Abstract
Aim
PEGylated components have been widely used to reduce particle aggregation in serum and extend circulation lifetime for lipid- and polymer-based gene-delivery systems. However, PEGylation is known to interfere with cell interaction and intracellular trafficking, resulting in decreased biological activity. In the present study, the effect of cholesterol domains on PEGylated liposome-mediated gene delivery was evaluated by PEGylating formulations with and without a cholesterol domain, and also by altering the location of PEG on the particle surface (i.e., within or excluded from the domain).
Materials and methods
Lipoplexes formulated with PEG–cholesterol or PEG–diacyl lipid were used to transfect various cell lines, including human and mouse cancer cells. Cellular uptake of lipoplexes was also quantified and compared with the transfection results.
Results
Our findings are consistent with previous work demonstrating that PEGylation reduces transfection rates; however, formulations in which PEG was incorporated into the cholesterol domain did not exhibit this detrimental effect. In some cell lines, the incorporation of PEG into the domain actually increased transfection rates, despite no enhancement of cellular uptake.
Discussion
These results suggest that the adverse alterations in intracellular trafficking that are a consequence of PEGylation may be avoided by utilizing delivery vehicles that allow PEG to partition into a cholesterol domain.
Cationic liposomes have been widely used for delivery of DNA and siRNA both in vitro and in vivo. One major problem associated with the use of cationic delivery vehicles is their strong interaction with blood components, which can dramatically lower the transfection efficiency [1–7]. Serum has been reported to exert its inhibitory effect by binding serum proteins to the particle surface, which leads to structural reorganization, aggregation and/or dissociation of the delivery vehicle [4,8–10]. The presence of nuclease in the serum can also degrade nucleic acids, resulting in a loss of supercoil content and biological activity [11]. In order to overcome the adverse effects of serum protein binding, PEGylated components are predominantly utilized to sterically shield the delivery vehicles from blood constituents [12–14].
It is well documented that the steric stabilization provided by PEGylation reduces particle aggregation in serum and extends circulation lifetime. However, PEGylation is known to interfere with trafficking involved in intracellular delivery and render vectors more susceptible to agitation-induced damage during processing [14–19]. Other studies have reported that PEGylation significantly reduces the cellular interaction and uptake of the lipid nanoparticles, resulting in decreased biological activity [20,21]. To overcome these shortcomings of PEGylation, strategies such as using PEG-chains that are removable in the endosomal compartment have been adopted in order to allow endosomal escape. One of the approaches is to adopt a pH-sensitive linker between the PEG moiety and lipid. The PEG chains are then removed in the endosomal compartment due to acid-catalyzed hydrolysis [22,23]. Another approach is to exchange PEG–lipid by modulating the hydrophobicity of the PEG–lipid conjugate. This can be achieved by varying the length of the alkyl chain of the lipid anchor [24] as the lipid portion of the conjugate determines its affinity for the lipid delivery vehicle.
As a potential alternative to PEGylation, lipoplexes with high levels of cholesterol have been shown to exhibit enhanced transfection in vitro and resistance to serum-induced aggregation [5,25]. Furthermore, studies have demonstrated that cholesterol domains form in lipoplexes with cholesterol contents above 52 wt% [25]. In the present study, the effect of cholesterol domains on PEGylated liposome-mediated gene delivery was evaluated by PEGylating formulations with and without a cholesterol domain, and also by altering the location of PEG on the particle surface. More specifically, we investigated the effects of conjugating PEG to either cholesterol or a diacyl lipid (1,2-distearoyl-sn-glycero-3-phosphoethanolamine [DSPE]), which results in a PEGylated component that is either incorporated into, or excluded from, respectively, the cholesterol domain. Lipoplexes incorporating PEG–cholesterol or PEG–DSPE were used to transfect various cell lines including human and mouse cancer cells. Cellular uptake of lipoplexes was also quantified and compared with the transfection results. Our findings are consistent with previous work showing that PEGylation reduces transfection rates; however, formulations in which PEG was incorporated into the cholesterol domain did not exhibit this detrimental effect. In some cell lines, the incorporation of PEG into the domain actually increased transfection rates, despite no enhancement of cellular uptake. These results suggest that the adverse alterations in intracellular trafficking that are a consequence of PEGylation may be avoided by utilizing delivery vehicles that allow PEG to be incorporated into a cholesterol domain.
Materials & methods
Materials
Luciferase plasmid DNA (5.9 kb) was generously provided by Valentis Inc. (CA, USA). N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethyl-ammonium chloride (DOTAP), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DOPE) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy[polyethylene glycol]-2000) (PEG2000-DSPE) were purchased from Avanti Polar Lipids (AL, USA). Cholesterol, cholesteryl chloroformate (CholCOCl), MeO-PEG2000-OH, and tetrabutyl ammomium chloride (TBACl) were purchased from sigma-aldrich (MO, USA). MeO-PEG2000-cholesterol was synthesized using the modified procedure described by Zhao et al. [26]. The identity of the MeO-PEG2000-cholesterol was confirmed by NMR (400 MHz; Bruker Avance III 400) and its purity was estimated to be >95%. The luciferase assay kit was obtained from Promega (WI, USA). Fetal bovine serum (FBS) was purchased from Mediatech Inc. (VA, USA) and was filtered with a 0.22 μm low-protein-binding cellulose acetate filter from Fisher Scientific (PA, USA) before use. All chemicals were of reagent grade or higher quality.
