Abstract
Three new ruthenium complexes with bidentate chloroquine analogue ligands, [Ru(η6-cym)(L1)Cl]Cl (1, cym = p-cymene, L1 = N-(2-((pyridin-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine), [Ru(η6-cym)(L2)Cl]Cl (2, L2 = N-(2-((1-methyl-1H-imidazol-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine) and [Ru(η6-cym)(L3)Cl] (3, L3 = N-(2-((2-hydroxyphenyl)methylimino)ethyl)-7-chloroquinolin-4-amine) have been synthesized and characterized. In addition, the X-ray crystal structure of 2 is reported. The antimalarial activity of complexes 1–3 and ligands L1, L2 and L3, as well as the compound N-(2-(bis((pyridin-2-yl)methyl)amino)ethyl)-7-chloroquinolin-4-amine (L4), against chloroquine sensitive and chloroquine resistant Plasmodium falciparum malaria strains was evaluated. While 1 and 2 are less active than the corresponding ligands, 3 exhibits high antimalarial activity. The chloroquine analogue L2 also shows good activity against both the choloroquine sensitive and the chloroquine resistant strains. Heme aggregation inhibition activity (HAIA) at an aqueous buffer/n-octanol interface (HAIR50) and lipophilicity (D, as measured by water/n-octanol distribution coefficients) have been measured for all ligands and metal complexes. A direct correlation between the D and HAIR50 properties cannot be made because of the relative structural diversity of the complexes, but it may be noted that these properties are enhanced upon complexation of the inactive ligand L3 to ruthenium, to give a metal complex (3) with promising antimalarial activity.
Introduction
Malaria has been afflicting mankind for 500 000 years, and in the 21st century the disease remains as lethal as ever.1 Recent reports by WHO estimate 234 million cases and 860 000 deaths in 2008, numbers that are representative for recent years.2 Although there is cause for optimism within some areas of the battle against malaria - especially as the funding available for malaria control has increased dramatically - serious obstacles remain, e.g. the resistance to common antimalarial drugs. The question of resistance is now more current than ever, after reports of resistance to artemisinins,3,4 drugs that are part of the recommended first line treatment for infection by the most lethal of the malaria parasites, Plasmodium falciparum.2
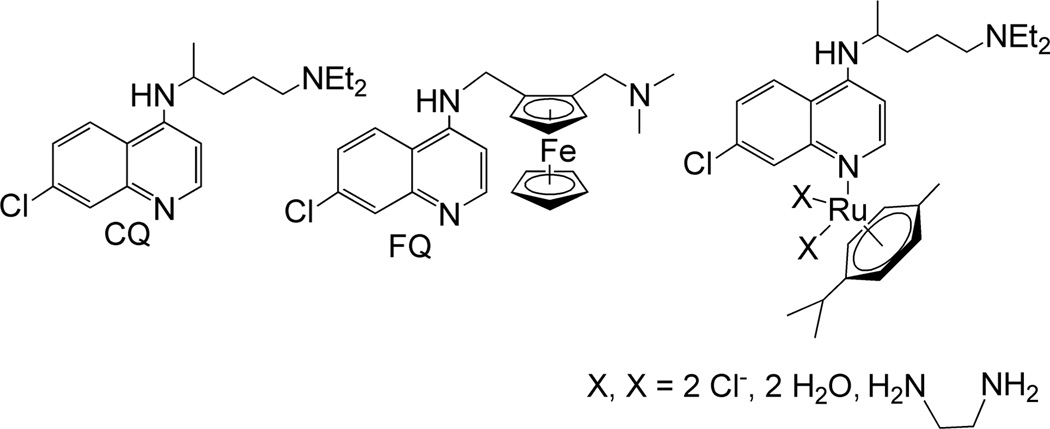
Chloroquine (CQ, Fig. 1) is one of the most successful antimalarial drugs and was for decades the primary chemotherapy used to treat malaria.5 In its erythrocytic phase, the malaria parasite digests red blood cells, releasing lethal free heme. In the acidic food vacuole of the parasite, heme is detoxified through a biomineralization process which converts it into a non-toxic crystalline dimer known as hemozoin.6 The commonly accepted mechanism of action of chloroquine and related 4-aminoquinoline drugs involves binding hematin and inhibiting its conversion into hemozoin.6–11 It has been shown that the 7-chloro-4-aminoquinoline nucleus of chloroquine is responsible for inhibition of hemozoin formation, while the basic quinoline nitrogen and the amine side chain assist accumulation of the molecule in the food vacuole.12,13
Figure 1.
Structures of chloroquine (CQ), ferroquine (FQ) and reported ruthenium arene complexes with chloroquine as ligand.
Widespread resistance has rendered chloroquine useless in most malaria-endemic countries, but it is still the recommended treatment for Plasmodium vivax malarial cases in parts of the world.2,14 Although the mechanism of resistance to chloroquine is not yet fully understood, it seems to be related to mutations in the Plasmodium falciparum chloroquine resistance transporter (PfCRT) transmembrane protein, located at the membrane of the digestive vacuole of the parasite.13,15–18 The mutated protein is able to recognize and expel chloroquine from the food vacuole by mechanisms that are still subject to debate19 and that results in a dramatic decrease of drug concentration at the active site. Therefore new antimalarial drugs capable of overcoming resistance are urgently needed. The ability of the PfCRT protein to extrude chloroquine from the digestive vacuole strongly depends on the lipophilicity of the drug as well as on its structural features.18,20,21 In addition, there is clear evidence indicating that the formation of hemozoin crystals occurs at water/lipid interfaces.22–24 Thus, the design of new antiplasmodial agents aimed at CQ-resistant parasites must take into consideration not only their heme aggregation inhibition activity (HAIA) but also structural and physicochemical factors.
Numerous chloroquine analogues have been synthesized during the last decades; one of the most original, and potentially most successful, being the organometallic derivative ferroquine (Fig. 1).25 Ferroquine exhibits a completely restored activity against resistant parasite strains and has entered phase IIb clinical trials in association with artesunate.25–28 Other reported organometallic and inorganic derivatives of chloroquine include ruthenocene compounds and half sandwich compounds of chromium and rhenium, as well as ruthenium, rhodium and gold coordination complexes.29–35
Ruthenium arene half sandwich complexes have recently received increasing attention as prospective metal-based anticancer drugs and a number of complexes, mostly with nitrogen and phosphorus ligands, are being investigated for their drug candidate potential.36–39 Combining the versatility of ruthenium arene compounds with the interesting properties of metal-chloroquine derivates, a family of ruthenium half sandwich compounds with chloroquine as ligand was recently reported (Fig. 1). These compounds showed an increased antimalarial activity against chloroquine resistant parasite strains compared to free chloroquine, as well as anticancer properties against several tumour cell lines.40 A detailed study of factors relating to the antimalarial mechanism of action of these complexes indicated that the increased activity was likely to originate primarily in the major change in lipophilicity and structure of the Ru complexes as compared to chloroquine itself (see Results and Discussion).41 However, the coordination of chloroquine in these complexes occurred through the quinoline nitrogen (Fig. 1), which has been shown to be crucial for the antimalarial activity.12 Here we report the synthesis and characterization of a new group of ruthenium arene complexes containing chloroquine analogue ligands with N,N- or Schiff base N,O-donor moieties, and an investigation of the antimalarial activity of the ruthenium complexes as well as the ligands. We also report some relevant physicochemical studies of their lipophilicity and heme aggregation inhibition ability in an attempt to better account for the observed biological properties.
