Abstract
The recent years have seen melanoma research undergo a renaissance. What was once viewed, at least in the metastatic setting, as an intractable and untreatable disease is now revealing its molecular weaknesses. 2011 was a landmark year for melanoma therapy with two new agents, the anti-CTLA4 antibody ipilimumab and the BRAF inhibitor vemurafenib, shown to confer a survival benefit in randomized phase III clinical trials. Forgotten in the recent flurry of interest that has accompanied the development of these drugs, melanoma is in fact an ancient disease that has long frustrated attempts at therapeutic intervention. In this article we trace the history of melanoma; from the earliest known cases of melanoma in pre-Colombian South America, through the explorations of the Victorian anatomists right up to the molecular biology revolution of the 20th century that allowed for the identification of the key driving events required for melanomagenesis. We further outline how observations about melanoma heterogeneity, first made over 190 years ago, continue to drive our efforts to reduce melanoma to the level of chronic, manageable disease and ultimately cure it entirely.
Keywords: BRAF, melanoma, surgery, oncogenes
Introduction
“As to the remote and exciting causes of melanosis, we are quite in the dark, nor can more be said of the methodus medendi. We are hence forced to confess the incompetency of our knowledge of the disease under consideration, and to leave to future investigators the merit of revealing the laws which govern its origin and progress….and pointing out the means by which its ravages may be prevented or repressed” - Thomas Fawdington, The Manchester Royal Infirmary, 1826.
When Thomas Fawdington wrote these words in 1826, medical science had very little to offer those with melanoma, or indeed most other ailments (1). This was an era that predated anesthesia and antiseptic agents. The nature of heredity and the structure of DNA had yet to be determined, and it was not yet appreciated that cancer arose from the transformation of normal cells. Today our understanding of melanoma is very different, it is now well known that melanoma is a malignancy of melanocytes, and that exposure to ultraviolet radiation is etiologic and that genetic polymorphisms in genes such as melanocortin receptor-1 (MC1R) and CDKN2A increase melanoma risk (2).
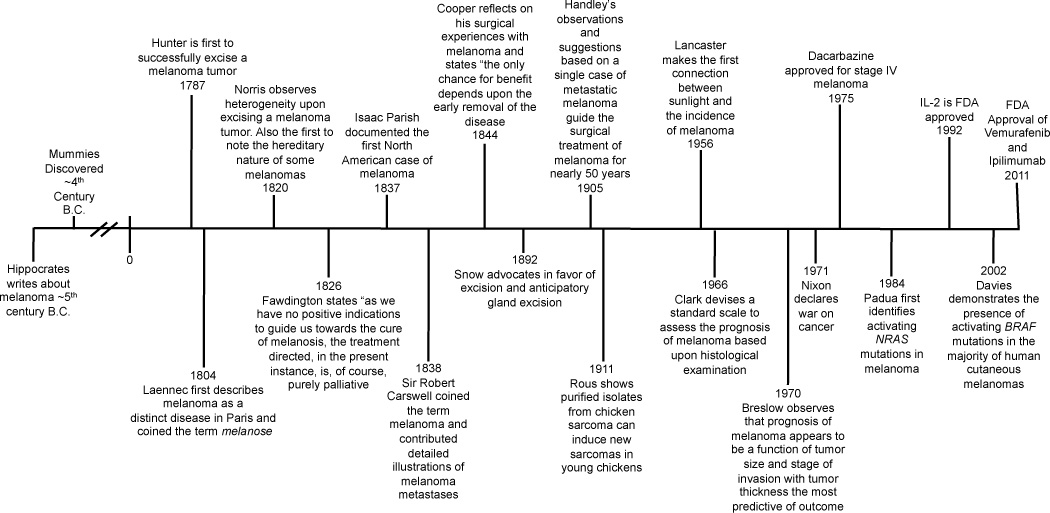
Until very recently, many in the melanoma field may have felt Fawdington’s despair at having little to offer patients with widespread metastatic disease. Today our accumulated knowledge is bearing fruit, we now understand a great deal about the genetic mutations that are responsible for sustaining melanoma and are able to subgroup melanoma patients and match genotypes with therapies (3, 4). Despite this progress, we still cannot answer some of Fawdington’s most important questions. Strategies for melanoma prevention are lacking and the molecular steps required to transform a melanocyte into a melanoma cell are still poorly understood. Even the targeted therapies that show so much promise only lead to objective responses in ~50% of patients who harbor BRAF mutations, and most of these are short-lived. (4). It is not the intention of this article to provide a comprehensive history of cancer, as Siddhartha Mukherjee has already covered this in detail in his excellent book The Emperor of All Maladies (5). We would also like to apologize to those with an interest in immunology, as, for the sake of brevity, we have neglected to include this in our discussion and refer the reader to a number of reviews, including (6). Instead, we trace some of the selected key events through history that have shaped our understanding of melanoma leading up to the present day discoveries of the key genetic mutations required for melanoma development and progression (Figure 1).
Figure 1.
Time-line of the key events in melanoma history.