Liposome & lipoplex preparation
DOTAP combined with cholesterol at different weight percentages (36–69%) was mixed in chloroform. The lipid mixture was dried under a stream of nitrogen gas and placed under vacuum (100 mTorr) for 2 h to remove residual chloroform, dried lipids were subsequently resuspended in autoclaved, distilled water and sonicated. Cationic liposomes were prepared immediately before use as previously described [5]. Lipoplexes were prepared by mixing equal volumes of DNA (40 μg/ml) and liposomes (0.5 mM), and incubated at room temperature for 15 min before transfection.
In vitro transfection assay
KB cells and MCF7/ADR cells were obtained from American Type Culture Collection (MD, USA). Neuro2A cells were a gift from Emmanuel Katsanis (University of Arizona, AZ, USA), and murine cell lines including B16, CT26 and 4T1 were generously provided by Steve Dow (Colorado State University, CO, USA). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. Cells were maintained in complete growth media supplemented with 10% FBS, 50 units/ml penicillin G and 50 μg/ml streptomycin sulfate. For in vitro transfection, cultures were freshly seeded at 3 × 105 cells/well in 12-well plates 24 h before transfection. Lipoplexes (40 μl) containing 0.8 μg DNA were incubated at room temperature for 15 min and then transferred into wells containing freshly washed cells in 50% FBS medium. The use of these relatively high-serum conditions for our transfection experiments was an effort to better simulate the environment experienced by the delivery systems upon intravenous injection, and has been shown to provide results from cell culture experiments that are more consistent with in vivo studies [5,25,27]. The cells were incubated with lipoplexes for 4 h before the medium was replaced with the medium containing 10% FBS. 40 h after transfection, the culture medium was discarded, and the cells were washed with phosphate-buffered saline (PBS) and then lyzed with 200 μl of lysis buffer (Promega). A total of 20 μl of cell lysis solution was used to assay for luciferase expression via a luciferase assay kit (Promega), according to the manufacturer’s protocol. The signal was quantified using a Monolight™ 2010 Luminometer (BD Biosciences, CA, USA). Protein contents were determined with a Bio-Rad protein assay kit (CA, USA) according to the manufacturer’s instructions. The absorbance was measured at 550 nm using a THERMOmax microplate reader (Molecular Devices, CA, USA).
Dynamic light scattering & zeta potential analysis
Lipoplexes at charge ratio of 4:1 prepared as previously described were diluted to a final volume of 500 μl with 10 mM 4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid, pH 7.4. To measure the size and zeta potential of lipoplexes after exposure to serum, lipoplexes were mixed with 100% FBS at equal volume and incubated for 30 min at room temperature before measurement. Diluted samples were transferred to a cuvette for dynamic light scattering analysis on a Nicomp 380 Zeta Potential/Particle Sizer (Particle Sizing Systems, CA, USA). Channel width was set automatically based on the rate of fluctuation of scattered light intensity and triplicate preparations were measured at room temperature for each formulation. Intensity-weighted Gaussian size distribution was fit to the autocorrelation functions, and particle size values were obtained as previously described [5]. Samples were then subjected to an electric field in a Nicomp 380 Zeta Potential/Particle Sizer for zeta potential determination.
Flow cytometry analysis of cellular uptake of lipoplexes
Plasmid DNA was labeled with fluorescein by Label IT® Nucleic Acid Labeling Kit according to the manufacturer’s manual (Mirus Bio LLC, WI, USA). Lipoplexes (1.6 μg fluorescein-labeled DNA) were incubated with 4 × 105 kb cells at 37°C for 4 h. After incubation, cells were washed with ice-cold PBS three times and collected by centrifugation at 300 × g for 5 min. The final cell pellets were resuspended by vortexing and fixed in 1% formalin in PBS. To quench the bound lipoplexes on the outer leaflet of the cell membrane, 0.1% Trypan blue solution was added into the final cell suspension. Flow cytometry analysis was conducted on a BD FACScalibur™ system (BD Bioscience, CA, USA) with the excitation at 488 nm and emission at 530 nm. A total of 50,000 cells were analyzed for each sample and the mean fluorescence intensity was determined by FlowJo software and interpreted as a measure of cellular uptake of lipoplexes.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to determine statistical significance (p < 0.05) among the mean values for transfection efficiency. A Tukey’s multiple comparison test was used to determine statistical significance (p < 0.05) between formulations.
Results
Particle size & zeta potential measurement
The particle size and zeta potential of lipoplexes containing 69% cholesterol and PEG conjugates (0.4 mol%) were measured at charge ratio (+/−) of 4:1 before and after exposure to serum (Table 1). Before exposure to serum, the zeta potentials of lipoplexes are comparably high at this charge ratio (≥30) and the particle sizes of all the formulations are approximately 200 nm. After exposure to serum for 30 min, particle sizes of all the formulations increased slightly while zeta potential decreased dramatically and became negative. These results indicate that the size of the lipoplexes are not dramatically altered by serum; however, the extra positive charge on the lipoplexes surface are neutralized after exposure to serum. In addition, the very similar changes in zeta potential observed with all formulations after serum exposure suggest that the location of PEG (within or excluded from the domain) does not appear to significantly alter the extent of serum protein binding.
Table 1.
