mouse MSCs were fixed with 100% ethanol for 15 minutes at room temperature and stained with 0.25% Alizarin Red (pH 6.4). Cells committed for chondrogenic lineage were fixed in 10% formalin and stained with 1% Toluidine Blue in NaCl (pH 2.4), which allows histochemical detection of bone and cartilage deposition. Images were acquired with a digital camera (Pixera, Los Gatos, CA).
RNA Isolation and Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
RNA isolation, cDNA synthesis, and qRT-PCR analysis was performed as described earlier (3). Briefly, total RNA was isolated from the cells using TRIZOL reagent (Invitrogen) and first strand cDNA was synthesized with reverse transcriptase and oligo(dT) priming using iScript cDNA Synthesis Kit (BioRad Laboratories, Hercules, CA). qRT-PCR for osteocalcin and collagen XI was performed in triplicate for each sample on CFX384 Real-Time PCR Detection System (BioRad) using SsoFastTM EvaGreenR Supermix (BioRad). Each reaction was normalized to 18S.
Protein Isolation/Western Blot
Following osteogenic and chondrogenic differentiation, MSCs were harvested in cell fractionation buffer (50 mM Tris, pH 7.4, protease inhibitors (Roche)). The cytoplasmic fraction was separated from the membrane fraction by ultracentrifugation at 100,000 × g for 2 hours. Following BCA Protein Assay (Pierce), 10 μg of the cytoplasmic proteins were resolved on a 10% SDS/PAGE and transferred to nitrocellulose membrane (BioRad). Subsequently after blocking in 5% milk in Tris buffer saline-Tween (TBS-Tween), membrane was incubated with primary antibodies β-catenin (1:1000, B D Biosciences, San Jose, CA) and β-actin (1:10,000, Sigma) at 4°C overnight. Species-specific secondary antibodies were used and chemiluminesence (PerkinElmer, NEL104) was detected by film. Densitometry analysis was performed using ImageJ version 1.38x (National Institutes of Health) software for quantification and β-actin was used for normalization.
Histochemistry and Morphometry
For histological analysis, PVA Sponges were cut in half and embedded with cut surface down while the other half was frozen for quantitative biochemical analysis. Toluidine Blue staining was performed on sponge sections. Images were photographed with a CoolSNAP Hq CCD camera (Photometrics). The positively stained area within the sponges was blindly scored by a pathologist. The range for scores was from zero to three, where zero meant no Toluidine Blue positive areas were identified and three meant there were prominent, brightly stained areas.
Statistical Analysis
The statistical significance between experimental groups and control was determined by paired t test or ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism (San Diego, CA). For MSC engraftment analysis, non-parametric Mann-Whitney test was performed on the log-transformed β-glucuronidase enzyme specific activity of experiment over control. Graphs show mean ± standard deviation and p<0.05 is considered statistically significant.