1) Melanoma history: defining the disease
The first recorded descriptions of melanoma (a word derived from the Greek melas, "dark" and oma “tumor”) in history appeared in the writings of Hippocrates of Cos in the 5th century, B.C., and later in those of the Greek physician Rufus of Eupheses (7). Although archeological evidence of cancer has been generally scant, tumors can be diagnosed from the skeletons of those afflicted by osteolytic bone metastases or bony osteosarcomas. The earliest physical evidence of melanoma comes from the diffuse melanotic metastases found in the skeletons of Pre-Colombian mummies (radiocarbon dated to be ~2400 years old) from Chancay and Chingas in Peru (7).
Between 1650–1760, the European medical literature, including the work of Highmore (1651), Bonet (1651), and Henrici and Nothnagel (1757), made numerous references to “fatal black tumors with metastases and black fluid in the body”. The Scottish surgeon John Hunter, working at St George’s Hospital Medical School, London is credited with the first surgical removal of a melanoma in the Western medical literature. In 1787, he operated upon and successfully removed a recurrent melanoma from the jaw of a 35-year old man. This case, as retold by Everard Home in his 1805 Observations on Cancer, reported the tumor to be “soft and black”, with Hunter labeling it as a “cancerous fungous excrescence” (8). Although it was not clear that Hunter knew what he was dealing with, the preserved tumor was later diagnosed as a melanoma in 1968, and is still housed in the Hunterian Museum at Lincolns Inn Fields in London, UK (9).
Rene Laennec, primarily famed as the inventor of the stethoscope, was the first to realize that melanoma was a distinct disease and unrelated to the black carbon deposits typically found in the lungs of patients upon autopsy (10, 11). In a lecture, given in Paris in 1804, and later published in 1806, he coined the term melanose to describe these tumors. His publication on melanoma led to a rift with his mentor Baron Dupuytren (the former surgeon to Napoleon Bonaparte) who claimed that his own work had not been sufficiently credited (12). Another student of Dupuytren, Jean Cruveilhier in his book Anatomie Pathologique du Corps Humain (published between 1829–1842) was the first to describe melanomas of the hand, foot and vulva (12).
One of the first thorough and insightful reports into the etiology and progression of “melanosis” was given in 1820 by Dr. William Norris, a general practitioner from Stourbridge, UK, under the description of “fungoid disease” (13). In his study, Norris followed a 59-year old male melanoma patient over 3 years, documenting both his disease progression and making detailed anatomical observations upon autopsy. In his examination of the cadaver, Norris made a number of seminal observations that continue to be of interest to melanoma biologists and clinicians to this day. Upon making an inscision through the original tumor Norris noted: “I found the texture to be heterogeneous; it was of a reddish and whitish brown tint throughout, not very unlike the internal structure of a nutmeg” (13). He also remarked upon the propensity of melanoma for widespread metastasis “On opening the abdomen I found numerous tubera of various sizes. To the eye of the morbid anatomist it was interesting to behold the tumors scattered in the utmost profusion in every direction” (13). Perhaps most significant of all, Norris was the first to note the heritable nature of some melanomas, some 50 years before Mendel presented his data on inheritance. Said Norris: “It is remarkable that this gentleman’s father died of a similar disease…This tumor, I have remarked, originated in a mole….my patient and his children had many moles on various parts of their bodies…. These facts, together with another case that has come under my notice…would incline me to believe that this disease is hereditary” (13). It is likely that Norris described the first ever case of familial atypical multiple mole melanoma syndrome, an inherited condition characterized by multiple nevi and a family history of melanoma associated in many cases with mutations in the CDKN2A gene.
In 1857, Norris expanded upon his earlier observations and described a further 8 cases of melanoma (14). From these he devised some general principles for melanoma epidemiology and clinical management. He was one of the first to propose a relationship between nevi and melanoma and a possible link between melanoma and exposure to environmental factors (such as industrial pollution), and made the observation that most of his patients had light colored hair and pale complexions (14). He further described that melanomas could be either pigmented or amelanotic and that they had a tendency to disseminate widely to many visceral organs. Norris further observed that neither surgery nor medical treatments were effective once the melanoma was widely dispersed. To control recurrence, Norris advocated a wide excision of the tumor and surrounding unaffected skin, as this was thought to be more effective at preventing the tumor’s regrowth (14).
In 1826, Thomas Fawdington published A Case of Melanosis, with General Observations on the Pathology of the Interesting Disease, in which he described a 30-year old patient who developed an ocular melanoma after receiving a blow to the eye. He stated at the time...”as we have no positive indications to guide us towards the cure of melanosis, the treatment directed, in the present instance, is, of course, purely palliative” (1).
Isaac Parish documented the first North American case of melanoma in 1837. His patient, a 43-year old widow, was admitted to Wills Hospital in Philadelphia, PA with a “fungous tumor” upon her toe (15). The melanoma was described to have originated from a “purple mark or mole about the size of a mulberry, which was supposed to have been congenital”. Despite the application of a poultice of ground elm, purgatives and leeches to the groin, the patient soon succumbed to her disease (15).