Particle size and zeta potential of DOTAP/cholesterol (wt/wt: 31/69) lipoplex at charge ratio of 4:1 before and after exposure to serum.
| Particle diameter (nm) | Zeta potential (mV) | |||
|---|---|---|---|---|
| In buffer | In serum | In buffer | In serum | |
| Non-PEGylated | 196.4 ± 12.4 | 235.8 ± 23.5 | +45.8 ± 5.1 | −16.5 ± 0.6 |
| PEG–DSPE | 198.9 ± 17.1 | 235.3 ± 14.7 | +37.2 ± 6.2 | −17.5 ± 0.4 |
| PEG–cholesterol | 193.8 ± 26.6 | 217.7 ± 13.1 | +34.8 ± 6.6 | −15.4 ± 1.3 |
Data represent mean ± one standard deviation of three replicates.
DOTAP: N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethylammonium chloride; DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine.
Effect of the PEG anchor on transfection
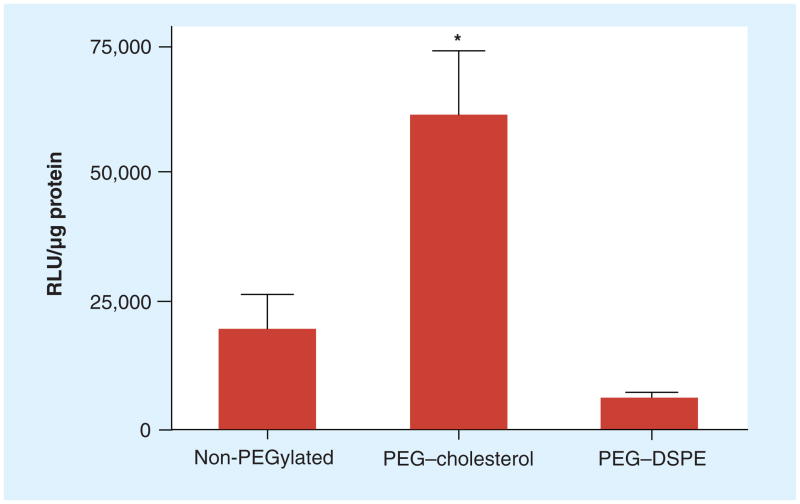
To test the effect of the PEG anchor on transfection, PEGylated lipoplexes with DOTAP/cholesterol (31:69, wt/wt) were prepared by incorporation of PEG–cholesterol or PEG–DSPE and used to transfect KB cells in the presence of 50% serum. Interestingly, PEG–cholesterol showed the highest transfection rate among the three formulations while lipoplexes with PEG–DSPE showed the lowest transfection. Surprisingly, PEG–cholesterol enhanced transfection rates threefold as compared with the non-PEGylated control (Figure 1). Although cell viability was not specifically measured in these experiments, we did not observe alterations in total protein content of the cell lysate, suggesting that neither of the PEG conjugates are highly toxic.
Figure 1. Transfection by DOTAP/cholesterol lipoplexes prepared with 69 wt% cholesterol.
KB cells were transfected by non-PEGylated lipoplexes and formulations incorporating 0.4 mol% PEG–cholesterol or PEG-DSPE in 50% serum. The data represent the mean ± one standard deviation of three replicates.
*p < 0.05 when compared with the non-PEGylated control.
DOTAP: N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethylammonium chloride;
DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine.
Effect of cholesterol domain on transfection by PEGylated lipoplexes
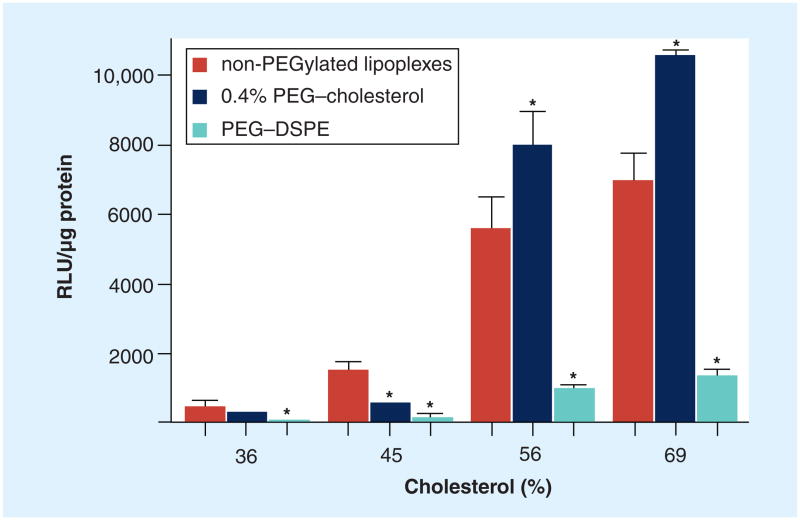
To evaluate the effect of cholesterol domain formation on transfection, PEGylated lipoplexes containing different cholesterol contents (36, 45, 56 and 69%) were prepared with either PEG–cholesterol or PEG–DSPE, and used to transfect KB cells in 50% serum. In all cases, the use of PEG–DSPE reduced transfection rates, consistent with previous reports [14,15,17,19]. Similar effects were observed with PEG–cholesterol in lipoplexes formulated with 36% and 45% cholesterol (Figure 2). In contrast, PEG–cholesterol significantly enhanced transfection when incorporated into lipoplexes possessing 56 and 69% cholesterol. As shown in our previous study [25], cholesterol domains are formed in lipoplexes with ≥52% cholesterol, whereas cholesterol domains are not observed in lipoplexes formulated with 36 and 45% cholesterol. Considering PEG–cholesterol is able to partition into the cholesterol domain while PEG–DSPE is excluded from such domains, the results suggest that presentation of the PEG molecule within the cholesterol domain facilitates transfection in KB cells. In this context, it should be noted that PEG–DSPE reduced the transfection efficiency in all the formulations regardless of the presence of a cholesterol domain, whereas the ability of PEG–cholesterol to enhance transfection was dependent upon cholesterol domain formation (Figure 2).