RESULTS
Pyrvinium inhibits Wnt signaling
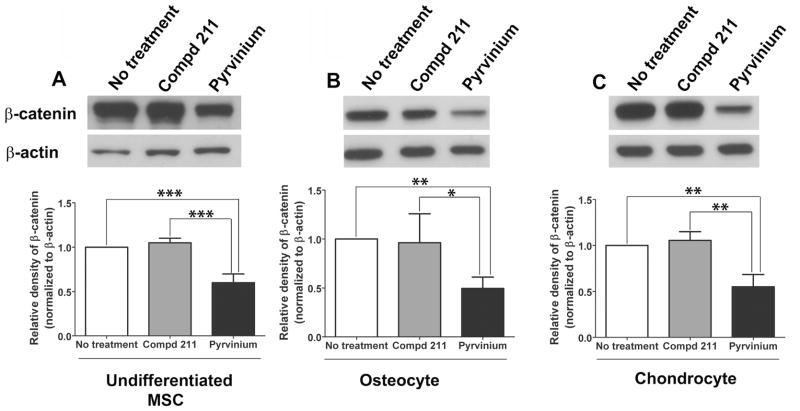
Previous studies by our laboratory and Thorne et al. (17, 18) have demonstrated that pyrvinium inhibits Wnt signaling by binding and activating casein kinase 1α. To assess the affect of pyrvinium on Wnt signaling during differentiation, MSCs were directed towards osteogenic and chondrogenic lineages in the presence and absence of compd 211 or pyrvinium. The molecular analog of pyrvinium, compd 211, does not inhibit Wnt signaling (17) and was used as a control. Western blot analysis demonstrated low levels of cytoplasmic β-catenin in the cells treated with pyrvinium. Pyrvinium inhibited cytoplasmic β-catenin levels by 48.5% in the cells committed for osteogenic lineage when compared to the cells treated with compd 211 (0.496 ± 0.115 SD vs. 0.963 ± 0.295 SD; p<0.05) (Figure 1B) and by 47.5% in the cells committed for chondrogenic lineage (Figure 1C) compared to control compd 211 treated cells (0.551 ± 0.133 vs. 1.05 ± 0.0959; p<0.005). Additionally, 43% inhibition (0.599 ± 0.1 vs. 1.05 ± 0.0515; p<0.0005) of β-catenin levels was also evident in the non lineage committed MSCs (Figure 1A). These results confirmed our previous finding that pyrvinium acts as a Wnt inhibitory molecule in MSCs. Also, these results identified that pyrvinium inhibited Wnt signaling in MSCs committed for lineage specification.
Figure 1. The effect of pyrvinium on Wnt signaling during basal, osteogenic and chondrogenic differentiation of MSCs in vitro.
Representative Western blot analysis and the quantification of the relative β-catenin protein levels in the cytoplasmic fractions of MSCs normalized to β-actin. Decreased β-catenin levels were identified in the cytoplasmic fractions of pyrvinium-treated MSCs committed towards osteogenic (B) and chondrogenic lineages (C) when compared to the untreated cells and the cells treated with the control compd 211; the similar effect was also identified in the undifferentiated cells (A). The statistical significance between experimental groups and control was determined by ANOVA followed by Tukey’s multiple comparison test; ***p<0.0005, **p<0.005, and *p<0.05, n =4.
Increased MSC engraftment in vivo and proliferation in vitro were mediated by pyrvinium
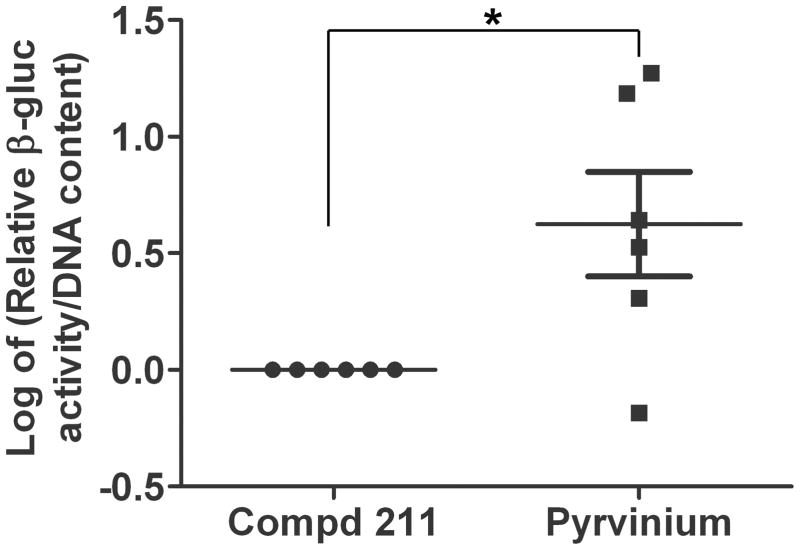
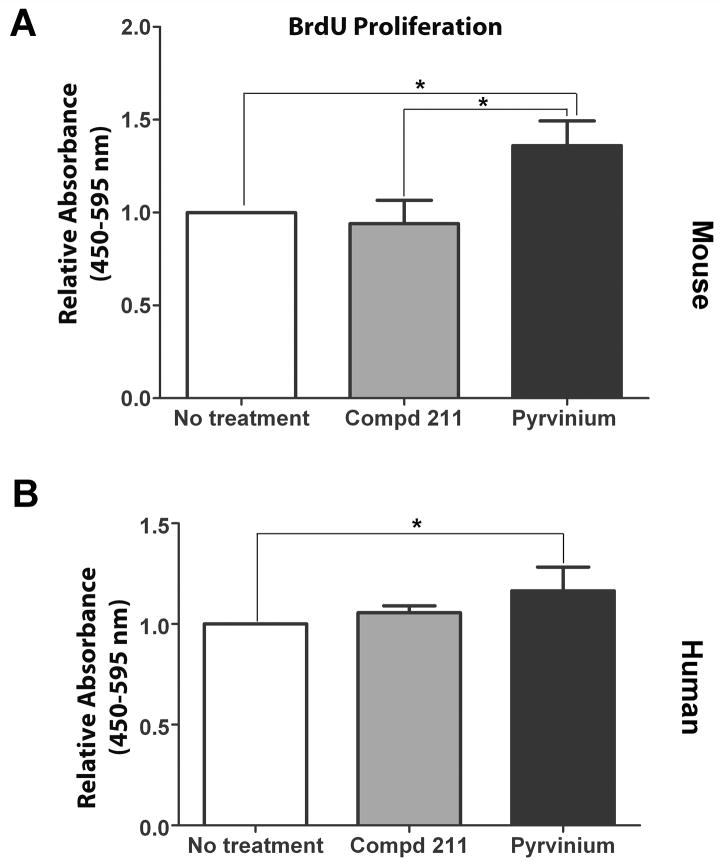