Sir Robert Carswell, a distinguished practitioner of pathology, was credited with coining the word melanoma in 1838. He also contributed depictions of various pathological conditions in his folio volume, Illustrations of the Elementary Forms of Disease, amongst them melanoma (16). This work was notable for including detailed drawings of melanoma metastases, including those from the brain from a patient “between seventy and eighty years of age, brought to the Hotel Dieu of Paris in a state of incomplete paralysis… The paralysis became complete and general and he died in a state of collapse and profound stupor…. Deep brown or black tumors….were found in several organs. The brain contained two in each hemisphere, as large as a hen’s egg” (16).
Many commentators at the time, including Breschet, Fawdington and Carswell, also noted the incidence of melanoma in many species of animal including horses, dogs and cats (16). In 1844, a British surgeon by the name Samuel Cooper published The First Lines of the Theory and Practice of Surgery (17). In 1840, Cooper recognized that the advanced stages of melanomas were untreatable and stated, “the only chance for benefit depends upon the early removal of the disease…” (17), a statement that is still largely true to this day.
In 1853, Sir James Paget, Consulting Surgeon to St. Bartholomew's Hospital, London gave a report on 25 cases of melanoma in which he described the transition of melanoma from a radial growth phase to a vertical growth phase “in some instances the growth is superficial, and the dark spot acquires a larger area and appears slightly raised by some growth beneath it: in other cases the mole rises and becomes very prominent” (18). In 1858, Oliver Pemberton detailed 60 melanoma cases collected from 1820–1857 describing their clinical characteristics and sites of metastases. He was also the first to describe melanoma in a black patient from the island of Madagascar (19).
2) The surgical management of melanoma: from the Victorian era to the present day
In the mid-late 19th century, treatments for melanoma consisted of ligature, excision via knife or scissors, chloride of zinc, extirpation, amputation, or the use of caustic agents to burn the tumor away. Around this time, surgical anesthesia first became available, with a report in the Lancet from 1851 detailing the removal of a recurrent melanoma from a 45-year woman of “melancholy appearance….rendered insensible by chloroform” (20). In 1892, Herbert Snow, a London surgeon advocated in favor of melanoma being treated by excision and anticipatory gland excision (what would now be considered prophylactic or elective lymph node dissection) (21). Snow believed removal of the primary melanoma alone to be ineffective treatment, noting that “it is essential to remove, whenever possible, those lymph glands which first receive the infective protoplasm” (21).
Developments in the surgical management of melanoma continued under the Scottish physician William Handley, a research fellow at the Middlesex Hospital, London. Handley spent two years researching the metastatic dissemination of breast cancer before he analyzed the lymphatic spread of a secondary melanoma deposit on a woman’s leg in 1905 (22). On the basis of one case of metastatic melanoma, he suggested the removal of two inches (or approximately 5 centimeters) of subcutaneous tissue down to the level of muscle fascia along with radical removal of lymph nodes. His work was published in the Lancet in 1907 and helped guide the surgical treatment of melanoma for nearly 50 years (22). Pringle, in 1908, took notice of Handley’s concept of melanoma’s lymphatic spread and was the first to adopt the principle of “excision and dissection in continuity”, removal to encompass the site of primary melanoma, the regional lymph nodes and a wide strip of skin, subcutaneous fat and fascia between the original lesion and the secondarily involved lymph nodes.
Although much of the work from the 19th century was critical in defining melanoma as a disease, the studies performed were descriptive in nature and gave little insight into its underlying etiology and mechanistic basis. The utility of careful observation, coupled with rigorous quantitative methods, proved critical in the latter part of the 20th century in defining melanoma prognosis and treatment. In 1966, Wallace Clark devised a standard scale to assess the prognosis of melanoma based upon histological examination. The system, called Clark’s levels, refers to the extent of downward invasion into five levels of the skin and subcutis, each one more deadly as the tumor cells invade deeper into the epidermis, dermis and ultimately the subcutaneous tissue. 5-year survival rates range from >99% for Clark level I melanoma (melanoma in situ) down to 55% for those presenting with a Clark level V melanoma. In 1969, Clark disproved the earlier widely-held assumption that all melanomas arose from nevi; his series of 209 cases showed that only 20 (9.6%) had the unquestioned presence of nevus cells.
In 1970, Alexander Breslow observed that prognosis of cutaneous melanoma appeared to be a function of both tumor size and level of invasion with tumor thickness the most significant measure of size. The Breslow thickness was defined as the total vertical depth of the melanoma from the granular layer of the epidermis to the area of deepest penetration into the skin. Clark’s level II lesions and lesions less than 0.76 mm in maximal thickness were noted to be associated with a more favorable prognosis. These criteria helped in stratifying patients to have or not have prophylactic lymph node dissection (and now sentinel node biopsy). The pioneering work of Clark and Breslow is still relevant to this day, with tumor thickness being a major prognostic factor for localized melanoma in the current version of the AJCC Melanoma Staging System (23).