Figure 2. Transfection of DOTAP/cholesterol lipoplexes prepared with 36, 45, 56 and 69 wt% cholesterol.
KB cells were transfected by non-PEGylated lipoplexes and with formulations incorporating 0.4 mol% PEG–cholesterol or PEG–DSPE in 50% serum. It is known that the 56 and 69% formulations possess a cholesterol domain, whereas the 36 and 45% formulations lack a domain. The data represent the mean ± one standard deviation of three replicates.
*p < 0.05 when compared with the non-PEGylated control.
DOTAP: N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethylammonium chloride;
DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine.
Effect of PEG–cholesterol concentration on transfection in KB cells
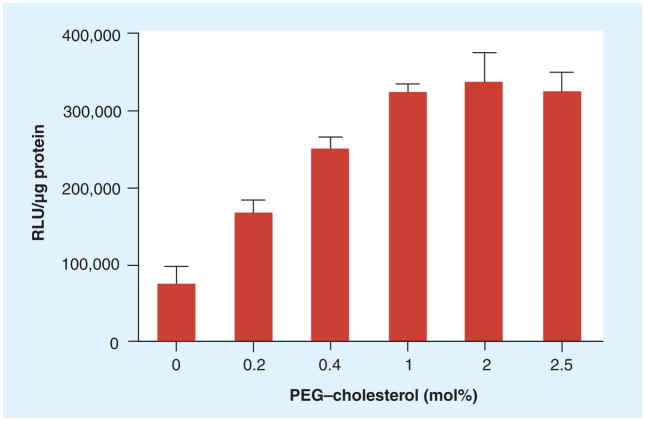
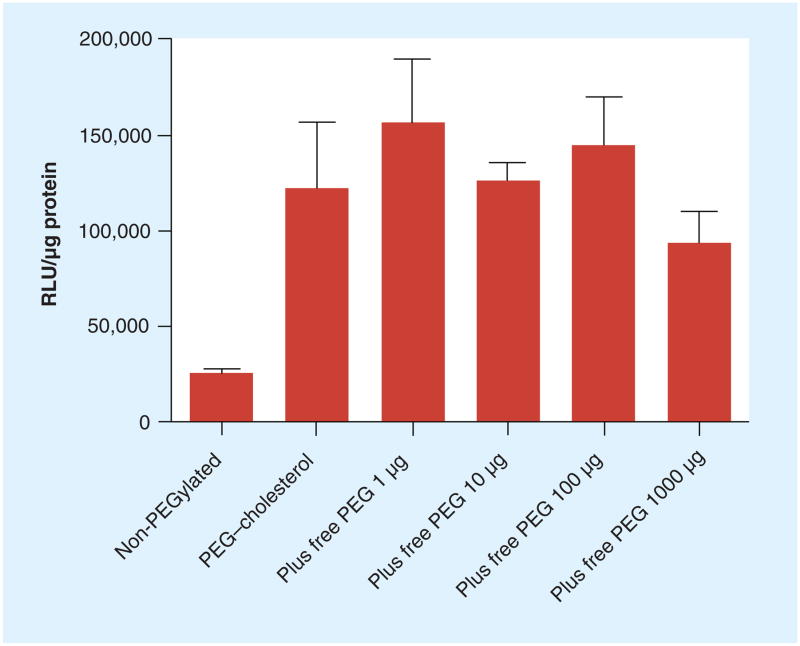
The ability of domain-containing lipoplexes incorporating PEG–cholesterol to significantly enhance transfection in KB cells proved very reproducible, and further investigation of this curious effect seemed warranted. Lipoplexes containing 69% cholesterol were prepared with PEG–cholesterol at concentrations ranging from 0 to 2.5 mol% and used to transfect KB cells in 50% serum. The transfection increased progressively from 0 to 1% PEG–cholesterol and reached a plateau above 1% (Figure 3). This apparent saturation suggests that the enhanced transfection by PEG–cholesterol may involve an interaction with a specific cellular component(s). To test this hypothesis, increasing quantities of free PEG were added into the transfection media with lipoplexes containing PEG–cholesterol. The data demonstrated that transfection efficiency remained constant regardless of the amount of free PEG added (Figure 4). It should be pointed out that the free PEG added into the transfection was in great excess to the PEG included in the lipoplexes, with 1000 μg of PEG being approximately 5000-fold that in the lipoplexes used for transfection.
Figure 3. Effect of PEG–cholesterol concentration in lipoplexes with DOTAP/cholesterol (31:69, wt/wt) on transfection of KB cells in 50% serum.
The data represent the mean ± one standard deviation of three replicates.
DOTAP: N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethylammonium chloride.
Figure 4. Effect of free MeO-PEG2000-OH on transfection of KB cells in 50% serum.
KB cells were transfected by lipoplexes with 69% cholesterol and 1% at doses of 1, 10, 100 and PEG–cholesterol in the presence of free PEG2000 1000 μg in each well of a 12-well plate. The data represent the mean ± one standard deviation of three replicates.