The PVA sponge model that mimics healing by secondary intention was used to assess the effect of pyrvinium on features of MSC-derived granulation tissue study and its effect on MSC survival and maintenance (i.e. engraftment) within the newly formed granulation tissue (24). Biochemical analysis of β-gluc enzyme activity was determined and relative fold change was calculated, log-transformed and analyzed for pyrvinium-treated over compd 211 treated MSC-loaded sponges implanted in the same animal. Since all wild type cells (including implanted MSCs) are β-gluc positive, this enabled us to quantify, by measuring β-gluc activity, the relative amount of MSC engraftment and distinguish that from infiltrating host cells (β-gluc negative). The relative intensity of β-gluc enzyme activity among multiple animals identified that pyrvinium enhanced the amount of MSC generated granulation tissue in the sponges by 2.4 fold (p<0.05) compared to compd 211-treated sponges (Figure 2). To further understand the effect of pyrvinium on the functionality of MSCs, we performed in vitro BrdU proliferation assay on mouse and human MSCs grown in the presence of pyrvinium and compd 211. Relative change in proliferation, measured by BrdU incorporation, was calculated for pyrvinium-treated cells over no treatment or control compd 211-treated cells. Pyrvinium treatment resulted in 1.4 fold increase in mouse MSC proliferation when compared to control compd 211 (p<0.05; Figure 3A). A modest increase in human MSC proliferation (1.16 fold) (Figure 3B) over no treatment was observed but no difference was identified in human MSC proliferation when compared to control compd 211. However, pyrvinium’s effect was specific to MSCs as we did not identify pyrvinium-induced enhancement in proliferation in HUVECs and NIH3T3 cells (data not shown).
Figure 2. Pyrvinium affects MSC engraftment in vivo.
β-gluc enzyme activity (MSC marker) normalized to total cellular DNA content of MSC sponge granulation tissue samples treated with pyrvinium or compd 211. Data here shows log-transformed values of β-gluc activity of pyrvinium-treated over compd 211-treated MSC-loaded sponges implanted in the same mouse (n=6). The statistical significance between experimental groups and control was determined by non-parametric Mann-Whitney test, *p<0.05 was considered statistically significant.
Figure 3. The effect of pyrvinium on MSC proliferation.