Less well recognized is how improvements in histopathologic prognostication led to advances in the surgical management of localized melanoma. As surgeons began to appreciate the greatly improved prognosis of thin melanomas (Clark’s level II or lesions <0.76 mm), it became clear that the very radical resections proposed by Handley and Pringle were excessive (24). This led to a series of randomized, prospective trials that have consistently supported the safety of narrower margins (1 cm for melanomas ≤1 mm and 2 cm for thicker melanomas) and the 5 cm margins advocated based on Handley’s observation have been entirely abandoned. Even more significantly, the relationship between tumor thickness and the likelihood of occult nodal metastasis led to the elimination of prophylactic lymph node removal. In its place, the pioneering technique of sentinel lymph node biopsy, developed by Donald Morton, provided a minimally-invasive way to stage the regional nodes and restrict node dissection to those patients with proven metastases (25, 26). Most recently, the histologic assessment of mitotic activity in the primary melanoma has resurfaced as a key prognostic factor (27), especially in thin lesions (23), and now serves as a guide for identifying thin melanomas at increased risk of nodal metastasis for sentinel node biopsy (28).
Today, histopathological observations, such as those made by Breslow and Clark, are being integrated with genetic data to give a comprehensive view of how oncogenic drivers of melanoma progression influence distinct pathological behavior (29).
3) Melanoma as a genetic disease: susceptibility genes and driver mutations
In 1956, Henry Lancaster, an Australian mathematician, made the first connection between sunlight (ultraviolet (UV) radiation exposure) and the incidence of melanoma. He observed that the risk of melanoma development, particularly in Caucasian populations, was directly associated with ‘latitude’ or the intensity of sunlight (30). Later work from Lancaster and Nelson demonstrated that 5 skin characteristics (skin color, texture, hair color, eye color, and reaction to sun) were of etiological importance in melanoma development (31). The risk of developing melanoma was particularly high when those of English/Celtic ancestry (typically with pale skin and a poor tanning response) migrated to areas of high UV exposure such as Australia, New Zealand and the southern United States.
Investigations into the genetics of mouse coat color led to the discovery that both the tanning response and hair/skin color in humans was regulated through the UV-mediated activation of a G-protein coupled receptor called melanocortin receptor 1 (MC1R) (2). It was later found that MC1R was highly polymorphic and that inherited variants of MC1R predicted both for skin/hair color as well as skin cancer risk in Caucasian populations (32, 33). Biochemical studies showed that the MC1R variants conveying the red hair/pale skin phenotype were associated with inefficient cyclic adenosine monophosphate (cAMP) signaling stimulation upon UV exposure and that this led to an impairment of melanin production in the skin and reduced photoprotection (34). Together, this work provided some mechanistic underpinning for Norris’s observations on the association between pale skin/hair color and melanoma development.
Some of the earliest genetic insights into melanoma etiology came from “melanoma families”, in which heritable germline mutations conveyed increased risk of melanoma development (35, 36). About 5–12% of all melanomas are estimated to be hereditary, with about 40% of these being associated with CDKN2A mutations (37, 38). The CDKN2A gene, located on chromosome 9p21.3, encodes for two genes, p14ARF and p16INK, which regulate cell cycle entry at the G1 checkpoint and stabilize p53 expression. To date, at least 178 melanoma families have been identified with CDKN2A mutations (35). Typically, these patients show multiple nevi and have a family history of melanoma (such as the family described by Norris in 1820). Melanoma predisposition genes continue to be identified, with a study in 2011 detailing a novel germline gain-of-function mutation in the melanocyte lineage specific microphthalmia transcription factor (MITF) at the sumoylation consensus site (E318K) (39).
The discovery of driver mutations in melanoma
One of the most significant intellectual shifts in the understanding of cancer development was the realization that tumors arise following the acquisition of genetic mutations. The first steps towards this came from the experiments of Peyton Rous, who in 1911 showed that purified isolates from chicken sarcoma filtered through sand (to exclude the involvement of bacteria) induced new sarcomas in young chickens (40, 41). At the time, Rous’s findings met with considerable hostility and skepticism, as it was commonly believed that cancers arose endogenously and could not result from a viral infection (41). Although viral infection only accounts for the minority of all human cancers, the work of Rous helped to provide a foundation that eventually linked viral oncogenes to their normal human cellular gene counterparts (proto-oncogenes). In 1977, Joan Brugge and Ray Erikson completed the puzzle of the Rous Sarcoma Virus by identifying the viral gene v-Src as being responsible for its transforming function (42). v-Src was later shown to be a protein tyrosine kinase, and more surprisingly very similar to the same c-Src protein that was expressed ubiquitously in every human cell (43). Together, these and other discoveries led to the “oncogene revolution” and the idea that tumor development occurred following the acquisition of mutations in normal cellular genes.
The early 1980s saw the identification of a new family of oncogenes, the Ras genes (44, 45). These were quite unlike the previously characterized receptor tyrosine kinases (RTKs; such as EGFR and PDGFR) and non-receptor tyrosine kinase (such as Src) and were instead small GTPases, molecular switches that linked the cell surface RTKs to downstream signaling pathways (45). Activating NRAS mutations were first identified in melanoma cell lines in 1984 and subsequently in short-term cell cultures grown from a melanoma patient (46, 47). The growth of multiple cell lines from different lesions on the same patient gave the first indication that melanoma was a genetically heterogeneous disease, with only 1 out of 5 specimens being found to harbor an NRAS mutation (47). Mutations in NRAS, KRAS and HRAS are now known to present in 20%, 2% and 1% of all melanomas, respectively (48). Ras genes acquire their transforming activity following the acquisition of a single point mutation that impairs their GTPase activity and leads to constitutive signaling through the MAPK, PI3K/AKT and Ral-GDS pathways (45, 49). Despite Ras genes being discovered more than 30 years ago, and the subject of an intensive research effort, strategies to therapeutically target these GTPases remain elusive.