Cellular uptake of PEGylated lipoplexes
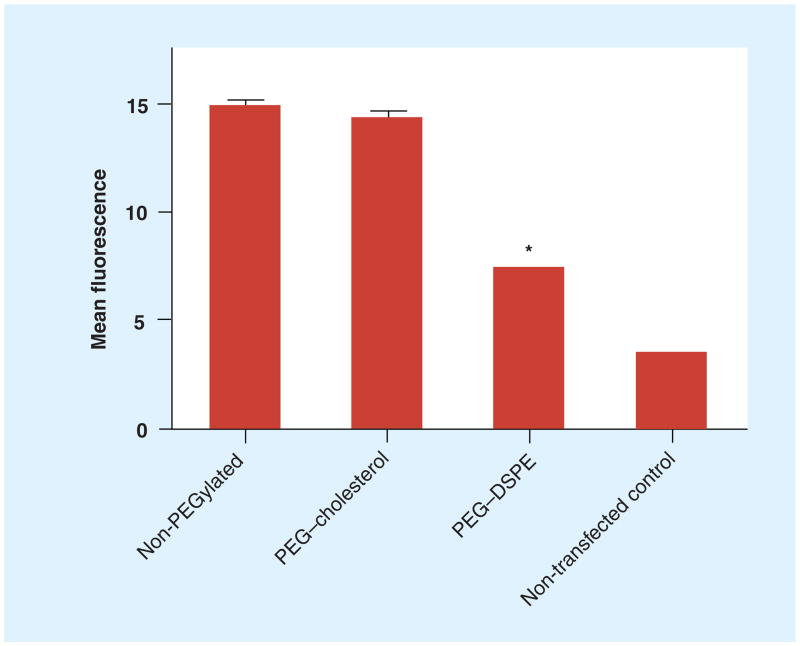
Liposomes with DOTAP/cholesterol (31:69, wt/wt) and PEG conjugate were complexed with fluorescein-labeled DNA to quantify the cellular uptake of lipoplexes by KB cells. After a 4-h incubation in 50% serum, cellular uptake of lipoplexes was measured by flow cytometry. In Figure 5, lipoplexes PEGylated with PEG–cholesterol demonstrated levels of cellular uptake comparable to the non-PEGylated control, whereas PEG–DSPE demonstrated significantly lower levels of uptake. The values of mean fluorescence appear to be very low, with the nontransfected control showing only 3.5 in mean fluorescence. This result indicates that the enhanced transfection induced by PEG–cholesterol in domain-containing lipoplexes (Figure 1) could not be explained by increased cell uptake.
Figure 5. Cellular uptake of DOTAP/cholesterol (31:69, wt/wt) lipoplexes with different PEG conjugates (0.4 mol%).
The mean fluorescence intensity measured by fluorescence-activated cell sorting was used to quantify the cellular uptake of lipoplexes. The data represent the mean ± one standard deviation of three replicates.
*p < 0.05 when compared with the non-PEGylated control.
DOTAP: N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethylammonium chloride;
DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine.
Enhanced transfection by PEG–cholesterol in different cell lines
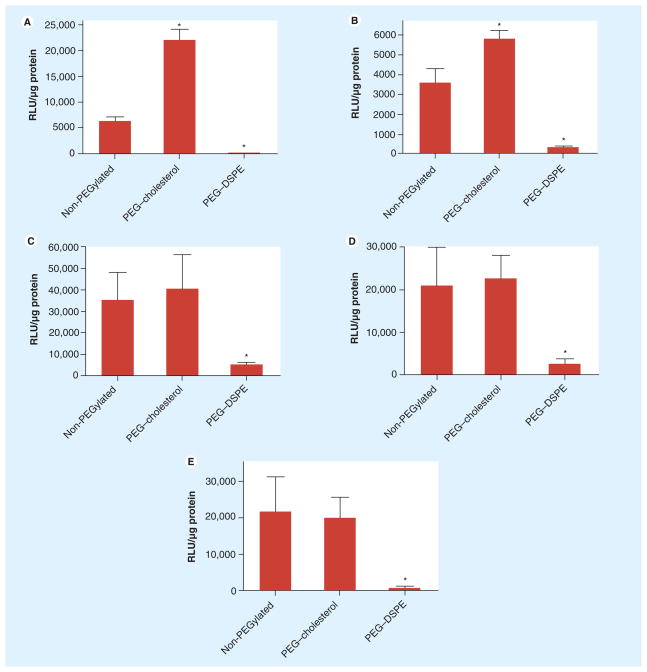
To determine whether the enhanced transfection by PEG–cholesterol was unique to KB cells, transfection experiments were performed on different cell lines. Lipoplexes containing DOTAP/cholesterol (31:69, wt/wt) were prepared with PEG–cholesterol and PEG–DSPE, respectively. Human breast cancer cells (MCF7/ADR) and murine cancer cells (Neuro2A, B16, CT26 and 4T1) were transfected in 50% serum. As summarized in Figure 6, the enhanced transfection was also observed in MCF7/ADR and Neuro2A cells, but not in the other cell lines. In all the cells tested, PEG–DSPE dramatically decreased transfection, consistent with reports in the literature [14,15,17,19]. Even in the cell lines where no enhanced transfection by PEG–cholesterol was observed, the PEG–cholesterol conjugate did not reduce transfection efficiency, in contrast to that observed with PEG–DSPE (Figures 6C–E). These results indicate that incorporation of PEGylated components within a cholesterol domain does not compromise transfection efficiency in vitro, and even improves transfection in some cell lines.
Figure 6. Transfection of DOTAP/cholesterol (31:69, wt/wt) lipoplexes with PEG–cholesterol in different cell lines.
(A) MCF7, (B) Neuro2A, (C) B16, (D) CT26 and (E) 4T1 cells were transfected with non-PEGylated lipoplexes and formulations incorporating 1% PEG–cholesterol or PEG–DSPE in 50% serum. The data represent the mean ± one standard deviation of three replicates.