In vitro proliferation of MSCs treated with pyrvinium or compd 211 was evaluated by BrdU incorporation. Pyrvinium enhanced both mouse (A) and human (B) MSC proliferation. Data here shows average of the relative BrdU incorporation indicating MSC proliferation in the presence or absence of different treatments. The statistical significance between experimental groups and control was determined by ANOVA followed by Tukey’s multiple comparison test. *p<0.05 was considered statistically significant.
Pyrvinium inhibits osteogenic and chondrogenic differentiation of MSCs
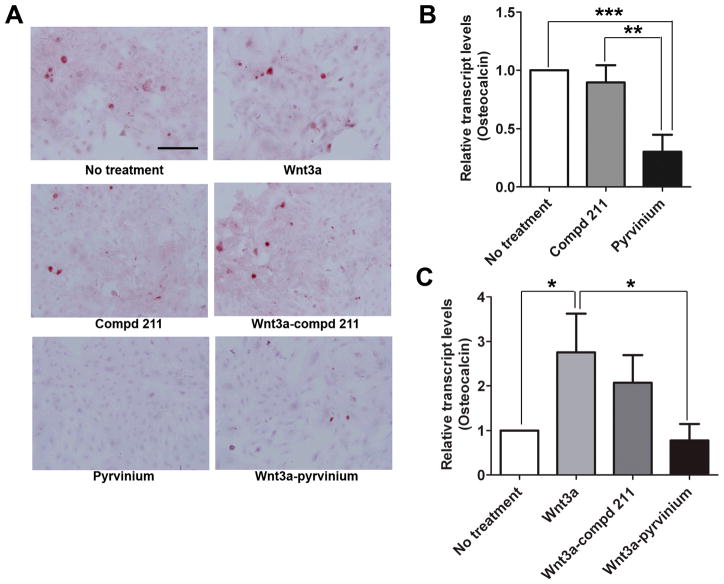
MSC differentiation can be accelerated by culturing them in differentiation-inducing culture conditions (25). Since pyrvinium enhanced MSC proliferation, we hypothesized that it does so by delaying the lineage commitment of MSCs. To investigate this phenomenon, MSCs were differentiated and the effect of pyrvinium on the in vitro multilineage commitment ability of MSCs was assessed. To evaluate osteogenic differentiation, calcification was analyzed by Alizarin Red staining (Figure 4A) and the qRT-PCR was done for the osteogenesis specific marker (osteocalcin) (Figure 4B). The amount of calcified matrix decreased in the presence of pyrvinium when compared to no treatment and control compd 211. The transcript levels of osteocalcin decreased by 66.5% in response to pyrvinium treatment when compared to control compd 211 (0.301 ± 0.148 SD vs 0.896 ± 0.148 SD; p<0.005). The particular role of Wnt signaling on MSC lineage commitment was confirmed by inducing MSC for osteogenic differentiation in the presence of recombinant Wnt3a. Indeed, we identified Wnt-induced enhancement in MSC osteocalcin levels by ~2.76 fold (p<0.05; Figure 4C). To identify that pyrvinium-induced decline in MSC osteocalcin levels is due the inhibitory effect of pyrvinium on Wnt signaling, MSCs were treated with pyrvinium and Wnt3a in combination. Undoubtedly, pyrvinium reduced osteocalcin levels by 71.7% in MSCs treated with Wnt3a and pyrvinium when compared to the osteocalcin levels of the cells treated with Wnt3a alone (0.780 ± 0.369 vs. 2.76 ± 0.863; p<0.05); the similar effect was not observed in the cells treated with compd 211 in the presence of Wnt3a.
Figure 4. The effect of pyrvinium on MSC differentiation towards osteogenic lineage in vitro.
(A) Representative images of Alizarin Red stained MSCs committed for osteogenic differentiation (magnification 20X, scale bar=2.0 mm). (B) Relative transcript levels of osteocalcin in MSCs treated with pyrvinium compared with compd 211 treated MSCs. (C) Change in transcript levels of osteocalcin in the presence of Wnt alone and in combination with compd 211 or pyrvinium when compared to no treatment. The statistical significance between experimental groups and control was determined by ANOVA followed by Tukey’s multiple comparison test. ***p<0.0005, **p<0.005, and *p<0.05, n =3.