Discoveries from the oncogene revolution have continued to shape melanoma biology in the 21st century. In 1983, Ulf Rapp cloned CRAF, the human homolog of the mouse retrovirus M3661-MSV derived v-raf oncogene (50). This was followed in 1986 by the discovery of the closely related ARAF and the 1988 identification of BRAF as the transforming gene in a sample of Ewing’s sarcoma (51, 52). Raf genes were found to encode for serine/threonine kinases that constitute one module of the mitogen activated protein kinase (Ras/Raf/MEK/ERK) signaling cascade (53). The importance of Raf in melanoma was demonstrated in 2002, when a systematic genetic screen identified activating BRAF mutations in the majority of human cutaneous melanomas (54). Most of the BRAF mutations noted (>85%) were a single amino acid substitution of valine for glutamic acid at the 600 position (the BRAF V600E mutation), which conferred constitutive activation of the kinase leading to downstream MAPK signaling (55, 56). Due to a sequencing error, the original mutation was reported at the 599 position (hence the description of V599E in earlier papers) before being later corrected (54). Mutant BRAF has since been shown to the major regulator of oncogenic behavior in ~50% of all melanomas through its effects upon growth, survival and motile behavior (55, 57–60). The identification in 2003 of similar activating BRAF mutations in otherwise unsuspicious nevi helped to solidify the link between nevi and melanoma that had been first suggested in the Victorian era (61). In addition, the observation that most nevi were in a state of permanent growth arrest following the acquisition of mutant BRAF (oncogene induced senescence) provided the mechanistic basis for why so few nevi ultimately progress to full-blown melanoma (62).
NRAS and BRAF mutational status is thought to be prognostic in melanoma, with the presence of a BRAF mutation being predictive for reduced survival in the metastatic setting in at least some series. The typical BRAF mutant melanoma patient is <55 years old and has only an intermittent pattern of acute UV exposure (63). In contrast, NRAS mutant melanoma patients tend to be older with a more chronic pattern of UV exposure. Together, 81% of all melanomas arising on the skin were found to have either a BRAF or an NRAS mutation, with those that were BRAF and NRAS wild-type frequently showing an increased copy number of cyclin dependent kinase-4 (CDK4) and cyclin D1 (64).
The discovery of activating NRAS and BRAF mutations in cutaneous melanoma led to the hunt for driver oncogenes in other histological subtypes of melanoma. It was noted that melanomas arising on the soles of the feet, subungual sites or mucous membranes had low rates of BRAF mutation and were instead often associated with genetic amplification of and/or activating mutations in the receptor tyrosine kinase c-KIT (65). These findings led to the initiation of clinical trials to evaluate imatinib in patients whose melanomas harbored c-KIT aberrations (66). Although responses were observed in some patients, sensitivity to imatinib seemed restricted to a limited subset of KIT mutations (66–68). At this time, other KIT and RTK inhibitors are undergoing clinical evaluation in this melanoma sub-group.
Ocular melanomas were also found to have a low incidence of BRAF mutations and instead exhibited activating mutations in the G-proteins GNAQ and GNA11 (69). Although today the driving oncogenic events have been identified for ~70% of all cutaneous melanomas, there remains a group of ~30% for which the initiating event remains obscure. It is hoped that the ongoing efforts of a number of labs around the world to sequence the entire melanoma genome will shed light upon this remaining mystery (70).
4) The development of medical treatments for melanoma
In the late 19th century cancer treatments were mostly palliative and largely ineffective. An important conceptual development in cancer therapy came from the German physician Paul Ehrlich who first postulated the idea of the “magic bullet”, a chemical compound that would only kill the disease-causing cell or organism, leaving the healthy tissues untouched (5). Although Ehrlich’s approach was initially successful at treating the microorganisms responsible for syphilis, he was unable to make any headway with cancer. His idea that selective chemical agents could eradicate defined sub-populations of cells resurfaced in light of the post-Great War observation that survivors of mustard gas (nitrogen mustard) attacks suffered from prolonged suppression of their bone marrow cells (5). These findings ultimately led to the advent of chemotherapy and the development of drugs such as anti-folates, alkylating agents and microtubule poisons, that selectively killed rapidly growing cells. Unfortunately for the patients receiving these therapies, many other normal cells in the body, such as those of the bone marrow, the hair follicles and the gut lining also showed rapid proliferation, and were similarly susceptible. Chemotherapy regimens became notorious for their noxious side effects and the promise of Ehrlich’s magic bullet was yet to be realized.
In 1968, a review of 650 melanoma cases in the West of England described intra-arterial melphalan as being the most effective systemic treatment available for widely disseminated melanoma (9). Its use was reserved only for advanced cases and was limited by high toxicity and the short-duration of action, typically less than 3 months. The authors concluded at this stage the use of chemotherapy was “difficult to reconcile the use of such….toxic immuno-suppresive agents” (9). The use of radiotherapy was not recommended.