Results and discussion
Synthesis and characterization
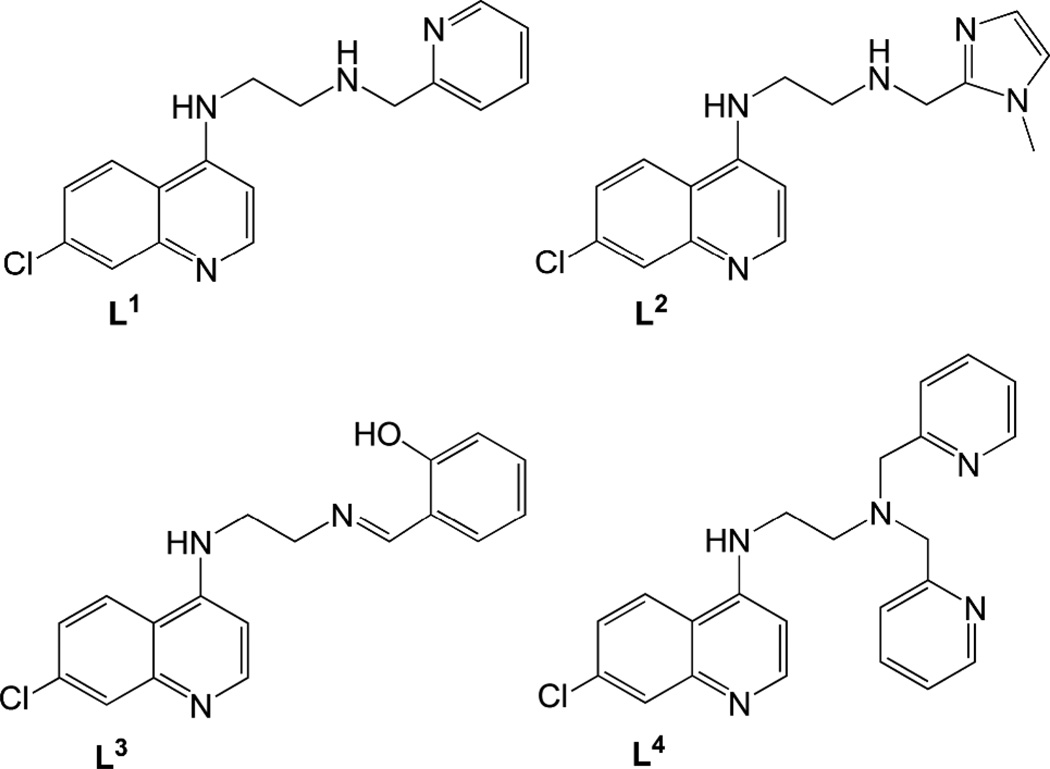
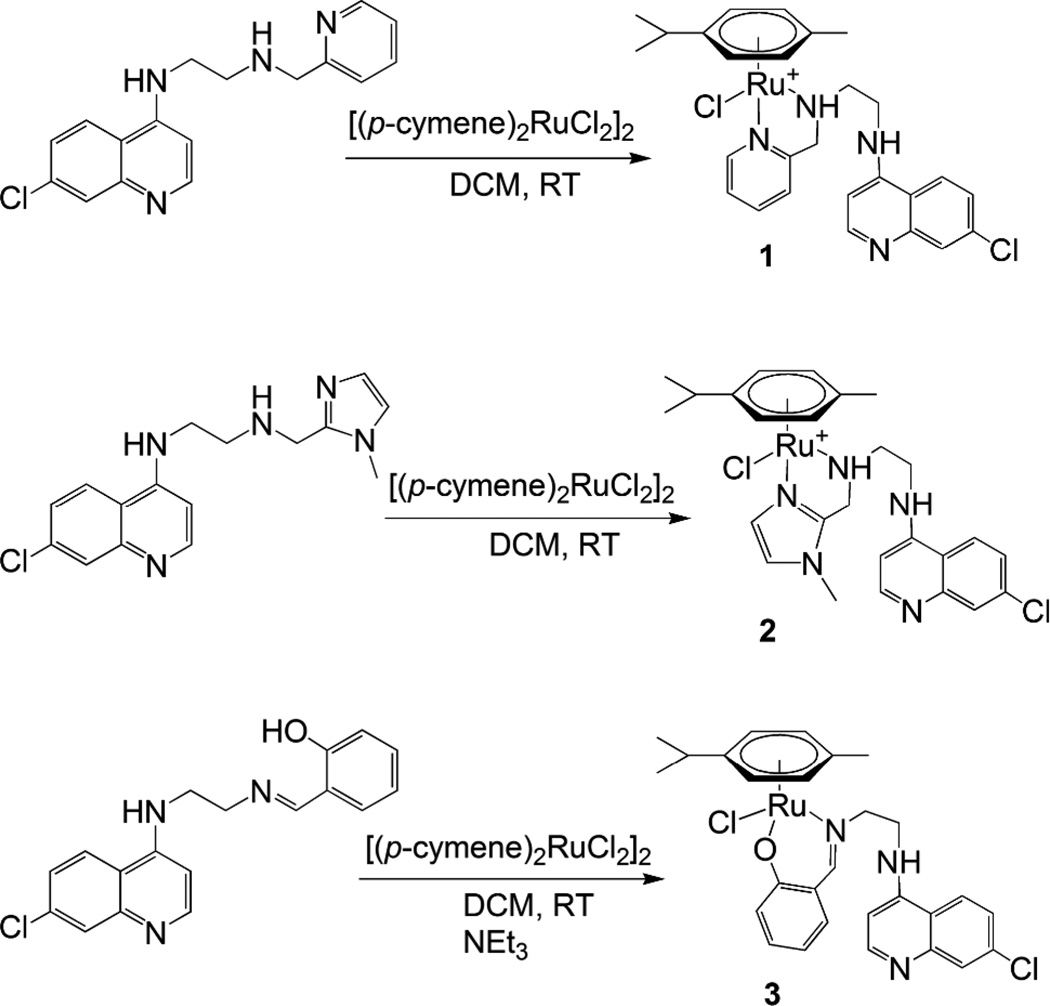
Ligands L1–L4 (Fig. 2) were synthesized following standard synthetic protocols (cf. Experimental section). To synthesize N-(2-((pyridin-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine (L1), N-(2-aminoethyl)-7-chloroquinolin-4-amine and 2-pyridine carboxaldehyde were stirred overnight at room temperature to form the imine, which was in turn reduced with sodium borohydride to give the desired product in good yield. However, when this reaction was carried out with 3 equivalents of aldehyde and sodium tris(acetoxy)borohydride as reducing agent, the disubstituted ligand N-(2-(bis((pyridin-2-yl)methyl)amino)ethyl)-7-chloroquinolin-4-amine (L4) was formed. The ligand N-(2-((1-methyl-1H-imidazol-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine (L2)was synthesized analogously to L1. In contrast, N-(2-((2-hydroxyphenyl)methylimino)ethyl)-7-chloroquinolin-4-amine (L3) was isolated in the imine form, after stirring the starting materials together in ethanol at room temperature. Reaction of ligands L1 or L2 with the [(p-cymene)RuCl2]2 dimer in 2:1 molar ratio in dichloromethane at room temperature gave the complexes [RuCl(p-cymene)(L)]Cl(L = L1(1); L2 (2)) in good yields (Scheme 1). For ligand L3, deprotonation by triethylamine followed by stirring with the ruthenium p-cymene dimer gave the complex [RuCl(p-cymene)(L3)] (3). All complexes were isolated as yellow or orange air-stable solids with good solubility in polar solvents.
Figure 2.
Structures of ligands L1–L4.
Scheme 1.
Synthesis of complexes 1–3.
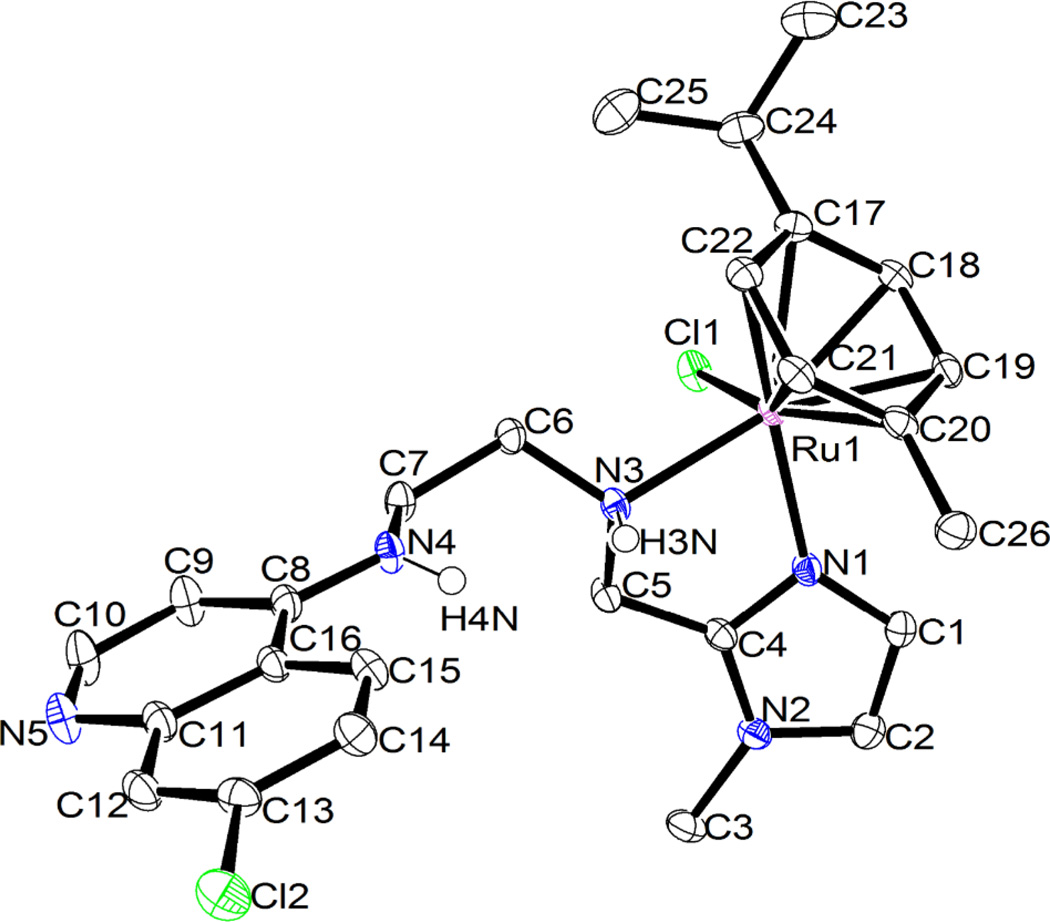
The structure of 2 could be confirmed by single crystal X-ray diffraction (Fig. 3, vide infra). The NMR and mass spectra of 1 and 3 strongly support that the structures of these complexes are analogous to that of 2. The mass spectra of complexes 1–3 show fragmentation patterns that are in agreement with bidentate coordination of ligands L1–L3 (see ESI), and half sandwich (η6-arene)Ru complexes of ligands based on a picolylamine motif that coordinates in a bidentate fashion to the ruthenium atom are previously known.42–44 Similarly, there are previous examples of complexes analogous to 3, with the ligand coordinating to ruthenium through the imine nitrogen and the hydroxyl oxygen.45–47
Figure 3.