*p < 0.05 when compared to the non-PEGylated control.
DOTAP: N-(1-[2,3-dioleoyloxy]propyl)-N,N,N-trimethylammonium chloride.
Discussion
To develop lipoplexes that efficiently transfer genes in the presence of serum, PEGylation has become the standard procedure in liposomal formulation to sterically shield the delivery vehicles from blood components and increase circulation lifetimes in vivo. This strategy has been utilized for decades in the development of liposome-based formulations and has been shown to increase circulation lifetimes and allow the accumulation of liposomes containing small-molecule drugs in tumors [28]. It is thought that PEGylation provides steric stabilization that ultimately reduces surface–surface interactions, including the aggregation of liposomes [29–31]. Although the enhanced circulation time of PEGylated liposomes allows the accumulation of lipoplexes in tumors, the influence of PEGylation on cellular uptake and intracellular trafficking compromises the ultimate delivery efficiency [14,15,17,19].
The commercially available PEGylated lipids are mostly PEG conjugated to phospholipids and ceramides, with PEG–DSPE being the most commonly used. In the present study, MeO-PEG2000–cholesterol was synthesized by linking the hydroxyl moiety in MeO-PEG2000-OH onto cholesterol via cholesteryl chloroformate to form a carbonate ester (i.e., ‘PEG–cholesterol’). As shown in Figure 1, transfection was not reduced in KB cells when lipoplexes containing a cholesterol domain were PEGylated with PEG–cholesterol. Instead, transfection was enhanced in comparison to lipoplexes PEGylated with PEG–DSPE and the non-PEGylated control. The data in Figure 3 indicate that the enhanced transfection by PEG–cholesterol in KB cells is dependent on the concentration of the conjugate, and the effect is saturated above 1%. However, as shown in Figure 4, free MeO-PEG2000-OH was not able to reduce transfection, suggesting that a specific interaction of PEG with a cellular component is not responsible for the observed enhancement of transfection with PEG–cholesterol. Furthermore, cellular uptake of lipoplexes with or without PEG–cholesterol was equivalent (Figure 5), suggesting that increased uptake cannot explain the enhanced transfection by PEG–cholesterol. Our results (Figure 1) are consistent with previous reports concluding that PEG–DSPE has a detrimental effect on intracellular trafficking [14]. By contrast, enhanced transfection by PEG–cholesterol suggests that incorporation of PEGylated components into a cholesterol domain does not trigger the same pathway as PEG–DSPE. Instead, PEG–cholesterol appears to promote more productive intracellular trafficking, which results in improved transfection. However, parallel experiments using confocal microscopy to assess changes in intracellular trafficking did not reveal consistent differences among the PEGylated formulations with regard to accumulation in endosomes or nuclei (data not shown). It should be pointed out that the PEG molecule we utilized is linked to cholesterol via a carbonate ester bond while the PEG–DSPE is linked via a carbamate bond. It has been reported that PEG–lipids with orthoester linkages are sensitive to the reduced pH of the endosomal compartment [32–34]. Thus, the carbonate ester linkage in our PEG–cholesterol conjugate may be more labile than the carbamate linkage in PEG–DSPE; these chemical differences may contribute to the enhanced transfection observed with the former conjugate. However, the inability of PEG–cholesterol to enhance transfection when incorporated into lipoplexes lacking a domain suggests that linker chemistry alone cannot explain the current observations. Similarly, after the lipoplexes were exposed to serum, zeta potentials became negative and the particle sizes remained comparable among all the formulations. Furthermore, the negative charge contributed by the very small amount of PEG–DSPE used in this study is small compared with the cationic lipid (DOTAP) content, and the surface charge remains strongly positive even after 1% PEG–cholesterol is incorporated into the formulation (zeta potential is reduced from +33.0 to +16.2 mV; data not shown). Therefore, we conclude that the slight chemical differences between these conjugates cannot explain their differential effects on transfection, and the mechanism responsible for our observations is not clear.
We feel it is important to reiterate that the beneficial effects of PEG–cholesterol were dependent on the presence of a cholesterol domain. This is consistent with our previous findings that when the targeting ligand, folate-cholesterol, was incorporated in the DOTAP/cholesterol formulation, the presence of the ligand within the cholesterol domain promotes more productive transfection in cultured cells [35]. In lipoplexes with 36 and 45% cholesterol that lack a cholesterol domain, PEG conjugates should be evenly distributed over the particle surface. In these formulations, the transfection efficiency was reduced by both PEG conjugates, consistent with the well-known adverse effects of PEGylation on delivery [14,15,17,19]. By contrast, enhanced transfection was observed with PEG–cholesterol in formulations that are known to possess a cholesterol domain (i.e., the 56 and 69% cholesterol formulations) [25]. Considering that the PEG–cholesterol conjugate would be expected to partition into the cholesterol domain while PEG–DSPE is excluded from the domain, our findings suggest that presentation of the PEG moieties in the cholesterol domain may alleviate (at least partially) the adverse effect on intracellular trafficking due to PEGylation.
