Abstract
Overeating is believed to result when the appetitive motivation to consume palatable food exceeds an individual’s capacity for inhibitory control of eating. This hypothesis was supported in recent studies involving predominantly normal weight women, but has not been tested in obese populations. The current study tested the interaction between food reward sensitivity and inhibitory control in predicting palatable food intake among energy-replete overweight and obese women (N=62). Sensitivity to palatable food reward was measured with the Power of Food Scale. Inhibitory control was assessed with a computerized choice task that captures the tendency to discount large delayed rewards relative to smaller immediate rewards. Participants completed an eating in the absence of hunger protocol in which homeostatic energy needs were eliminated with a bland preload of plain oatmeal, followed by a bogus laboratory taste test of palatable and bland snacks. The interaction between food reward sensitivity and inhibitory control was a significant predictor of palatable food intake in regression analyses controlling for body mass index and the amount of preload consumed. Probing this interaction indicated that higher food reward sensitivity predicted greater palatable food intake at low levels of inhibitory control, but was not associated with intake at high levels of inhibitory control. As expected, no associations were found in a similar regression analysis predicting intake of bland foods. Findings support a neurobehavioral model of eating behavior in which sensitivity to palatable food reward drives overeating only when accompanied by insufficient inhibitory control. Strengthening inhibitory control could enhance weight management programs.
Keywords: Obesity, Reward, Eating behavior, Delay discounting, Inhibitory control
Introduction
Overeating often occurs in the absence of true physiological hunger, and the sensory properties of palatable food can promote the desire to eat independent of actual energy needs (1). The feeding system is highly responsive to signals of palatable food available in the environment, and palatable food cues can easily overwhelm the body’s relatively weak homeostatic satiety mechanisms (2). Individual differences exist in one’s degree of sensitivity to food reward, a term which encompasses both the sensory pleasure associated with eating and the degree to which food elicits the motivation to eat (3). Studies have linked food reward sensitivity to stronger food cravings (4), preferences for sweet and fatty foods (5), greater food intake in laboratory studies (6), and higher body weight in adults and children (5,7,8). The motivational component of food reward is largely mediated by the mesolimbic dopamine system, the brain’s “reward circuit” which also mediates the motivation to engage in sex, gambling, and substance use [see reviews by (3,9)]. Neurobiological and genetic factors which influence mesolimbic dopamine signaling are associated with obesity (6,10,11).
Though reward sensitivity has received significant attention as a risk factor for overeating and obesity, evidence also supports a role for inhibitory processes in the control of palatable food intake. We (12) and others (13,14) have posited that the capacity to inhibit food intake is an example of an executive function governed by the prefrontal cortex (PFC). A relative deficiency in inhibitory control is thought to increase vulnerability to overeating when exposed to palatable food cues, which has clear implications for weight control. This notion is supported by neuroimaging studies showing that stronger neural activation of the PFC following food intake is associated with lower body mass (15,16), decreased food craving (17), and successful weight loss (18). In another neuroimaging study, overweight adolescent girls performed more poorly on a task measuring the ability to inhibit behavioral responses to appetizing food cues relative to normal weight adolescents, and also showed decreased activation of prefrontal regions associated with inhibitory control (19).
Based on findings such as those noted above, overconsumption of palatable food would be most likely to occur in the context of greater food reward sensitivity and lower inhibitory control (12). Three recent studies have found direct support for this hypothesized interaction at the neurobiological and behavioral levels. Hare et al (20) found that non-obese dieters who made food choices based on their perceived health benefits rather than their taste showed greater PFC activation when making such choices, and neuroimaging revealed that the PFC modulated the activity of brain areas associated with reward processing. In a study of predominantly normal weight college students, Nederkoorn et al (21) found that food reward sensitivity was prospectively associated with weight gain over a 1-year period only for those who performed poorly on a behavioral response task measuring inhibitory control. A third study conducted by Rollins, Dearing, & Epstein (22) found that the willingness of non-obese women to work for access to palatable food (reflecting food reward sensitivity) was predictive of actual palatable food intake only among those who also demonstrated diminished inhibitory control on a delay discounting task. Though all of the above studies have involved non-obese populations, these findings support the notion that the impact of food reward sensitivity on overeating is modulated by the capacity for inhibitory control.
The current study tested the hypothesized interaction between food reward sensitivity and inhibitory control in predicting palatable food intake in the absence of physiologic hunger among overweight and obese women. Inhibitory control was measured using a delay discounting task similar to that used by Rollins et al. (22). Delay discounting refers to the tendency to prefer smaller immediate rewards (e.g., $60 right now) to larger delayed rewards (e.g., $100 next month). Delay discounting has been conceptualized as the result of “competition” between an “impulsive” neurobehavioral system that favors pursuit of immediate rewards, and a “reflective” executive system that inhibits impulsive behavior to maximize long-term gains (23,24). Neuroimaging studies implicate the mesolimbic dopamine system and PFC, respectively, as the primary components of these systems (25–28). Different conceptualizations of delay discounting exist in the research community. In our view, the evidence noted above is consistent with the premise that delay discounting is a facet of impulsivity that reflects inadequate inhibitory control of reward processes in decision making.