ORTEP drawing of the molecular structure of 2·(CH3)2CO, showing the atom numbering scheme. Thermal ellipsoids are drawn at the 50 % probability level. Solvent of crystallization, the counter ion (Cl−) and all hydrogens except those of the secondary amines (N3 and N4) have been omitted for clarity (cf. ESI). The ruthenium centre has the RRu configuration, according to the ligand priority sequence η6-arene > Cl > Nimidazole > Namine, and the chiral aminic nitrogen shows a SN configuration. Relevant bond lengths (Å) and angles (°): Ru-N(1) 2.078(1); Ru-N(3) 2.169(1); Ru-Cl(1) 2.422(3); Ru-arene centroid 1.66; N(1)-Ru-N(3) 76.47(5); N(1)-Ru-Cl(1) 87.07(3); N(3)-Ru-Cl(1) 85.31(3).
NMR spectroscopy
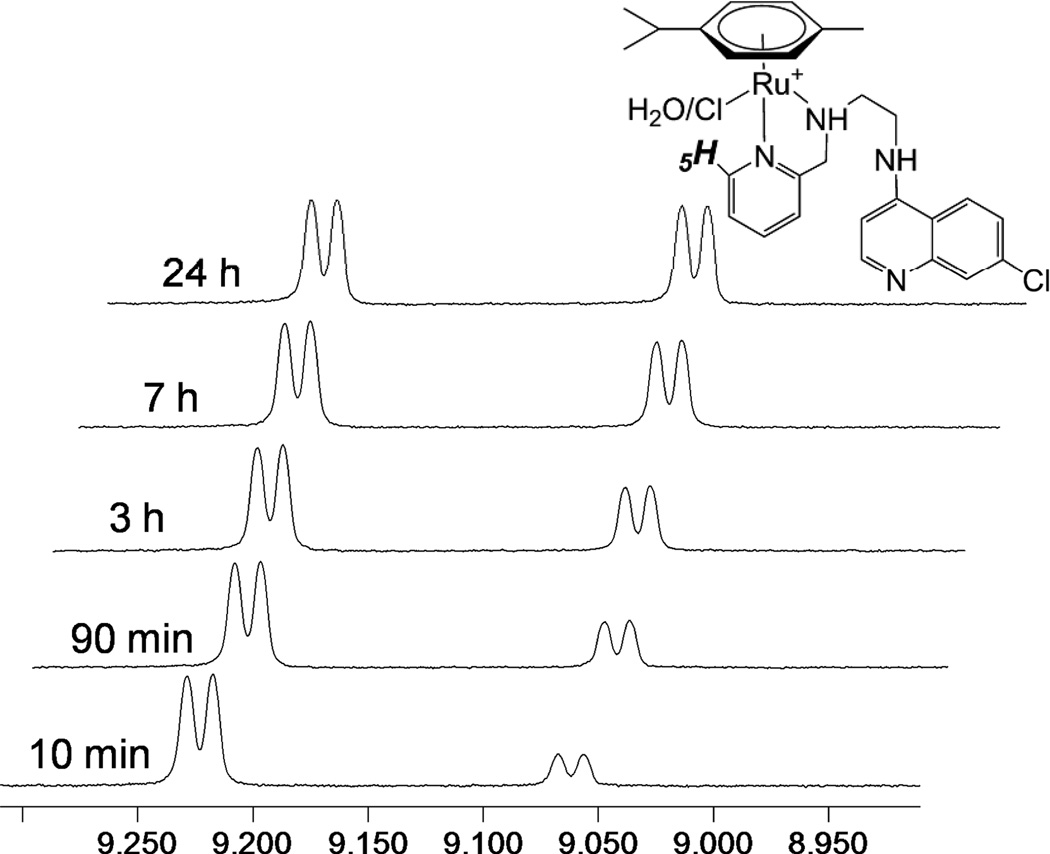
Unsymmetrical N,N- and N,O-donor ligands induce chirality at the metal atom upon coordination to the (η6-p-cymene)Ru fragment, resulting in loss of two-fold symmetry of the p-cymene moiety. Hence, in the 1H NMR spectra of 1–3 the aromatic protons of the p-cymene are observed as four separate doublets and the isopropyl group as two doublets, one for each methyl group. For 1 and 2, the methylene protons adjacent to the coordinated amine show diastereotopic splitting, consistent with a bidentate coordination mode. In the 1H NMR spectrum of 1 in CDCl3, one set of peaks was present, but upon dissolution of 1 in DMSO-d6 two sets of signals of different relative intensities were observed. The less intense signal set increased with time until equilibrium was reached, after which the distribution of species remained stable; this is demonstrated in Figure 4, which shows the emergence of the second set of signals for the H5 proton of the pyridine moiety over time. A similar behaviour was observed in CD3OD. The solution behaviour of 2 differed slightly from that of 1, as the emergence of the second set of signals in the 1H NMR spectrum was slower. Five days after dissolution in DMSO-d6, the relationship between the two species was 1:0.17 for 2, while for 1 a 1:1 relationship was reached within 24 h. It is possible that this difference may be related to the larger ring strain of the imidazole relative to the pyridine. In both cases, both sets of signals differed from those of the free ligand, so decomplexation was ruled out as a possible explanation for the observed equilibrium. Complex 3 displayed only one set of signals in DMSO-d6, as well as in CDCl3. The most likely explanation for the observed solution behaviour of 1 and 2 is the exchange of the coordinated chloride for a molecule of solvent (DMSO, MeOH, vide infra). The alternative explanation that the two observed species correspond to two diastereomers may be ruled out since only one species is observed in CDCl3.
Figure 4.
H5 region of the 1H NMR spectrum of complex 1 at 10 min, 90 min, 3 h, 7 h and 24 h after dissolution in d6-DMSO.
Conductivity measurements
In order to gain further knowledge of the behaviour of the new complexes in solution and better interpret the NMR spectroscopy results, their electrical conductance was measured. Molar conductivity values of 279 and 293 ohm−1cm2mol−1 for 1 mM aqueous solutions of 1 and 2, respectively, are within the normal range for three ions and indicate that these two complexes undergo rapid hydrolysis to form [Ru(p-cymene)(ligand)(H2O][Cl]2; these conductivity values remain essentially unchanged after 3 h (299 and 306 ohm−1cm2mol−1 for 1 and 2, respectively). For complex 3, a conductivity value of 249 ohm−1cm2mol−1 (or 266 ohm−1cm2mol−1 after 3 h) also indicates the presence of three ions in solution, rather than the two expected for aquation of the complex to yield [Ru(p-cymene)(L3)(H2O)][Cl]; a possible explanation for this unusual value is that the phenoxy group of the ligand is detached in solution and replaced by a second water molecule. The 1H NMR spectrum of 3 in D2O shows that the ligand is no longer bidentate, as the p-cymene peak pattern is that of a symmetric complex (i.e. two doublets for the aromatic protons). Signals for the coordinated ligand of 1:1 intensity to the p-cymene are also observed.
More interesting, the molar conductivity values of 1 mM solutions of the various complexes in DMSO, the solvent in which two sets of NMR signals were observed (and which was used in biological experiments, see Experimental section), are 48.3 ohm−1cm2mol−1 for 1 and 52.1 ohm−1cm2mol−1 for 2, respectively. These values are intermediate between those corresponding to two and three ions,48,49 which indicates partial displacement of the coordinated chloride by DMSO to produce equilibrium mixtures of [RuCl(p-cymene)(ligand)][Cl] and [Ru(p-cymene)(ligand)(DMSO)][Cl]2 (Eq. 1). Such equilibria are fully consistent with the two independent sets of signals appearing in the NMR spectra of 1 and 2. On the other hand, the conductivity of 3 (33.8 ohm−1cm2mol−1) corresponds to two ions in solution, most likely resulting from full displacement of the chloride to yield exclusively [Ru(p-cymene)(L3)(DMSO)]Cl (Eq. 1).
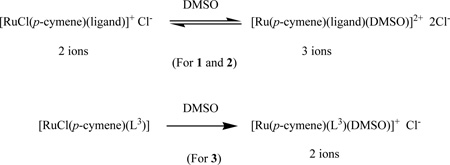 |
Eq.1 |
X-ray crystallography
The ruthenium complex 2 was characterised by X-ray crystallography. Suitable crystals were grown from acetone at −20 °C. The molecular structure of 2 is shown in Fig. 3 and relevant crystallographic data are summarized in Table 1. To our knowledge, this crystal structure is unique in being the first half sandwich complex with a pendant 4-amino-7-chloroquinoline moiety; the only previously reported structures are of metallocene aminoquinoline conjugates.30 The complex crystallizes as a racemate, and each molecule possesses the expected piano stool geometry, with the two nitrogens of the imidazole and neighbouring amine and the chloro ligand as the legs of the stool. The counter ion Cl− (omitted in Fig. 3) is engaged in hydrogen bonding with the two NH groups framing the ethylene spacer (cf. ESI). There are very few examples of piano stool ruthenium arene complexes with a bidentate imidazole ligand,50,51 but the molecular structure of 2 closely resembles corresponding picolylamine complexes with respect to the immediate coordination environment of the metal.42,43,45 As is expected in this class of structures, the arene ring of the coordinated p-cymene is essentially planar, with the distance between the ruthenium atom and the p-cymene ring centroid being 1.66 Å and Ru-Carene bonds ranging between 2.165(2) and 2.213(2) Å. This agrees well with similar complexes.43,52,53 The Ru-Cl, Ru-Namine and Ru-Nimidazole bonds (2.422(3), 2.169(1) and 2.078(1) Å, respectively) are also similar to distances reported for related complexes. The Ru-Namine bond is longer than the Ru-Nimidazole bond, a pattern that mirrors corresponding picolylamine structures.42,43,45
Table 1.