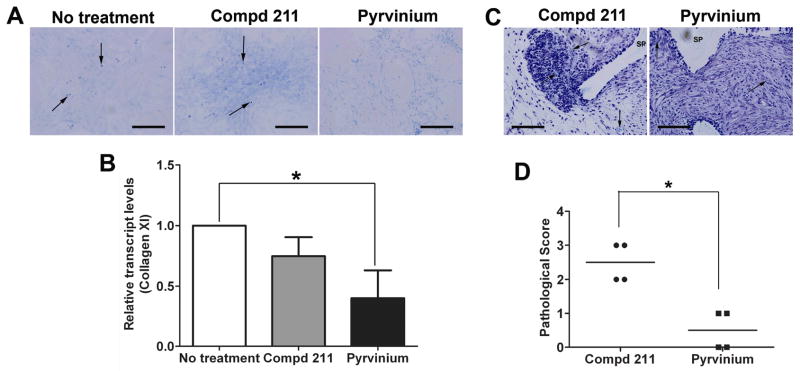
Chondrogenic differentiation was identified by Toluidine Blue staining of sulphated glycosaminoglycans produced by MSCs in vitro (Figure 5A) and in the MSC-loaded sponges in vivo (Figure 5C). The quantitative real time assessment of chondrogenic specific marker, collagen XI, was also performed (Figure 5B). The transcript levels of collagen XI decreased by 60% (0.401 ± 0.230 SD vs. 1 ± 0; p<0.05)) with the pyrvinium treatment; the effect not observed in the presence of the control compd 211. Moreover, decreased Toluidine Blue staining (for chondrogenesis) was evident in the MSCs cultured in vitro and in MSC-loaded sponges in vivo that were treated with pyrvinium. The histological scores between pyrvinium and compd 211 treated MSC-loaded sponges depicted significant decrease of 80% (0.5 ± 0.577 SD vs. 2.5 ± 0.577 SD; p<0.05) in Toluidine Blue staining levels (Figure 5D) in the presence of pyrvinium. GAPDH transcript levels were unaltered in the MSCs committed for differentiation with different treatments (osteogenic and chondrogenic; data not shown) and served as an internal control. These results identify the inhibitory role of pyrvinium on MSC differentiation.
Figure 5. The effect of pyrvinium on MSC differentiation towards chondrogenic lineage in vitro and in vivo.
(A) Representative images of Toluidine Blue stained MSCs committed for chondrogenic differentiation (magnification 20X, scale bar=2.0 mm). (B) Relative transcript levels of collagen XI in MSCs treated with pyrvinium when compared with control (no treatment). (C) Representative images of calcified matrix stained with Toluidine Blue within granulation tissue of MSC-loaded PVA sponges treated with pyrvinium or compd 211 (magnification 40X, scale bar=2.0 mm). Arrows point towards sulphated glycosaminoglycan-positive areas. (D) Histological score of the amount of Toluidine Blue positive staining within MSC-loaded sponges treated with pyrvinium compared with compd 211. The statistical significance between experimental groups and control was determined by ANOVA followed by Tukey’s multiple comparison test or t test, **p<0.005, *p<0.05, n=3.