In the decades that followed this report, the situation seemed to change little (71). The FDA-approval of the alkylating agent dacarbazine in 1975 for disseminated melanoma established a new standard treatment, albeit one associated with partial responses at best and median survivals that ranged from 5–11 months and an overall 1-year survival of 27% (72). A 2008 analysis of 3 decades of phase II co-operative group clinical trials revealed little difference in response rates between any investigational agent evaluated and dacarbazine (71). Immunotherapy approaches such as high dose interleukin-2 (IL-2), FDA-approved in 1992, were also only effective in small numbers of patients (6% of patients had complete responses) and were associated with high levels of toxicity (73). However, following the FDA-approval of the anti-CTLA-4 antibody ipilimumab in 2011, it now seems that immunotherapy is efficacious against at least a subset of metastatic melanomas (74, 75).
The ideas that Ehrlich first postulated were finally realized for cancer with the development of imatinib mesylate (Gleevec) for chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) (76, 77). Both of these tumors shared the important characteristic of being “addicted” to one single oncogene: Bcr-Abl in the case of CML and the c-KIT receptor in the case of GIST (77, 78). The ability of imatinib to induce significant levels of levels of tumor regression in CML and GIST offered a new paradigm in which impressive results could be achieved provided the effects of the driver oncogene could be inhibited.
The identification of mutant BRAF as a major driver of melanoma progression raised hopes that BRAF could be to melanoma what BCR-ABL was to CML (79). Initial attempts at targeting BRAF using inhibitors with low potency against the mutant protein (such as sorafenib) met with little success and prompted skepticism over the approach (80–82). A turning point was reached with the development of vemurafenib, a BRAF kinase inhibitor with high specificity for mutant BRAF in preclinical models of melanoma (83, 84). This agent progressed rapidly through early phase trials, with astoundingly rapid and significant levels of tumor shrinkage seen in patients whose melanomas harbored BRAF V600E mutations (85, 86). In a randomized phase III trial of 675 patients, vemurafenib went head-to-head with dacarbazine, the long-standing standard of care for disseminated melanoma (4). Again, the results were striking and the trial was stopped early when a clear survival benefit was seen for vemurafenib. The overall response rate for vemurafenib was 48% compared to 5% for dacarbazine (4). On the basis of this and the equally compelling phase II trial data, vemurafenib was FDA-approved approved in late 2011 as the first ever “mutation specific” therapy for melanoma.
The story of targeting mutant BRAF does not end here. Although the results seen to the BRAF targeted agents were highly encouraging, nearly all patients treated eventually showed resistance and most progressed on therapy (4, 86, 87). The resistance mechanisms observed in melanoma cells following BRAF inhibitor treatment are quite distinct to those seen following targeted therapy treatment in other cancers and secondary BRAF mutations (so-called “gatekeeper” mutations) have been lacking. Instead, a wide variety of potential resistance mechanisms have been reported including BRAF truncations, RTK signaling, NRAS mutations and MEK mutations (88–91). There is also some evidence of multiple different resistance mechanisms occurring concurrently within the same melanoma lesion (88). It thus seems likely that the heterogeneity of melanoma, first observed by Norris in 1820, may complicate future attempts to manage drug resistance and therapeutic escape (47, 92–94). The issue of genetic heterogeneity is likely to be critical in the case of patients who harbor both BRAF mutant and NRAS mutant clones in their melanomas, as BRAF inhibitors have been shown to paradoxically stimulate the growth of NRAS mutant and RTK-driven cancer cell lines (95–97).
The future: therapeutic strategies to achieve durable responses in disseminated melanoma
As BRAF and c-KIT inhibitors continue their development, and new drug combinations become available for cutaneous melanomas with NRAS mutations and ocular melanoma, the issue of managing drug resistance will grow ever more important. Effective strategies to eradicate heterogeneous populations of melanoma cells are urgently needed and may require a greater understanding of therapeutic escape at the single cell level. Although our understanding of melanoma heterogeneity at the molecular level is currently limited there is evidence that minor subsets of melanoma cells exist (such as those expressing the histone demethylase JARID1B), and that these undergo clonal expansion after drug treatment (98). Other work has pointed to the role of epigenetics in the adoption of a drug tolerant state in minor populations of cells that allows for therapeutic escape (99).
The revolution in genomics that shaped research in the 20th century has revealed melanoma to be a heterogeneous group of cancers with distinct mutational profiles. This, together with knowledge about melanoma susceptibility and environmental risk factors has helped to answer some of the questions first posed by Fawdington in 1826. We are still only beginning to understand how the complex constellation of mutations found in any given melanoma determines the disease history and what drug combinations should be used to treat each of these genotypes. We are confident that as we move forward, our rapidly evolving knowledge will allow us to bring melanoma to level of a chronic, manageable disease and not the intractable “black cancer” of old that struck fear into the hearts of those who observed it. Beyond that, perhaps a cure for the majority of patients with disseminated melanoma will not be far behind.