Crystal data for 2·(CH3)2CO.
| 2 | |
|---|---|
| empirical formula | C29H38Cl3N5ORu |
| fw | 680.06 |
| temp (K) | 100(2) |
| λ(Å) | 0.71073 |
| cryst syst | Triclinic |
| space group | P 1̄ |
| a (Å) | 11.3740(4) |
| b (Å) | 12.0484(4) |
| c (Å) | 12.0484(4) |
| α (deg) | 99.6620(10) |
| β (deg) | 106.562(2) |
| γ (deg) | 114.2640(10) |
| V (Å3) | 1511.68(12) |
| Z | 2 |
| ρcalc (Mg/m3) | 1.494 |
| µ(Mo Kα) (mm−1) | 0.815 |
| No. reflns. | 33271 |
| Unique reflns. | 10277 |
| GOOF (F2) | 1.023 |
| Rint | 0.0260 |
| R1a (I ≥ 2σ) | 0.0277 |
| wR2b (I ≥ 2σ) | 0.0609 |
R1 = Σ||Fo| − |Fc||/Σ|Fo|.
wR2 = [Σ[w(Fo2 − Fc2)2]/Σ[w(Fo2)2]]1/2.
Antimalarial activity and cytotoxicity towards normal mammalian cells
To our knowledge, the antimalarial activities of ligands L1–L4 have not been reported previously.54 Therefore, the antimalarial activities of complexes 1–3 as well as of ligands L1–L4 were evaluated in vitro against chloroquine sensitive (D10) and chloroquine resistant (Dd2) Plasmodium falciparum strains (Table 2). Ligand L4 was included in the antimalarial study as a comparison to L1, even though a corresponding metal complex had not been synthesized. The standard drug chloroquine diphosphate was used as reference in all assays. Ligands L1 and L2 show good activity against both parasite strains, with the IC50-values for L1 (against D10) being just above those for chloroquine. Ligand L4 exhibited medium activity against both strains (resistance index, RI = 1.0, cf. Table 2), while L3 was active against the chloroquine sensitive (CQS) strain, but inactive against the chloroquine resistant (CQR) strain. Complexes 1 and 2 showed medium activity against D10 (CQS) and 3–4 times lower activity against Dd2 (CQR, relative to D10), while complex 3 is very active against the CQS strain, though less active against the CQR strain. The complexes and ligands were screened for cytotoxicity against a mammalian cell line, Chinese Hamster Ovarian (CHO), with emetine as reference drug. Ligands L1 and L2 showed low cytotoxicity (IC50 = 144 µM and IC50 = 240 µM, Table 2), while L3 and L4 showed no measurable cytotoxicity at the highest tested concentration. Correspondingly, complexes 1 and 2 also showed no cytotoxicity at the highest tested concentration. Complex 3 showed higher cytotoxicity than the other two complexes (IC50 = 89 µM). In summary, all tested samples exhibited low or no cytotoxicity.
Table 2.
IC50-values of L1–L4 and 1–3 tested in vitro for antiplasmodial activity against chloroquine sensitive (D10) and chloroquine resistant (Dd2) strains of P. falciparum and for cytotoxicity on Chinese Hamster Ovarian (CHO) cells.
| Compound | D10: IC50 (µM) | Dd2: IC50 (µM) | CHO: IC50 (µM) | RIa | SIb |
|---|---|---|---|---|---|
| L1 | 0.03 ± 0.002 | 0.22 ± 0.03 | 144 ± 50 | 7.3 | 4800 |
| L2 | 0.19 ± 0.03 | 0.25 ± 0.06 | 240 ± 65 | 1.3 | 1263 |
| L3 | 0.55 ± 0.30 | >30 | >300 | ND | ND |
| L4 | 1.3 ± 0.1 | 1.3± 0.1 | >240 | 1.0 | ND |
| 1 | 7.69 ± 0.07 | 21.9 ± 1.0 | >160 | 2.8 | ND |
| 2 | 4.53 ± 0.69 | 19.7 ± 0.4 | >160 | 4.3 | ND |
| 3 | 0.07 ± 0.007 | 1.3 ± 0.1 | 89 ± 19 | 18.6 | 1271 |
| CQ | 0.025 ± 0.009 (n = 7) | 0.143 ± 0.014 (n = 3) | − | ||
| Emetine | - | - | 0.25 ± 0.03 (n = 2) |
ND = not determined, n = number of data sets averaged
RI (Resistance Index) = IC50 Dd2/ IC50 D10
SI (Selectivity Index) = IC50 CHO/IC50 D10
Ligands L1 54 and L2 both show promising antimalarial activity, even though they are less active than chloroquine against the CQR strain. Ligand L2 is previously unreported and exhibits very low toxicity against mammalian cells (Selectivity index = 1263, cf. Table 2). Compared to the corresponding ligands, the complexes [RuCl(p-cymene)(L)]Cl (L = L1 (1); L2 (2)) had significantly lower antimalarial activity. This is not surprising, since by blocking two of the nitrogens of the ligand, the ruthenium fragment in 1 and 2 might impede the ability of the complexes to accumulate in the food vacuole, resulting in low activity. However, for complex [RuCl(p-cymene)(L3)] (3) the relationship is reversed; the activity of the complex is notably higher than that of the ligand. The resistance index for 3 is high (RI = 18), but this is most likely a consequence of the lack of activity against the CQR strain for L3. The activity of 3 is considerably higher than that of 1 and 2 (particularly against the CQS strain). Conductivity data (vide supra) suggest that the behaviour of 3 in aqueous solution differs slightly from that of 1 and 2, which might be related to activity.
Even though firm conclusions cannot be drawn based on results from two parasite strains, a few points are worth noticing regarding the cross-resistance with chloroquine of the reported complexes and ligands.55 Ligand L1 is fully cross-resistant with chloroquine, but exchange of the terminal pyridine group for a methylimidazole as in L2 results in a compound that is not cross-resistant. Most notable is the very high resistance of the Dd2 strain to L3, which could suggest that L3 is transported out of the food vacuole of the parasite to a higher extent than the other ligands. The level of resistance to L3 is only partly decreased by metal complexation. These results suggest that coordination to a metal atom may limit cross-resistance with chloroquine, but does not necessarily eliminate it. More importantly, the antimalarial activity data of L3 and 3 indicates that attachment of the bulky metal group to L3 does not prevent interaction with the chloroquine resistance transporter, PfCRT. The limited number of ligands and corresponding ruthenium arene complexes prevents a clear correlation between structure and cross-resistance.
Heme aggregation inhibition activity (HAIA) and water-octanol distribution coefficients
As mentioned in the introduction, the design of new antiplasmodial agents aimed at overcoming chloroquine resistance must take into consideration the heme aggregation inhibition activity (HAIA), as well as relevant physicochemical factors, such as lipophilicity. The antimalarial potential of the Ru-chloroquine complexes depicted in Fig. 1 against resistant strains of the Plasmodium falciparum parasite has been demonstrated.41,56 The incorporation of a metal centre induced important structural modifications together with an increase in the lipophilicity of the drug and an enhancement of the HAIR50 values (drug to hemin ratio required to inhibit 50% of heme aggregation). This combination of features were claimed to be responsible for the high activity observed against resistant parasites. However, complexation of the metal to chloroquine (CQ) in those cases took place through the quinoline nitrogen, which resulted in an undesirable decreased basicity of the new drugs, a crucial property for pH trapping in the acidic vacuole. In the new series of complexes reported herein, Ru(II) is coordinated to the chloroquine analogues in a way that does not block the basic quinoline nitrogen, while the enhancement of the lipophilicity compared to the free ligand is still possible, and they were thus expected to display interesting biological properties. It was therefore important to gather experimental data on the above-mentioned physicochemical features of the new compounds.
The results of water-octanol distribution coefficients (D) as a measure of lipophilicity, and HAIR50 measurements are shown in Table 3 for the ligands and metal complexes, in comparison with chloroquine. Most of the ligands and their Ru complexes display an increased lipophilicity at the acidic conditions of the digestive vacuole (pH 4.9) with respect to chloroquine, which might be a good indicator of activity against resistant parasites. On the other hand, L1 shows similar lipophilicity to chloroquine, whereas 2 is clearly more hydrophilic. Moreover, with the only exception of L3, all the ligands and the corresponding metal complexes are more than twice as potent as chloroquine in their HAIA ability at an aqueous acetate/n-octanol interface,41 as seen by the values of HAIR50. Interestingly, the HAIA ability is also enhanced with reference to the Ru-chloroquine complexes previously described by us (Fig. 1),41,56 again with the sole exception of L3.
Table 3.
Results for heme aggregation inhibition at an acetate buffer (pH=4.9)/n-octanol interface (HAIR50) and water/n-octanol distribution coefficient (D) measurements.
| Compound | HAIR50a | D(pH=4.9)b | D(pH=6.6) |
|---|---|---|---|
| L1 | 0.95 | 0.15 ± 0.01 | 4.24 ± 0.27 |
| 1 | 1.00 | 0.28 ± 0.04 | 8.84 ± 0.82 |
| L2 | 1.05 | 1.68 ± 0.09 | 2.82 ± 0.16 |
| 2 | 0.86 | 0.007 ± 0.002 | 2.06 ± 0.78 |
| L3 | 3.45 | 0.35 ± 0.02 | 5.91 ± 0.48 |
| 3 | 1.20 | 0.40 ± 0.02 | 1.99 ± 0.10 |
| L4 | 0.87 | 3.41 ± 0.62 | 4.39 ± 0.54 |
| CQc | 2.92 | 0.15 ± 0.01 | 6.61 ± 0.64 |
HAIR50 is the drug to hemin ratio required to inhibit 50% of heme aggregation in comparison to a control experiment with no drug.