DISCUSSION
Wound healing is an intricate phenomenon that takes place in multiple overlapping steps, including, inflammation, cellular proliferation, and remodeling of the injured tissue which leads to the formation of granulation tissue. Granulation tissue encompasses extracellular matrix support incorporating the infiltration of multiple cell types including immune cells, fibroblasts, and vascular endothelial cells. Substantial granulation tissue deposition is required in order to heal the wounded tissue. Wound healing could be improved by the implantation and engraftment of MSCs within the granulation tissue deposited in the wounded area. MSCs have been utilized for wound repair in several preclinical and clinical trials of tissue regeneration (26, 27). Although pre-clinical studies in rodent models of ischemic injury have recognized significant benefits of stem cell therapy, its translation for clinical efficacy is still in its infancy (6). Presently there are more than 150 ongoing clinical trials that involve exogenous human MSCs (http://clinicaltrials.gov/ct2/home; accessed on 09/30/11) (28). However, varying degrees of success have been obtained in clinical trials where MSCs have been shown to repair infarcted myocardium, bone, and soft tissue (8). Poor engraftment, low potency, and low survival of transplanted MSCs are still major challenges that limit the clinical application of MSC-based therapy (29). In light of these observations, we hypothesized that the therapeutic effects of MSCs can be augmented with mechanisms to extend their survival and function. In the present study we evaluated the strategy of using a small molecule Wnt inhibitor to enhance MSC engraftment and effectiveness in wound granulation tissue.
Self-renewal is a key factor in stem cell maintenance which allows cells to proliferate and engraft while preventing lineage commitment (30). Wnt signaling has been shown to modulate stem cell self-renewal in the intestinal stem cell niche (31) as well as in hematopoietic stem cells (9, 32). Wnt signaling also directs self-renewal and differentiation of Islet1-expressing precursor cells in neo- and postnatal hearts (33). Several groups have indicated the role of Wnt signaling in MSC lineage specification (30, 34), but the role of canonical inhibition of Wnt signaling with respect to MSC self-renewal has only been reported recently by our group (18). Over expression of a Wnt inhibitory protein, sFRP2, was shown to increase the proliferation and engraftment of MSCs in vivo (3). Based on these studies we speculate that pharmacological inhibition of Wnt signaling will delay differentiation and promote engraftment of MSCs probably by maintaining the self-renewal capability of MSCs.
A pharmacological compound, pyrvinium, has recently been identified as a potent small molecule inhibitor of Wnt pathway (17) and has been demonstrated by our group to improve endogenous granulation tissue (19). The role of the Wnt inhibitory molecule on the functional relevance of MSCs was assessed by utilizing a PVA sponge model of repair and regeneration. The PVA sponge model is an extensively used model for wound healing studies (24). It mimics wound healing by secondary intention. PVA sponges provide an inert substance that can be implanted subcutaneously thereby stimulating the growth of new tissues within the sponge as well as in the surrounding areas of the sponge referred as granulation tissue (35). Our goal in this study was to determine whether pyrvinium affects the ability of MSCs to engraft within the granulation tissue deposited in the sponges. Indeed we found that pyrvinium administration enhanced the amount of MSC engraftment within the granulation tissue (demonstrated by enhanced β-gluc activity in pyrvinium-treated sponges compared with control compd 211), indicating the functional relevance of Wnt inhibition as a therapeutic strategy to enhance MSC function following transplantation in injured tissues. The control compd 211 and the pyrvinium-treated MSC-loaded sponges utilized in the present study were implanted in the same animal. One cannot rule out the possibility of cross-contamination of MSCs from one sponge to another; however the rationale behind using all the conditions in the same animal was to rule out the variability that may arise when using multiple animals.
We also investigated the effect of pyrvinium on MSC self-propagation and identified a modest increase in mouse MSC proliferation. Previously, we have shown that sFRP2 significantly increased proliferation of human as well as mouse MSCs (3). In addition, Dkk1, a Wnt pathway inhibitor, has been shown to increase human MSC proliferation in vitro (36) but only marginally affected mouse MSC proliferation (3). Data from non-stem cells also connect Wnt inhibition with enhanced proliferation and reduced fibrosis. For example studies done by Liu et al. indicated that continuous exposure to Wnt3a results in inhibiting the proliferation of primary mouse embryonic fibroblasts and further triggers senescence (37). The robust affect on MSC proliferation, observed with other Wnt inhibitors, was not identified in the present study. However, pyrvinium administration clearly improved MSC generated granulation tissue in our sponge model. These observations provide a clue that the cell numbers generated in the presence of a Wnt inhibitor in vitro may not be consistent with the better engraftment of cells in vivo. These somewhat inconsistent findings could be due to that fact that these various strategies differ in their mechanism of action. Some of them (i.e. sFRP2) act upstream and theoretically affect both canonical and non-canonical Wnt pathway in contrast to pyrvinium (38). Nonetheless, our study indicates a positive role of a Wnt-inhibitor, pyrvinium, in stem cell maintenance and engraftment.