Another interesting finding of this study is that the enhanced transfection by PEG–cholesterol was not unique to KB cells, a human carcinoma of the nasopharynx. Transfection in MCF7/ADR (human breast cancer cell line) and Neuro2A (murine neuroblastoma cell line) cells was also significantly enhanced by PEG–cholesterol. Although PEG–cholesterol did not improve transfection in other murine cancer cell lines (B16, CT26 and 4T1), it did not have a negative effect on transfection in these cell lines. Our experiments do not allow us to definitively determine whether the maintenance of transfection in these cell lines is caused by avoidance of detrimental trafficking, or if transfection enhancement due to the incorporation of this conjugate into domain-containing lipoplexes is equal in magnitude to the adverse effects. Regardless of the exact mechanism(s) involved, these results demonstrate that the ability of cholesterol domains to increase transfection rates is further enhanced by the incorporation of PEG in the domain. Considering that transfection was enhanced despite constant cellular uptake, we conclude that the inclusion of PEG in cholesterol domains likely promotes more productive intracellular trafficking of particles that results in increased transfection efficiency.
Future perspective
PEGylation has been commonly adopted as surface modification of drug- and gene-delivery systems to improve the stability and circulation time in vivo. However, it has been shown that the influence of PEGylation on cellular uptake and intracellular trafficking compromises the ultimate delivery efficiency. Furthermore, PEGylated liposomes have been linked to accelerated blood clearance and an immune response after repeated injection [36]. Considering these issues, it would be beneficial to develop alternative strategies to PEGylation for increasing circulation lifetimes. In this context, high levels of cholesterol in lipid-based formulations have been shown to reduce aggregation in serum and result in longer circulation lifetimes [5,8,27]. Our current work indicates that PEGylation in a cholesterol domain may be advantageous for intracellular delivery, although the mechanism behind the enhanced transfection is unclear. This alternative strategy offered the potential of exploiting lipid domains to compartmentalize different functionalities within a delivery vehicle. Therefore, PEGylated formulations incorporating PEG–cholesterol, instead of the typically used PEG–DSPE, is worthy of further examination. Future work on the modes of cell binding and uptake might be also explored.
Executive summary.
Size of PEGylated lipoplexes were not dramatically altered by serum, but surface charge was.
Enhanced transfection in KB cells by PEG–cholesterol in domain-containing lipoplexes is dependant on PEG–cholesterol concentration.
The enhanced transfection induced by PEG–cholesterol in domain-containing lipoplexes could not be explained by increased cell uptake.
Confinement of PEG to a lipid domain does not compromise transfection efficiency in vitro, and even improves transfection in some cell lines.
Enhanced uptake of PEG–cholesterol indicates unknown pathways of intracellular uptake that may be exploited for delivery.
Acknowledgments
We thank Emmanuel Katsanis and Steve Dow for providing the murine cell lines.
Key Terms
- Liposome
Spherical, self-closed structures formed by one or several concentric lipid bilayers
- Transfection
The process of introducing DNA or RNA into cells
- PEGylation
The incorporation of components that are covalently linked to polyethylene glycol
- Lipoplex
Liposome/nucleic acids complex
- Cholesterol domain
Cholesterol-rich area on cell membrane or artificial membrane
- Gene delivery
Delivery of foreign DNA (gene) into cells
- Intracellular trafficking
The physical translocation within the target cell, including interactions with various organelles and transport systems
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This work was supported by grants #BES-0433811 (NSF) and EB0005476–01A2 (NIH-NIBIB) to Thomas Anchordoquy and also supported in part via the Medicinal Chemistry Core facility (MFW) via Colorado CTSA grant 5UL1RR025780 from NCRR/NI The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Zelphati O, Uyechi LS, Barron LG, Szoka FC., Jr Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim Biophys Acta. 1998;1390(2):119–133. doi: 10.1016/s0005-2760(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 2.Thierry AR, Rabinovich P, Peng B, Mahan LC, Bryant JL, Gallo RC. Characterization of liposome-mediated gene delivery: expression, stability and pharmacokinetics of plasmid DNA. Gene Ther. 1997;4(3):226–237. doi: 10.1038/sj.gt.3300350. [DOI] [PubMed] [Google Scholar]
- 3.Yang JP, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997;4(9):950–960. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 4.Yang JP, Huang L, Yang JP, Huang L. Time-dependent maturation of cationic liposome-DNA complex for serum resistance. Gene Ther. 1998;5(3):380–387. doi: 10.1038/sj.gt.3300596. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Zhang Y, Anchordoquy TJ. The role of lipid charge density in the serum stability of cationic lipid/DNA complexes. Biochim Biophys Acta. 2004;1663(1–2):143–157. doi: 10.1016/j.bbamem.2004.03.004. Demonstrates that high cholesterol contents impart serum resistance to lipoplexes. [DOI] [PubMed] [Google Scholar]
- 6.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 7.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7(12):1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 8.Crook K, Stevenson BJ, Dubouchet M, Porteous DJ. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther. 1998;5(1):137–143. doi: 10.1038/sj.gt.3300554. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6(4):585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler JJ, Palmer L, Ossanlou M, et al. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6(2):271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 11.Wolff JA, Dowty ME, Jiao S, et al. Expression of naked plasmids by cultured myotubes and entry of plasmids into T tubules and caveolae of mammalian skeletal muscle. J Cell Sci. 1992;103 (Pt 4):1249–1259. doi: 10.1242/jcs.103.4.1249. [DOI] [PubMed] [Google Scholar]