D(pH) = [compound] in n-octanol/[compound] in water at the given pH.
As reported in ref.41
Due to the high number of variables introduced in the design of the new drug candidates tested (presence or absence of metal moiety, charge and/or substantially different structural features) an internal correlation among the new family of chloroquine analogues and/or their metal complexes regarding the HAIA ability and lipophilicity is not obvious. However, it may be noted that complexation to the metal did not induce a significant modification of the HAIA or an increase in lipophilicity at pH 4.9 of the ligands in the pairs L1-1, L2-2, contrary to what was previously observed for other Ru-CQ drug candidates,41,56 and contrary to the results obtained for L3-3.Furthermore, no clear correlations were found between HAIR50 or lipophilicity with the antiplasmodial activity against CQS or CQR Plasmodium falciparum parasites. Again, this is most likely a consequence of the complexity of the systems, i.e. the fact that the final antimalarial activity is the result of a multifaceted balance of heme aggregation inhibition activity, lipophilicity, structural features, solubility and general pharmacokinetic behaviour when the drug candidates are within biological systems. However, it is again worth noting the case of L3 and 3. The complexation of an inactive ligand, which results in a notable increase of the HAIA ability at the interface and a slight increase of lipophilicity at the acidic vacuole conditions, gives a metal compound with a significantly enhanced antimalarial activity. This indicates that, for the series of compounds reported herein, only when the incorporation of a metal moiety induces important changes in the lipophilicity and/or the HAIA ability of an inactive ligand, there is a good correlation with the differences observed in the final overall antiplasmodial activity of the complex.
Another feature which might contribute to the increased activity of 3 relative to L3 is aqueous solubility at physiological pH. Coordination to an arene metal unit as a means to increasing aqueous solubility and bioavailability of ligands with known biological activity has been documented in the literature.42,57 Even though lipophilicity at vacuolar pH increases upon coordination of L3 to the metal fragment, lipophilicity at pH 6.6 decreases. As L3 is virtually insoluble in water at neutral pH, the increased aqueous solubility of 3 could be one of the factors involved in the higher antimalarial activity.
Conclusion
A small family of ruthenium arene half sandwich complexes with chloroquine analogue ligands was synthesized and characterized by NMR spectroscopy, mass spectrometry and, in one case, X-ray crystallography. These complexes differ from previously reported ruthenium complexes with antimalarial activity, as the quinoline group is pendant, leaving the quinoline nitrogen uncoordinated. In the case of the ligands L1 and L2, coordination to the metal fragment did not result in increased antimalarial activity, but these ligands themselves displayed significant activity. However, complex 3 was considerably more active than L3 and showed good activity, particularly against the chloroquine sensitive strain of P. falciparum. The lipophilicity and heme aggregation inhibition activity at an aqueous buffer/n-octanol interface (HAIR50) were measured for all ligands and complexes but a general correlation with the in vitro antimalarial activity could not be established. In the case of the L3-3 couple, the enhancement of the antiplasmodial activity upon coordination to a metal centre was related to an increase in lipophilicity and the HAIA ability at the buffer/n-octanol interface. The high antimalarial activity of 3 is a promising result that warrants more research, including further study of the structure activity relationships of (η6-arene)Ru complexes with bidentate chloroquine analogue ligands. It is conceivable that structural optimization of L3 could give a complex with high activity also against chloroquine resistant strains.
Experimental
General
All synthetic procedures were performed under dry nitrogen using standard Schlenk and vacuum-line techniques. Solvents used were dried by distillation over appropriate drying reagents and stored over molecular sieves under nitrogen. All chemicals were purchased from Sigma Aldrich and used as received. [(p-cymene)RuCl2]258 and N1-(7-chloroquinolin-4-yl)ethane-1,2-diamine59 were prepared according to literature methods. NMR spectra were recorded on a Varian Inova 500 MHz spectrometer using the solvent resonance as internal standard for 1H NMR and 13C NMR shifts. Infrared spectra were recorded on a Nicolet Avatar 360 FT-IR spectrometer. Electrospray ionization (ESI) and high resolution mass spectra were recorded using a Waters Micromass Q-Tof micro mass spectrometer or an LC-MS Agilent 6220-TOF coupled with 1200 series HPLC. Conductivity values were obtained using 1 mM solutions of the complexes in water or DMSO at various time intervals using an Oaklon pH/Conductivity meter. Elemental analysis was performed by Mikroanalytische Laboratorium Kolbe, Mülheim an der Ruhr.
X-ray Structure Determinations
The crystal was immersed in cryo-oil, mounted in a Nylon loop, and measured at a temperature of 100 K. The X-ray diffraction data were collected on a Bruker AXS Kappa ApexII Duo diffractometer using Mo Kα radiation (λ= 0.71073 Å). The APEX260 program package was used for cell refinements and data reductions. The structures were solved by direct methods using SHELXS-97.61 A semi-empirical absorption correction based on equivalent reflections (SADABS)62 was applied to the data. Structural refinements were carried out using SHELXL-97.61 The NH hydrogen atoms were located from the difference Fourier map but constrained to ride on their parent atom, with Uiso = 1.5·Ueq(parent atom). Other hydrogen atoms were positioned geometrically and were also constrained to ride on their parent atoms, with C–H = 0.95–1.00 Å, and Uiso = 1.2–1.5 ·Ueq(parent atom). The crystallographic details are summarized in Table 1.
Syntheses
N-(2-((pyridin-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine (L1)
N1-(7-chloroquinolin-4-yl)ethane-1,2-diamine (0.800 g, 3.6 mmol) was dissolved in methanol (30 mL). 2-pyridinecarboxaldehyde (0.34 mL, 3.6 mmol) was added dropwise and the solution was stirred for 16 h at room temperature. Sodium borohydride (0.272 g, 7.2 mmol) was added and the reaction mixture was stirred for an additional hour. The solvent was removed in vacuo and the residue was dissolved in EtOAc (150 mL). The organic phase was washed with brine (3×50 mL), dried over Na2SO4 and evaporated. The product was purified by silica gel flash chromatography eluting with ethyl acetate-methanol-triethylamine 8:1:1 (v/v). Product was obtained as a beige solid (0.876g, 81%). 1H NMR (500 MHz, CDCl3) δ 8.58 (d, 1H, J=4.4Hz), 8.52 (d, 1H, J=5.3Hz), 7.95 (d, 1H, J=2.1Hz), 7.81 (d, 1H, J=8.9Hz), 7.65 (dt, 1H, J=1.7Hz, J=7.7Hz), 7.36 (dd, 1H, J=2.1Hz, J=8.9Hz), 7.27 (d, 1H, J=8.0Hz), 7.19 (dd, 1H, J=5.1Hz, J=6.7Hz), 6.38 (d, 1H, J=5.4Hz), 6.09 (br s, 1H), 3.99 (s, 2H), 3.37 (m, 2H), 3.08 (m, 2H), 2.05 (br s, 1H); 13C NMR (125 MHz, CDCl3) δ 159.2, 151.9, 150.0, 149.3, 149.0, 136.6, 134.8, 128.5, 125.1, 122.4, 122.2, 121.5, 117.4, 99.1, 54.2, 47.0, 42.3; IR (KBr) vmax/cm−1 3221m (br), 3067w, 2962w, 2839w, 1613m (7-chloroquinoline), 1582s (7-chloroquinoline), 1452m, 1431m, 1380m, 1333w, 1282w, 1249w, 1204w, 1140w, 1112w, 1082w, 849w, 807w, 763m; HRMS (ES+) m/z calcd. for C17H18N4Cl 313.12145, found 313.12215.
N-(2-((1-methyl-1H-imidazol-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine (L2)
N1-(7-chloroquinolin-4-yl)ethane-1,2-diamine (0.300 g, 1.35 mmol) and 1-methyl-2-imidazole carboxaldehyde (0.150 g, 1.35 mmol) were dissolved in methanol (30 mL) and a few molecular sieves (3Å) were added. The solution was stirred for 18 h at room temperature. Sodium borohydride (0.102 g, 2.7 mmol) was added and the reaction mixture was stirred for an additional hour. Molecular sieves were filtered off and the solvent was removed in vacuo. The residue was dissolved in EtOAc (50 mL). The organic phase was washed with brine (3×25 mL), dried over Na2SO4 and evaporated. Product was obtained as a white/yellow solid (0.396g, 92%). 1H NMR (500 MHz, CD3OD) δ 8.34 (d, 1H, J=5.6Hz), 8.09 (d, 1H, J=9.0Hz), 7.77 (d, 1H, J=2.1Hz), 7.39 (dd, 1H, J=2.2Hz, J=9.0Hz), 6.98 (d, 1H, J=1.3Hz), 6.86 (d, 1H, J=1.3Hz), 6.52 (d, 1H, J=5.7Hz), 3.90 (s, 2H), 3.68 (s, 3H), 3.46 (t, 2H, J=6.3Hz), 2.96 (t, 2H, J=6.3Hz); 13C NMR (125 MHz, CD3OD) δ 152.8, 152.5, 149.7, 147.6, 136.4, 127.6, 127.1, 126.1, 124.4, 123.0, 118.8, 99.8, 47.9, 45.2, 43.5, 33.1; IR (KBr) vmax/cm−1 3260m (br), 3255m, 3063w, 2961w, 2832w, 1613m (7-chloroquinoline), 1581s (7-chloroquinoline), 1549m (7-chloroquinoline), 1452m, 1429m, 1376m, 1289m, 1250w, 1216w, 1138m, 1106m, 1082w, 960w, 852m, 812w, 729m; HRMS (ES+) m/z calcd. for C16H19N5Cl 316.1329, found 316.1340.