Acknowledgement
The authors would like to thank Dr Jane Messina for her valuable comments and constructive criticism of this manuscript.
Funding: Work in the Smalley lab is supported by The National Cancer Institute (U54 CA143970-01 and R01 CA161107-01), The Harry Lloyd Trust and the State of Florida (09BN-14).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fawdington T. A Case of Melanosis, with General Observations on the Pathology of the Interesting Disease. London: Longman, Orme, Brown, Robinson and Bent; 1826. [Google Scholar]
- 2.Beaumont KA, Wong SS, Ainger SA, Liu YY, Patel MP, Millhauser GL, et al. Melanocortin MC receptor in human genetics and model systems. Eur J Pharmacol. 2011;660:103–110. doi: 10.1016/j.ejphar.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smalley KS, Nathanson KL, Flaherty KT. Genetic subgrouping of melanoma reveals new opportunities for targeted therapy. Cancer Res. 2009;69:3241–3244. doi: 10.1158/0008-5472.CAN-08-4305. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee S. The emperor of all maladies: a biography of cancer. New York Scribner: Scribner; 2010. [Google Scholar]
- 6.Weber J. Immunotherapy for melanoma. Current Opinion in Oncology. 2011;23:163–169. doi: 10.1097/CCO.0b013e3283436e79. [DOI] [PubMed] [Google Scholar]
- 7.Urteaga O, Pack G. On the Antiquity of Melanoma. Cancer. 1966;19:607–610. doi: 10.1002/1097-0142(196605)19:5<607::aid-cncr2820190502>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Home E. Observations on cancer, case VIII. London: 1805. [Google Scholar]
- 9.Bodenham DC. A study of 650 observed malignant melanomas in the South-West region. Ann R Coll Surg Engl. 1968;43 [PMC free article] [PubMed] [Google Scholar]
- 10.Laennec RTH. Extrait au memoire de M Laennec, sur les melanoses. Vol. 1. Paris: Bull L'Ecole Societie de Medicine; 1812. p. 24. [Google Scholar]
- 11.Roguin A. Rene Theophile Hyacinthe Laennec: the man behind the stethoscope. Clin Med Res. 2006;4:230–235. doi: 10.3121/cmr.4.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denkler K, Johnson J. A lost piece of melanoma history. Plast Reconstr Surg. 1999;104:2149–2153. doi: 10.1097/00006534-199912000-00032. [DOI] [PubMed] [Google Scholar]
- 13.Norris W. Case of Fungoid Disease. Edinb Med Surg J. 1820;16:562–565. [PMC free article] [PubMed] [Google Scholar]
- 14.Norris W. Eight cases of Melanosis with pathological and therapeutical remarks on that disease. London: Longman; 1857. [Google Scholar]
- 15.Parrish I. Case of melanosis. Am J Med Sci. 1837;20:266. [Google Scholar]
- 16.Carswell R. Illustrations of the elementary forms of disease. London: Longman, Orme, Brown, Greene and Longman; 1838. [Google Scholar]
- 17.Cooper S. The First Lines of the Theory and Practice of Surgery. London: Longman; 1840. [Google Scholar]
- 18.Paget J. Lectures on Surgical Pathology. London: Longman, Brown, Green and Longman; 1853. p. 639. [Google Scholar]
- 19.Pemberton O. On melanosis. London: Churchill; 1858. [Google Scholar]
- 20.Recurrence of a of a melanotic tumour; removal. Lancet. 1851;1:622. [Google Scholar]
- 21.Snow H. Melanotic cancerous disease. Lancet. 1892;2:872–874. [Google Scholar]
- 22.Handley WS. The pathology of melanotic growths in relation to their operative treatment. Lancet. 1907;1 [Google Scholar]
- 23.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breslow A, Macht SD. Optimal size of resection margin for thin cutaneous melanoma. Surg Gynecol Obstet. 1977;145:691–692. [PubMed] [Google Scholar]
- 25.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Archives of surgery. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 26.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. The New England journal of medicine. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 27.Azzola MF, Shaw HM, Thompson JF, Soong SJ, Scolyer RA, Watson GF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97:1488–1498. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 28.Sondak VK, Taylor JM, Sabel MS, Wang Y, Lowe L, Grover AC, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Annals of Surgical Oncology. 2004;11:247–258. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS medicine. 2008;5:e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancaster HO. Some geographical aspects of the mortality from melanoma in Europeans. Med J Aust. 1956;43:1082–1087. [PubMed] [Google Scholar]
- 31.Lancaster HO, Nelson J. Sunlight as a cause of melanoma; a clinical survey. Med J Aust. 1957;44:452–456. [PubMed] [Google Scholar]
- 32.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nature genetics. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 33.Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Human molecular genetics. 1997;6:1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- 34.Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Human molecular genetics. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- 35.Mize DE, Bishop M, Resse E, Sluzevich J. Familial atypical multiple mole melanoma syndrome. Bethesda, MD: National Center for Biotechnology Information; 2009. [PubMed] [Google Scholar]
- 36.Kraemer K. Dysplastic nevi as precursors to hereditary melanoma. The Journal of dermatologic surgery and oncology. 1983;9:619–622. doi: 10.1111/j.1524-4725.1983.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril MF, et al. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Research. 2006;66:9818–9828. doi: 10.1158/0008-5472.CAN-06-0494. [DOI] [PubMed] [Google Scholar]