Our next step was to tease out the effect of pyrvinium on Wnt signaling in MSCs committed for lineage specification. Enhancement of the cytoplasmic expression of β-catenin is a hallmark of Wnt activation (39). Decreased levels of β-catenin in the presence of pyrvinium have been previously shown by our laboratory (18) and Thorne et al (17). Indeed, we validated our previous findings and identified the inhibitory effect of pyrvinium, indicated by decreased cytoplasmic β-catenin levels, on Wnt signaling during MSC osteogenic and chondrogenic differentiation. For the therapeutic efficacy of MSCs, it is essential to maintain a balance between stem cell maintenance and lineage commitment. In order to augment their engraftment within a wound injury, MSCs must proliferate, self renew, and survive longer while delaying differentiation (30). We showed that pyrvinium inhibited the differentiation of MSCs induced for lineage commitment along chondrogenic and osteogenic lineages in vitro and towards chondrogenic lineage in vivo. Although, trilineage differentiation is a hallmark of MSC biology, our previous work has shown that Wnt inhibition does not affect the potential of MSCs to differentiate into adipogenic lineage (18); hence not considered for this study. Canonical Wnt signaling induces the expression of osteocalcin, indicating enhancement of osteogenic differentiation of MSCs (40) and has also been shown to be involved in early chondrogenesis (41). Additionally, inhibition of Wnt signaling through Dkk-1 augments human MSC proliferation without premature differentiation (36). These observations support our conclusion that increased proliferation in pyrvinium-treated MSCs in vitro and better engraftment in vivo is due to delayed differentiation of MSCs via inhibition of canonical Wnt signaling. While we have shown that MSCs treated with pyrvinium have proliferative potential, our results do not exclude the likelihood that pyrvinium mediated Wnt inhibition may have a multifactorial effect which could contribute to the better engraftment of MSCs in vivo.
There is a crucial necessity to develop approaches that will increase the survival and maintenance of MSCs in the wounded area. In the last few years, several attempts have been made to improve the survival and efficiency of MSCs. These methods include but are not limited to the preconditioning of MSCs with growth factors, hypoxic environment and other factors (42, 43). Wisel et al. demonstrated that pharmacological preconditioning of rat MSCs with an anti-ischemic drug, trimetazidine, enhances survival and improves heart function (44). Importantly, preconditioning of MSCs with a pharmacological Wnt inhibitor has not been studied yet. Our studies suggest that a small molecule Wnt inhibitor administered in conjunction with MSCs may improve their therapeutic efficacy. The exploitation of a small molecule Wnt inhibitor could aid in the development of therapeutic reagents that could be valuable in the improvement of cell-based therapies for regenerative medicine. However, it warrants future investigations in other wound injury models including myocardial infarction.
Acknowledgments
This work was supported by NIH grants R01-HL088424, HL08842402S1, RO1-GM081635; and Veterans Affairs merit award (PPY).