N-(2-((2-hydroxyphenyl)methylimino)ethyl)-7-chloroquinolin-4-amine (L3)
N1-(7-chloroquinolin-4-yl)ethane-1,2-diamine (2.026 g, 9.14 mmol) was dissolved in ethanol (90 mL). Salicylaldehyde (0.95 mL, 8.96 mmol) was added dropwise and the solution was stirred at room temperature for 45 min. The solvent was evaporated and the residue was washed with cold ethanol and petroleum ether. The product was obtained as a yellow solid (2.24 g, 77%). 1H NMR (500 MHz, CDCl3) δ 12.99 (br s, 1H), 8.58 (d, 1H, J=5.3Hz), 8.37 (s, 1H), 7.97 (d, 1H, J=0.9Hz), 7.60 (d, 1H, J=8.9Hz), 7.36 (m, 2H), 7.22 (d, 1H, J=7.5Hz), 6.98 (d, 1H, J=8.3Hz), 6.89 (t, 1H, J=7.5Hz), 6.51 (d, 1H, J=5.3Hz), 5.20 (br s, 1H), 3.95 (m, 2H), 3.74 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 167.2, 160.9, 152.0, 149.2, 149.1, 135.1, 132.8, 131.6, 129.0, 125.7, 120.7, 119.0, 118.5, 117.2, 117.1, 99.3, 57.7, 43.5; IR (KBr) vmax/cm−1 3405s (O–H), 2940w, 2855w, 1630m (N=C), 1613m (7-chloroquinoline), 1578s (7-chloroquinoline), 1541w (7-chloroquinoline), 1508w, 1449w, 1425w, 1379w, 1278m, 1210w, 1143w, 895w, 761m; HRMS (ES+) m/z calcd. for C18H17N3OCl 326.1060, found 326.1059.
N-(2-(bis((pyridin-2-yl)methyl)amino)ethyl)-7-chloroquinolin-4-amine (L4)
N1-(7-chloroquinolin-4-yl)ethane-1,2-diamine (0.200g, 0.9 mmol) was dissolved in dichloromethane (12 mL). 2-pyridinecarboxaldehyde (0.17 mL, 1.8 mmol) was added dropwise and the solution was stirred at room temperature for 30 min. Sodium tris(acetoxy)borohydride (0.900g, 4.1 mmol) was added and the reaction mixture was stirred for 17 h. A second portion of sodium triacetoxyborohydride (0.290 g, 1.3 mmol) was added and the stirring was continued for 3 h. Saturated aqueous Na2CO3 (7 mL) was added and when gas evolution subsided, the organic phase was washed with additional aqueous Na2CO3 (2×10 mL). The organic phase was dried over Na2SO4 and evaporated. The crude product was purified by silica gel flash chromatography eluting with ethyl acetate-methanol-triethylamine 83:07:10 (v/v). The pure product was obtained as a brown oil (0.221 g, 61%). 1H NMR (500 MHz, CDCl3) δ 8.57 (ddd, 2H, J=0.8Hz, J=1.6Hz, J=4.9Hz), 8.46 (d, 1H, J=5.4Hz), 8.32 (d, 1H, J=9.0Hz), 7.97 (d, 1H, J=2.1Hz), 7.56 (dt, 2H, J=1.8Hz, J=7.7Hz), 7.48 (br s, 1H), 7.45 (dd, 1H, J=2.2Hz, J=8.9Hz), 7.36 (d, 2H, J=7.8Hz), 7.15 (ddd, 2H, J=1.0Hz, J=4.9Hz, J=7.4Hz), 6.25 (d, 1H, J=5.5Hz), 3.97 (s, 4H), 3.30 (m, 2H), 3.00 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 158.9, 151.9, 149.8, 149.2, 149.0, 136.6, 134.3, 128.3, 124.9, 123.2, 122.5, 122.3, 117.7, 98.7, 59.8, 51.1, 40.5; IR (KBr) vmax/cm−1 3199m (br), 3058m, 3006m, 2942w, 1611m (7-chloroquinoline), 1581s (7-chloroquinoline), 1546m (7-chloroquinoline), 1449w, 1433m, 1367w, 1283w, 1226m, 1150m, 1076w, 850w, 759m; HRMS (ES+) m/z calcd. for C23H23N5Cl 404.16365, found 404.16356.
(η6-p-cymene)(N-(2-((pyridin-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine)chlororuthenium(II) chloride, [RuCl(cymene)(L1)]Cl (1)
[(p-cymene)RuCl2]2 (0.200 g, 0.32 mmol) and L1 (0.204 g, 0.64 mmol) were dissolved in dichloromethane (20 mL) and stirred at room temperature overnight. The solvent volume was reduced to approx. 3 mL and the product was precipitated with diethyl ether. The product was isolated via filtration and washed with diethyl ether and petroleum ether. The product was obtained as a fine, yellow solid (0.357g, 91%). 1H NMR (500 MHz, CDCl3) δ 9.56 (br s, 1H), 8.98 (d, 1H, J=9.1Hz), 8.89 (d, 1H, J=5.2Hz), 8.52 (d, 1H, J=5.4Hz), 8.10 (br s, 1H), 7.95 (d, 1H, J=0.8Hz), 7.83 (dt, 1H, J=1.4Hz, J=7.8Hz), 7.52 (dd, 1H, J=2.1Hz, J=9.0Hz), 7.40 (t, 1H, J=6.9Hz), 7.30 (d, 1H, J=7.7Hz), 6.33 (d, 1H, J=5.4Hz), 5.98 (d, 1H, J=5.9Hz), 5.91 (d, 1H, J=6.1Hz), 5.55 (d, 1H, J=6.0Hz), 5.39 (d, 1H, J=6.1Hz), 4.62 (dd, 1H, J=6.1Hz, J=16.1Hz), 3.93 (dd, 1H, J=5.4Hz, J=16.2Hz), 3.62 (m, 2H), 3.56 (m, 1H), 3.13 (m, 1H), 2.78 (hept, 1H, J=6.9Hz), 2.04 (s, 3H), 1.08 (d, 3H, J=7.0Hz), 1.02 (d, 3H, J=6.9Hz); 13C NMR (125 MHz, CDCl3) δ 161.2, 153.6, 151.4, 150.3, 149.0, 139.2, 135.3, 127.6, 125.7, 125.1, 124.9, 121.8, 117.9, 105.7, 98.3, 97.1, 84.7, 84.3, 83.9, 82.8, 58.7, 53.7, 41.1, 30.8, 22.6, 21.4, 18.0; IR (KBr) vmax/cm−1 3410m (br), 3060w, 2964w, 1610m (7-chloroquinoline), 1580s (7-chloroquinoline), 1535w (7-chloroquinoline), 1451m, 1372w, 1330w, 1141w, 1088w, 765w; MS (ES+) m/z 583 (M+, 100%), 548 (30, MH - Cl); HRMS (ES+) m/z calcd. for C27H31N4Cl2Ru 583.0969, found 583.0985; Elemental analysis (%) calcd. for C27H31N4Cl3Ru∙0.8CH2Cl2: C 48.61, H 4.78, N 8.16, found: C 48.65, H 4.83, N 8.29.
(η6-p-cymene)(N-(2-((1-methyl-1H-imidazol-2-yl)methylamino)ethyl)-7-chloroquinolin-4-amine)chlororuthenium(II) chloride, [RuCl(cymene)(L2)]Cl (2)
This compound was synthesized from [(p-cymene)RuCl2]2 (0.100 g, 0.16 mmol) and L2 (0.103 g, 0.32 mmol) using the same procedure employed for synthesis of 1 (vide supra). The product was obtained as a fine, yellow solid (0.192 g, 95%). 1H NMR (500 MHz, CDCl3) δ 9.09 (br s, 1H), 8.99 (d, 1H, J=9.0Hz), 8.54 (d, 1H, J=5.4Hz), 8.34 (br s, 1H), 7.96 (d, 1H, J=2.1Hz), 7.50 (dd, 1H, J=2.2Hz, J=9.0Hz), 7.20 (d, 1H, J=1.6Hz), 6.91 (d, 1H, J=1.5Hz), 6.36 (d, 1H, J=5.5Hz), 5.87 (d, 1H, J=5.6Hz), 5.55 (d, 1H, J=6.0Hz), 5.36 (d, 1H, J=6.1Hz), 5.28 (d, 1H, J=5.7Hz), 4.27 (m, 1H), 3.91 (m, 1H), 3.65 (m, 3H), 3.57 (s, 3H), 3.45 (m, 1H), 2.89 (hept, 1H, J=6.9Hz), 2.14 (s, 3H), 1.19 (d, 3H, J=6.9Hz), 1.16 (d, 3H, J=7.0Hz); 13C NMR (125 MHz, CDCl3) δ 151.2, 150.3, 149.6, 148.9, 135.5, 128.1, 127.6, 125.8, 125.1, 123.0, 117.9, 106.0, 98.1, 96.1, 83.2, 82.6, 81.0, 55.4, 46.2, 41.0, 34.8, 30.9, 22.9, 21.3, 18.1; IR (KBr) vmax/cm−1 3411m, 3258m, 3092w, 2964w, 2867w, 1612w (7-chloroquinoline), 1579s (7-chloroquinoline), 1538w (7-chloroquinoline), 1513w, 1451m, 1421, 1363, 1327, 1138w, 1088w, 869w; HRMS (ES+) m/z calcd. for C26H32N5Cl2Ru 586.1078, found 586.1077; Elemental analysis (%) calcd. for C26H32N5Cl3Ru∙0.6CH2Cl2: C 47.47, H 4.97, N 10.41, found: C 47.47, H 5.02, N 10.14.