- 38.Parker JF, Florell SR, Alexander A, DiSario JA, Shami PJ, Leachman SA. Pancreatic carcinoma surveillance in patients with familial melanoma. Archives of dermatology. 2003;139:1019–1025. doi: 10.1001/archderm.139.8.1019. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama S, Woods SL, Boyle GM, Aoude LG, MacGregor S, Zismann V, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rous P. A Sarcoma of the Fowl Transmissible by an Agent Separable from the Tumor Cells. The Journal of experimental medicine. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin GS. The hunting of the Src. Nature reviews Molecular cell biology. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 42.Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977;269:346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- 43.Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parada LF, Weinberg RA. Presence of a Kirsten murine sarcoma virus ras oncogene in cells transformed by 3-methylcholanthrene. Molecular and Cellular Biology. 1983;3:2298–2301. doi: 10.1128/mcb.3.12.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nature reviews Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 46.Padua RA, Barrass N, Currie GA. A novel transforming gene in a human malignant melanoma cell line. Nature. 1984;311:671–673. doi: 10.1038/311671a0. [DOI] [PubMed] [Google Scholar]
- 47.Albino A, LeStrange R. Transforming ras genes from human melanoma: a manifestation of tumor heterogeneity? Nature. 1984;308:69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- 48.Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS, et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 2010;70:5549–5557. doi: 10.1158/0008-5472.CAN-09-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smalley KS. Understanding Melanoma Signaling Networks as the Basis for Molecular Targeted Therapy. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.177. [DOI] [PubMed] [Google Scholar]
- 50.Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH, Jr, et al. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikawa S, Fukui M, Ueyama Y, Tamaoki N, Yamamoto T, Toyoshima K. B-raf, a new member of the raf family, is activated by DNA rearrangement. Molecular and Cellular Biology. 1988;8:2651–2654. doi: 10.1128/mcb.8.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huebner K, ar-Rushdi A, Griffin CA, Isobe M, Kozak C, Emanuel BS, et al. Actively transcribed genes in the raf oncogene group, located on the X chromosome in mouse and human. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:3934–3938. doi: 10.1073/pnas.83.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 54.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 55.Wellbrock C, Ogilvie L, Hedley D, Karasarides M, Martin J, Niculescu-Duvaz D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–2342. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 56.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Research. 2003;63:5198–5202. [PubMed] [Google Scholar]
- 57.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–2233. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Research. 2011;71:2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 62.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 63.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 64.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 65.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 66.Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, et al. KIT as a therapeutic target in metastatic melanoma. JAMA : the journal of the American Medical Association. 2011;305:2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008 doi: 10.1111/j.1755-148X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 68.Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–2051. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 69.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 72.Yang A, Chapman P. The History and Future of Chemotherapy for Melanoma. Hamatology/Oncology Clinics of N America. 2009;23:583–597. doi: 10.1016/j.hoc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 74.Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nature reviews Drug discovery. 2011;10:411–412. doi: 10.1038/nrd3463. [DOI] [PubMed] [Google Scholar]
- 75.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 77.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 78.Duensing S, Duensing A. Targeted therapies of gastrointestinal stromal tumors (GIST)--the next frontiers. Biochem Pharmacol. 2010;80:575–583. doi: 10.1016/j.bcp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 80.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 81.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 83.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flaherty KT, Puzanov I, Kim KB, Ribas A, MacArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smalley KS, Sondak VK. Melanoma--an unlikely poster child for personalized cancer therapy. N Engl J Med. 2010;363:876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- 87.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whittaker S, Kirk R, Hayward R, Zambon A, Viros A, Cantarino N, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Science translational medicine. 2010;2 doi: 10.1126/scitranslmed.3000758. 35ra41. [DOI] [PubMed] [Google Scholar]
- 91.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011 doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sensi M, Nicolini G, Petti C, Bersani I, Lozupone F, Molla A, et al. Mutually exclusive NRASQ61R and BRAFV600E mutations at the single-cell level in the same human melanoma. Oncogene. 2006;25:3357–3364. doi: 10.1038/sj.onc.1209379. [DOI] [PubMed] [Google Scholar]
- 93.Jovanovic B, Egyhazi S, Eskandarpour M, Ghiorzo P, Palmer JM, Bianchi Scarra G, et al. Coexisting NRAS and BRAF mutations in primary familial melanomas with specific CDKN2A germline alterations. J Invest Dermatol. 2010;130:618–620. doi: 10.1038/jid.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin J, Goto Y, Murata H, Sakaizawa K, Uchiyama A, Saida T, et al. Polyclonality of BRAF mutations in primary melanoma and the selection of mutant alleles during progression. Br J Cancer. 2011;104:464–468. doi: 10.1038/sj.bjc.6606072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the ongenic B-RAF inhibitor, PLX4720, in mutant N-Ras melanoma cell lines. Oncogene. 2010;30:366–371. doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]