References
- 1.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 2.Tropel P, Noel D, Platet N, Legrand P, Benabid A, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. A Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–71. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (sFRP2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfaro MP, Young PP. Lessons from Genetically Altered Mesenchymal Stem Cells (MSCs); Candidates for Improved MSC-Directed Myocardial Repair. Cell Transplant. 2011 doi: 10.3727/096368911X612477. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Coletta AP, Abdellah AT, Cullington D, Clark AL, Rigby AS. Clinical trials update from the American Heart Association 2007: CORONA, RethinQ, MASCOT, AF-CHF, HART, MASTER, POISE and stem cell therapy. Eur J Heart Fail. 2008;10(1):102–8. doi: 10.1016/j.ejheart.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–14. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 11.Oerlemans MI, Goumans MJ, van Middelaar B, Clevers H, Doevendans PA, Sluijter JP. Active Wnt signaling in response to cardiac injury. Basic Res Cardiol. 2010;105(5):631–41. doi: 10.1007/s00395-010-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadigan KM. Wnt-beta-catenin signaling. Curr Biol. 2008;18(20):R943–7. doi: 10.1016/j.cub.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. {beta}-Catenin Downregulation Is Required for Adaptive Cardiac Remodeling. Circ Res. 2007;100(9):1353–62. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 14.Malekar P, Hagenmueller M, Anyanwu A, Buss S, Streit MR, Weiss CS, Wolf D, Riffel J, Bauer A, Katus HA, Hardt SE. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension. 2010;55:939–45. doi: 10.1161/HYPERTENSIONAHA.109.141127. [DOI] [PubMed] [Google Scholar]
- 15.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44(47):15495–503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- 17.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM, 3rd, Lee E. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6(11):829–36. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaro MP, Vincent A, Saraswati S, Thorne CA, Hong CC, Lee E, Young PP. sFRP2 Suppression of Bone Morphogenic Protein (BMP) and Wnt Signaling Mediates Mesenchymal Stem Cell (MSC) Self-renewal Promoting Engraftment and Myocardial Repair. J Biol Chem. 2010;285(46):35645–53. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5(11):e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140(1):138–43. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 21.Hofling AA, Vogler C, Creer MH, Sands MS. Engraftment of human CD34+ cells leads to widespread distribution of donor-derived cells and correction of tissue pathology in a novel murine xenotransplantation model of lysosomal storage disease. Blood. 2003;101(5):2054–63. doi: 10.1182/blood-2002-08-2597. [DOI] [PubMed] [Google Scholar]
- 22.Glaser JH, Sly WS. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973;82(6):969–77. [PubMed] [Google Scholar]
- 23.Young PP, Vogler C, Hofling AA, Sands MS. Biodistribution and efficacy of donor T lymphocytes in a murine model of lysosomal storage disease. Mol Ther. 2003;7(1):52–61. doi: 10.1016/s1525-0016(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 24.Cooney R, Iocono J, Maish G, Smith JS, Ehrlich P. Tumor necrosis factor mediates impaired wound healing in chronic abdominal sepsis. J Trauma. 1997;42:415–20. doi: 10.1097/00005373-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21(10):1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 26.Barry FP. Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2003;69(3):250–6. doi: 10.1002/bdrc.10021. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 2004;15(5):406–10. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.U.S. National Library of Medicine USNIoH, U.S. Department of Health & Human Services. Clinicaltrials.gov: Lester Hill National Center for Biomedical Communications. [Google Scholar]
- 29.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satija NK, Gurudutta GU, Sharma S, Afrin F, Gupta P, Verma YK, Singh VK, Tripathi RP. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16(1):7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 31.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 32.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7(10):1048–56. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 33.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1(2):165–79. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T, Matsuoka AJ, Shimomura A, Koehler KR, Chan RJ, Miller JM, Srour EF, Hashino E. Wnt Signaling Promotes Neuronal Differentiation From Mesenchymal Stem Cells Through Activation of Tlx3. Stem Cells. 2011;29(5):836–46. doi: 10.1002/stem.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel J, Gudehithlu KP, Dunea G, Arruda JA, Singh AK. Foreign body-induced granulation tissue is a source of adult stem cells. Transl Res. 2010;155(4):191–9. doi: 10.1016/j.trsl.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Gregory CA, Harpreet S, Perry AS, Prockop DJ. The wnt signalling inhibitor Dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–78. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen JK, Mailida D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 38.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1–2):1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical Wnt signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 41.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 42.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2011;16(2):159–68. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 43.Khan M, Akhtar S, Mohsin SN, Khan S, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2011;20(1):67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 44.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009;329 (2):543–50. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]