(η6-p-cymene)(N-(2-((2-hydroxyphenyl)methylimino)ethyl)-7-chloroquinolin-4-amine)chlororuthenium(II), [RuCl(cymene)(L3)] (3)
L3 (0.053 g, 0.16 mmol) was dissolved in dichloromethane (10 mL) and triethylamine (0.023 mL, 0.16 mmol) was added. The solution was stirred at room temperature for 30 min and [(p-cymene)RuCl2]2 (0.050 g, 0.08 mmol) was added. Stirring at ambient temperature continued overnight. The solvent volume was reduced to approx. 2 mL and the product was precipitated with diethyl ether. The precipitate was isolated via filtration and washed with water (2–3 mL), diethyl ether and petroleum ether. The product was dried under vacuum and obtained as an orange solid (0.065 g, 67%). 1H NMR (500 MHz, CDCl3) δ 8.37 (d, 1H, J=4.3Hz), 8.02 (d, 1H, J=1.5Hz), 7.85 (d, 1H, J=9.0Hz), 7.51 (br s, 1H), 7.39 (s, 1H), 7.22 (dd, 1H, J=1.5Hz, J=9.1Hz), 7.15 (m, 1H), 6.91 (d, 1H, J=8.5Hz), 6.55 (d, 1H, J=3.9Hz), 6.48 (d, 1H, J=7.7Hz), 6.27 (t, 1H, J=7.3Hz), 5.57 (d, 1H, J=6.2Hz), 5.52 (d, 1H, J=6.2Hz), 5.46 (d, 1H, J=5.4Hz), 5.09 (d, 1H, J=5.4Hz), 4.57 (m, 1H), 4.27 (m, 2H), 3.97 (m, 1H), 2.75 (hept, 1H, J=6.9Hz), 2.29 (s, 3H), 1.24 (d, 3H, J=6.9Hz), 1.14 (d, 3H, J=6.9Hz); 13C NMR (125 MHz, CDCl3) δ 166.0, 165.0, 154.8, 143.1, 139.5, 139.2, 135.6, 134.9, 127.8, 124.7, 122.1, 120.3, 118.6, 115.4, 114.7, 100.9, 98.8, 98.5, 87.7, 83.1, 81.2, 80.6, 44.8, 43.8, 30.6, 22.9, 21.6, 18.9; IR (KBr) vmax/cm−1 3422m (br), 3236m, 3056w, 2961w, 1615s (7-chloroquinoline), 1587m (7-chloroquinoline), 1536w (7-chloroquinoline), 1467m, 1449m, 1318w, 1213w, 1146w, 1091w, 1018w, 877w, 758w; MS (ES+) m/z 596 (M+H, 100%), 560 (25, M-HCl); HRMS (ES+) m/z calcd. for C28H30N3Cl2ORu 596.0809, found 596.0822.
Heme Aggregation Inhibition Activity (HAIA) at a water/n-octanol interface
Inhibition of the heme aggregation near the interface of aqueous buffer/ n-octanol mixtures was studied following a previously published method.41 To establish a base line for the aggregation process, hemin was dissolved in 0.1 M NaOH solution to generate hematin and acetone was added until the acetone:water ratio was 4:6; the final solution contained 15 mg hematin/ml. A sample of this solution (200 µl) was carefully introduced close to the interface between n-octanol (2 ml) and aqueous acetate buffer (5 ml; pH 4.9) in a cylindrical vial with an internal diameter of 2.5 cm. The mixture was incubated at 37 °C for 60 min and at the end of the incubation period it was stirred to ensure the transfer of all solid particles to the aqueous layer. The product (β-hematin) was isolated by centrifugation. The pellet was collected and washed with DMSO (4 ml), centrifuged again for 20 min, washed with 2 ml of ethanol and finally dissolved in 25 ml of 0.1 M NaOH for spectrophotometric quantification. For the aggregation inhibition activity measurements the appropriate amount of each drug (23 mM in n-octanol) to yield [drug]:[hemin] ratios in the range 1–6 was added to the acetate buffer/n-octanol mixture; after stirring for 30 min to equilibrate the drug between the two phases, the hematin solution was added close to the interface and the procedure was followed as described above. All experiments were performed in quadruplicate.
Determination of Partition Coefficients
The distribution of all complexes between n-octanol and water was studied by use of the stir-flask method.63–65 A mixture of 10 ml n-octanol and 10 ml of water (each saturated in the other) was stirred for 30 min at the desired temperature after adding the right amount of the sample to be analyzed (~ 0.7 mmol). The pH was adjusted to the desired value by addition of either a 0.1 M solution of H3PO4 or NaOH (10–20 µl). Once the equilibrium was reached, the organic and aqueous phases were separated and centrifuged. Finally, the concentration of drug in each phase was measured spectrophotometrically in order to determine values of D = [drug](in n-octanol)/[drug](in water). Experiments were carried out in quadruplicate.
Determination of in vitro antiplasmodial activity
Two strains of Plasmodium falciparum were used in this study - the chloroquine sensitive strain D10 and the chloroquine resistant strain Dd2. Continuous in vitro cultures of asexual erythrocyte stages of P. falciparum were maintained using a modified method of Trager and Jensen.66 Quantitative assessment of antiplasmodial activity in vitro was determined via the parasite lactate dehydrogenase assay using a modified method described by Makler.67 The test samples were tested in triplicate on one occasion. The test samples were prepared as a 20 mg/ml stock solution in 100% DMSO. Samples were tested as a suspension if not completely dissolved. Stock solutions were stored at −20°C. Further dilutions were prepared on the day of the experiment. Chloroquine (CQ) was used as the reference drug in all experiments. A full dose-response was performed for all compounds to determine the concentration inhibiting 50% of parasite growth (IC50–value). Test samples 1 and 2 were tested at a starting concentration of 100 µg/ml, which was then serially diluted 2-fold in complete medium to give 10 concentrations; with the lowest concentration being 0.2 µg/ml. The same dilution technique was used for all samples. Test samples L3 and L4 were tested at a starting concentration of 10 µg/ml. Test samples L1 and 3 were tested at a starting concentration of 1000 ng/ml. Test sample L2 was tested at a starting concentration of 100 ng/ml against the CQS strain and 1000 ng/ml against the CQR strain. CQ was tested at a starting concentration of 100 ng/ml against the CQS strain and 1000 ng/ml against the CQR strain. The highest concentration of solvent to which the parasites were exposed to had no measurable effect on the parasite viability (data not shown).
Cytotoxicity assay
Cytotoxicity was assessed against a Chinese Hamster Ovarian (CHO) cell line using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide (MTT) assay.68 The test samples were tested in triplicate on one occasion. The test samples were prepared as a 20 mg/ml stock solution in 100% DMSO. Dilutions were prepared on the day of the experiment. Emetine was used as the reference drug in all experiments. The initial concentration of emetine was 100 µg/mL, which was serially diluted in complete medium with 10-fold dilutions to give six concentrations, the lowest being 0.001 µg/mL. The same dilution technique was applied to all the test samples. The highest concentration of solvent to which the cells were exposed had no measurable effect on the cell viability (data not shown). The 50% inhibitory concentration (IC50) values were obtained from full dose-response curves, using a nonlinear dose-response curve fitting analysis via Graph Pad Prism v.4.0 software.
Acknowledgements
This research has been funded by the Swedish International Development Cooperation Agency (SIDA) and the Swedish Research Council (VR). Financial support from the NIH through Grant # 5SC1GM89558-2 to R.A. S.-D. is gratefully acknowledged.
Footnotes
Electronic Supplementary Information available:
References
- 1.Carter R, Mendis KN. Clin. Microbiol. Rev. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Malaria Report 2009. http://www.who.int/malaria/world_malaria_report_2009.