- 12.Torchilin VP, Omelyanenko VG, Papisov MI, et al. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994;1195(1):11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 13.Torchilin VP, Shtilman MI, Trubetskoy VS, et al. Amphiphilic vinyl polymers effectively prolong liposome circulation time in vivo. Biochim Biophys Acta. 1994;1195(1):181–184. doi: 10.1016/0005-2736(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 14.Harvie P, Wong FM, Bally MB. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J Pharm Sci. 2000;89(5):652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Hyvonen Z, Ronkko S, Toppinen MR, Jaaskelainen I, Plotniece A, Urtti A. Dioleoyl phosphatidylethanolamine and PEG–lipid conjugates modify DNA delivery mediated by 1,4-dihydropyridine amphiphiles. J Control Release. 2004;99(1):177–190. doi: 10.1016/j.jconrel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Monck MA, Mori A, Lee D, et al. Stabilized plasmid-lipid particles: pharmacokinetics and plasmid delivery to distal tumors following intravenous injection. J Drug Target. 2000;7(6):439–452. doi: 10.3109/10611860009102218. [DOI] [PubMed] [Google Scholar]
- 17.Shi F, Wasungu L, Nomden A, et al. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. Biochem J. 2002;366(Pt 1):333–341. doi: 10.1042/BJ20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam P, Monck M, Lee D, et al. Stabilized plasmid-lipid particles for systemic gene therapy. Gene Ther. 2000;7(21):1867–1874. doi: 10.1038/sj.gt.3301308. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong TK, Girouard LG, Anchordoquy TJ. Effects of PEGylation on the preservation of cationic lipid/DNA complexes during freeze-thawing and lyophilization. J Pharm Sci. 2002;91(12):2549–2558. doi: 10.1002/jps.10255. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande MC, Davies MC, Garnett MC, et al. The effect of poly(ethylene glycol) molecular architecture on cellular interaction and uptake of DNA complexes. J Control Release. 2004;97(1):143–156. doi: 10.1016/j.jconrel.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126(1):77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Huang Z, MacKay JA, Grube S, Szoka FC., Jr Low-pH-sensitive poly(ethylene glycol) (PEG)-stabilized plasmid nanolipoparticles: effects of PEG chain length, lipid composition and assembly conditions on gene delivery. J Gene Med. 2005;7(1):67–79. doi: 10.1002/jgm.634. [DOI] [PubMed] [Google Scholar]
- 23.Shin J, Shum P, Thompson DH. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG-lipids. J Control Release. 2003;91(1–2):187–200. doi: 10.1016/s0168-3659(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 24.Webb MS, Saxon D, Wong FM, et al. Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): effects on the pharmacokinetics of liposomal vincristine. Biochim Biophys Acta. 1998;1372(2):272–282. doi: 10.1016/s0005-2736(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 25▪▪.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochim Biophys Acta. 2008;1778(10):2177–2181. doi: 10.1016/j.bbamem.2008.04.009. First demonstration that cholesterol nanodomains can be exploited for enhanced delivery. [DOI] [PubMed] [Google Scholar]
- 26.Zhao XB, Muthusamy N, Byrd JC, Lee RJ. Cholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomes. J Pharm Sci. 2007;96(9):2424–2435. doi: 10.1002/jps.20885. [DOI] [PubMed] [Google Scholar]
- 27▪.Zhang Y, Bradshaw-Pierce EL, Delille A, Gustafson DL, Anchordoquy TJ. In vivo comparative study of lipid/DNA complexes with different in vitro serum stability: effects on biodistribution and tumor accumulation. J Pharm Sci. 2008;97(1):237–250. doi: 10.1002/jps.21076. Demonstrates that lipoplexes formulated with high cholesterol accumulate in tumors to a greater extent than PEGylated formulations. [DOI] [PubMed] [Google Scholar]
- 28.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci USA. 1988;85(18):6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪▪.Dos Santos N, Allen C, Doppen AM, et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochim Biophys Acta. 2007;1768(6):1367–1377. doi: 10.1016/j.bbamem.2006.12.013. Studies the mechanism(s) by which PEG-lipids achieve prolonged circulation time in vivo. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone SA, Masin D, Mayer L, Bally MB. Surface-associated serum proteins inhibit the uptake of phosphatidylserine and poly(ethylene glycol) liposomes by mouse macrophages. Biochim Biophys Acta. 2001;1513(1):25–37. doi: 10.1016/s0005-2736(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Allen C, Dos Santos N, Gallagher R, et al. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol) Biosci Rep. 2002;22(2):225–250. doi: 10.1023/a:1020186505848. A ‘dysopsonization’ phenomenon where PEG actually promotes binding of certain proteins that then mask the vehicle is discussed. [DOI] [PubMed] [Google Scholar]
- 32.Mamot C, Drummond DC, Noble CO, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs. in vivo Cancer Res. 2005;65(24):11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 33.Choi JS, MacKay JA, Szoka FC., Jr Low-pH-sensitive PEG-stabilized plasmid-lipid nanoparticles: preparation and characterization. Bioconjug Chem. 2003;14(2):420–429. doi: 10.1021/bc025625w. [DOI] [PubMed] [Google Scholar]
- 34.Masson C, Garinot M, Mignet N, et al. pH-sensitive PEG lipids containing orthoester linkers: new potential tools for nonviral gene delivery. J Control Release. 2004;99(3):423–434. doi: 10.1016/j.jconrel.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Xu L, Anchordoquy TJ. Effect of cholesterol nanodomains on the targeting of lipid-based gene delivery in cultured cells. Mol Pharm. 2010;7(4):1311–1317. doi: 10.1021/mp100097b. The ability to locate ligands within the cholesterol nanodomain enhances transfection rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm. 2008;354(1–2):56–62. doi: 10.1016/j.ijpharm.2007.11.005. The detrimental effects of PEGylation on eliciting an immune response are demonstrated. [DOI] [PubMed] [Google Scholar]