- 3.Dondorp AM, Yeung S, White L, Nguon C, Day NPJ, Socheat D, von Seidlein L. Nat. Rev. Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ. N. Engl. J. Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hastings IM, Bray PG, Ward SA. Science. 2002;45:2001–2002. doi: 10.1126/science.1077573. [DOI] [PubMed] [Google Scholar]
- 6.Ursos LMB, Roepe PD. Med. Res. Rev. 2002;22:465–491. doi: 10.1002/med.10016. [DOI] [PubMed] [Google Scholar]
- 7.de Villiers KA, Egan TJ. Molecules. 2009;14:2868–2887. doi: 10.3390/molecules14082868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan TJ, Marques HM. Coord. Chem. Rev. 1999;190–192:493–517. [Google Scholar]
- 9.Leed A, DuBay K, Ursos LMB, Sears D, De Dios AC, Roepe PD. Biochemistry. 2002;41:10245–10255. doi: 10.1021/bi020195i. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal PJ, editor. Antimalarial Chemotherapy: mechanism of action, resistance and new directions in drug discovery. New Jersey: Humana Press; 2001. [DOI] [PubMed] [Google Scholar]
- 11.Chong CR, Sullivan DJ. Biochem. Pharmacol. 2003;66:2201–2212. doi: 10.1016/j.bcp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden J. J. Med. Chem. 2000;43:283–291. doi: 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- 13.Cheruku SR, Maiti S, Dorn A, Scorneaux B, Bhattacharjee AK, Ellis WY, Vennerstrom JL. J. Med. Chem. 2003;46:3166–3169. doi: 10.1021/jm030038x. [DOI] [PubMed] [Google Scholar]
- 14.Ridley RG. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez CP, McLean JE, Stein W, Lanzer M. Biochemistry. 2004;43:16365–16373. doi: 10.1021/bi048241x. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez CP, McLean JE, Rohrbach P, a Fidock D, Stein WD, Lanzer M. Biochemistry. 2005;44:9862–9870. doi: 10.1021/bi050061f. [DOI] [PubMed] [Google Scholar]
- 17.Roepe PD. Biochemistry. 2011;50:163–171. doi: 10.1021/bi101638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Schalkwyk DA, Egan TJ. Drug Res. Updates. 2006;9:211–226. doi: 10.1016/j.drup.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Bray PG, Mungthin M, Hastings IM, Biagini GA, Saidu DK, Lakshmanan V, Johnson DJ, Hughes RH, Stocks PA, O’Neill PM, Fidock DA, Warhurst DC, Ward SA. Mol. Microbiol. 2006;62:238–251. doi: 10.1111/j.1365-2958.2006.05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warhurst DC. Malaria J. 2003;2:31–43. doi: 10.1186/1475-2875-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warhurst D, Craig J, Adagu I, Meyer D, Lee S. Malaria J. 2003;2:26–30. doi: 10.1186/1475-2875-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan TJ. J. Inorg. Biochem. 2008;102:1288–1299. doi: 10.1016/j.jinorgbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Egan TJ. Mol. Biochem. Parasitol. 2008;157:127–136. doi: 10.1016/j.molbiopara.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Hoang AN, Ncokazi KK, a de Villiers K, Wright DW, Egan TJ. Dalton Trans. 2010;39:1235–1244. doi: 10.1039/b914359a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biot C, Glorian G, Maciejewski LA, Brocard JS, Domarle O, Blampain G, Millet P, Georges AJ, Lebibi J. J. Med. Chem. 1997;40:3715–3718. doi: 10.1021/jm970401y. [DOI] [PubMed] [Google Scholar]
- 26. http://clinicaltrials.gov/ct2/show/NCT00988507.
- 27. http://clinicaltrials.gov/ct2/show/NCT00563914.
- 28.Biot C, Chavain N, Dubar F, Pradines B, Trivelli X, Brocard J, Forfar I, Dive D. J. Organomet. Chem. 2009;694:845–854. [Google Scholar]
- 29.Beagley P, Blackie MAL, Chibale K, Clarkson C, Moss JR, Smith PJ. Dalton Trans. 2002:4426–4433. [Google Scholar]
- 30.Beagley P, Blackie MAL, Chibale K, Clarkson C, Meijboom R, Moss JR, Smith PJ, Su H. Dalton Trans. 2003:3046–3051. [Google Scholar]
- 31.Sánchez-Delgado RA, Navarro M, Pérez HA, Urbina JA. J. Med. Chem. 1996;39:1095–1099. doi: 10.1021/jm950729w. [DOI] [PubMed] [Google Scholar]
- 32.Navarro M, Pérez HA, Sánchez-Delgado RA. J. Med. Chem. 1997;40:1937–1939. doi: 10.1021/jm9607358. [DOI] [PubMed] [Google Scholar]
- 33.Navarro M, Vásquez F, Sánchez-Delgado RA, Pérez HA, Sinou V, Schrével J. J. Med. Chem. 2004;47:5204–5209. doi: 10.1021/jm049792o. [DOI] [PubMed] [Google Scholar]
- 34.Arancibia R, Dubar F, Pradines B, Forfar I, Dive D, Klahn AH, Biot C. Bioorg. Med. Chem. 2010;18:8085–8091. doi: 10.1016/j.bmc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Glans L, Taylor D, de Kock C, Smith PJ, Haukka M, Moss JR, Nordlander E. J. Inorg. Biochem. 2011;105:985–990. doi: 10.1016/j.jinorgbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 36.van Rijt SH, Sadler PJ. Drug Discov. Today. 2009;14:1089–1097. doi: 10.1016/j.drudis.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartinger CG, Dyson PJ. Chem. Soc. Rev. 2009;38:391–401. doi: 10.1039/b707077m. [DOI] [PubMed] [Google Scholar]
- 38.Süss-Fink G. Dalton Trans. 2010;39:1673–1688. doi: 10.1039/b916860p. [DOI] [PubMed] [Google Scholar]
- 39.Gasser G, Ott I, Metzler-Nolte N. J. Med. Chem. 2011;54:3–25. doi: 10.1021/jm100020w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajapakse CSK, Martínez A, Naoulou B, Jarzecki AA, Suárez L, Deregnaucourt C, Sinou V, Schrével J, Musi E, Ambrosini G, Schwartz GK, Sánchez-Delgado RA. Inorg. Chem. 2009;48:1122–1131. doi: 10.1021/ic802220w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez A, Rajapakse CSK, Jalloh D, Dautriche C, Sánchez-Delgado RA. J. Biol. Inorg. Chem. 2009;14:863–871. doi: 10.1007/s00775-009-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid WF, John RO, Mühlgassner G, Heffeter P, Jakupec MA, Galanski M, Berger W, Arion VB, Keppler BK. J. Med. Chem. 2007;50:6343–6355. doi: 10.1021/jm701042w. [DOI] [PubMed] [Google Scholar]
- 43.Carmona D, Lamata MP, Viguri F, Rodríguez R, Lahoz FJ, Dobrinovitch IT, Oro LA. Dalton Trans. 2008:3328–3338. doi: 10.1039/b802381f. [DOI] [PubMed] [Google Scholar]
- 44.Dickson SJ, Paterson MJ, Willans CE, Anderson KM, Steed JW. Chem. Eur. J. 2008;14:7296–7305. doi: 10.1002/chem.200800772. [DOI] [PubMed] [Google Scholar]
- 45.Govender P, Renfrew AK, Clavel CM, Dyson PJ, Therrien B, Smith GS. Dalton Trans. 2011;40:1158–1167. doi: 10.1039/c0dt00761g. [DOI] [PubMed] [Google Scholar]
- 46.Brunner H, Oeschey R, Nuber B. Dalton Trans. 1996:1499–1508. [Google Scholar]
- 47.Brunner H, Zwack T, Zabel M, Beck W, Böhm A. Organometallics. 2003;22:1741–1750. [Google Scholar]
- 48.Geary WJ. Coord. Chem. Rev. 1971;7:81–122. [Google Scholar]
- 49.Apelblat A. J. Solution Chem. 2011;40:1234–1257. [Google Scholar]
- 50.Govindaswamy P, Carroll P, Sinha C, Kollipara MR. J. Coord. Chem. 2007;60:97–107. [Google Scholar]
- 51.Mishra H, Mukherjee R. J. Organomet. Chem. 2006;691:3545–3555. [Google Scholar]
- 52.Filak LK, Mühlgassner G, Jakupec MA, Heffeter P, Berger W, Arion VB, Keppler BK. J. Biol. Inorg. Chem. 2010;15:903–918. doi: 10.1007/s00775-010-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grgurić-Sipka S, Ivanović I, Rakić G, Todorović N, Gligorijević N, Radulović S, Arion VB, Keppler BK, Tesić ZL. Eur. J. Med. Chem. 2010;45:1051–1058. doi: 10.1016/j.ejmech.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 54.97/18193. Int. Pat. 1997
- 55.We thank a reviewer for drawing our attention to these factors relating to the cross-resistance with chloroquine,.
- 56.Martínez A, Rajapakse CSK, Naoulou B, Kopkalli Y, Davenport L, Sánchez-Delgado RA. J. Biol. Inorg. Chem. 2008;13:703–712. doi: 10.1007/s00775-008-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmid WF, John RO, Arion VB, Jakupec MA, Keppler BK. Organometallics. 2007;26:6643–6652. [Google Scholar]
- 58.Bennett MA, Smith AK. J. Chem. Soc. Dalton Trans. 1974;2:233–241. [Google Scholar]
- 59.Yearick K, Ekoue-Kovi K, Iwaniuk DP, Natarajan JK, Alumasa J, de Dios AC, Roepe PD, Wolf C. J. Med. Chem. 2008;51:1995–1998. doi: 10.1021/jm800106u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.APEX2 - Software Suite for Crystallographic Programs. Madison, Wisconsin, USA: Bruker AXS Inc.; 2009. [Google Scholar]
- 61.Sheldrick GM. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 62.Sheldrick GM. SADABS - Bruker AXS scaling and absorption correction. Madison, Wisconsin, USA: Bruker AXS Inc.; 2008. [Google Scholar]
- 63.Danielsson LG, Zhang YH. Trends Anal. Chem. 1996;15:188–196. [Google Scholar]
- 64.Rappel C, Galanski M, Yasemi A, Habala L, Keppler BK. Electrophoresis. 2005;26:878–884. doi: 10.1002/elps.200410053. [DOI] [PubMed] [Google Scholar]
- 65.OECD. Guidelines for testing of chemicals. Paris: OECD; 1995. [Google Scholar]
- 66.Trager W, Jensen JB. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 67.Makler MT, Hinrichs DJ. Am. J. Trop. Med. Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 68.Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]