Though prior studies have observed greater delay discounting among obese women relative to lean women (29,30), the interaction of food reward sensitivity and inhibitory control on food intake has only been examined in non-obese individuals. It is important to test this hypothesis among overweight and obese individuals because there is a greater need to engage inhibitory control mechanisms when confronted with palatable food in this population. In the current study, overweight and obese women completed a self-report measure of food reward sensitivity, and consumed a preload of bland food in order to eliminate the homeostatic need for energy and thereby isolate the impact of reward on palatable food consumption (1). We hypothesized that food reward sensitivity would predict greater palatable food intake during a bogus laboratory taste test only for women who demonstrated low inhibitory control on the delay discounting task. As consumption of bland food is believed to be driven by homeostatic energy needs rather than hedonic reward processes, we hypothesized that intake of bland items would be unrelated to food reward sensitivity, inhibitory control, and their interaction.
Methods
Participants
Healthy overweight and obese women were recruited for a study of “dieting and decision making.” Study advertisements were posted as flyers on medical center campuses and electronically on community posting forums (i.e., craigslist.org). To be eligible, individuals had to be between ages 18 and 45 and have a body-mass index (BMI) between 25.0 and 39.9 kg/m2. Exclusion criteria, which were assessed in a telephone screening interview, included peri- or postmenopausal status; pregnancy or lactation in the past six months; adherence to any structured weight control diet within the past 30 days; allergies or sensitivities to common foods; unwillingness to consume study foods, including plain oatmeal; history of obesity surgery; clinically significant symptoms of depression, anxiety, or mania in the past 30 days; symptoms of eating pathology (e.g., underweight, binge eating, purging behavior) at any time in the past 5 years; and medical conditions or use of medications affecting appetite, metabolism, digestion, or cognitive functioning. Two individuals were excluded from participation upon arriving to the laboratory when it was found that their objectively measured height and weight did not place them in the eligible BMI range. The final sample comprised 62 overweight and obese women. Participants were compensated $50 (US) for their time. The Institutional Review Boards of Rush University Medical Center, University of Arizona, and Arizona State University approved study procedures.
Procedures
The procedures described in this report occurred during the initial laboratory visit within a larger study of diet adherence. After midnight on the morning of this visit, participants were required to fast from all food and drink except water, and abstain from caffeine, energy drinks, alcohol, non-prescription medication, and strenuous physical activity. They were also instructed to avoid nicotine within 1 hour of participation. Experimental sessions were scheduled to begin at 11:30 (±30 min) to minimize the effect of time of day on food intake and to reduce the duration of fasting needed to standardize energy intake prior to the session. Height and weight were measured in light clothing upon arrival to the laboratory. Participants were then queried to verify their compliance with the pre-session instructions listed above. Once compliance was confirmed, participants were offered a large bowl (approximately 690 g after preparation) of warm, plain oatmeal prepared with water and asked to eat slowly until they were “comfortably full.” This preload was designed to eliminate acute physiological hunger prior to the taste test while providing only a minimal degree of orosensory pleasure. All participants verbally confirmed feeling comfortably full at the time they discontinued eating. Following the oatmeal preload, participants completed self-report measures and then received instructions on how to complete the delay discounting task described below. Participants took a 3-minute break following the delay discounting task prior to proceeding to the laboratory taste test.
Laboratory taste test
The taste test was administered approximately 30–45 minutes after participants had begun consuming the oatmeal preload, or about 15–20 minutes after having reached “comfortable fullness”. We did not assess subjective hunger ratings prior to or during the taste test because drawing participants’ attention to their reduced hunger level would be expected to influence food intake during the taste test, which was specifically designed to assess the motivation to consume palatable food independent of hunger. Therefore, to account for individual differences in hunger, we relied on participants’ report of achieving satiety during the oatmeal preload and controlled for the amount of oatmeal consumed in statistical models. The laboratory taste test was introduced by the experimenter as a way to assess taste perception. Participants were asked to sample six snack foods (Table 1) and provide palatability ratings on a rating form. Four of the items offered were considered palatable (potato chips, salted peanuts, chocolate kisses, raisins) and two were considered “bland” (soup crackers, regular Cheerios). Snacks were removed from all packaging and served on separate plates. Drinking water was provided and participants were invited to request additional food (though none did). To encourage snack intake, participants were told that all uneaten food would be discarded. The experimenter left the room for 6 minutes while participants ate. Upon returning, the experimenter asked if the participant would like more time to complete the taste test, and left for an additional 6 minutes if participants responded in the affirmative (n=4). At no time were participants made aware that their snack intake was being measured.
Table 1.
Characteristics of study foods.
| Amount presented g/kcals | Energy density (kcals/g) | Palatability M (SD) | |
|---|---|---|---|
| Palatable items | |||
| Potato chips | 50.0/268.0 | 5.4 | 68.0 (23.5) |
| Salted peanuts | 150.0/910.5 | 6.1 | 56.4 (27.4) |
| Chocolate kisses | 90.0/439.2 | 4.9 | 78.5 (18.9) |
| Raisins | 100.0/325.0 | 3.3 | 60.3 (26.5) |
| Bland items | |||
| Soup crackers | 65.0/273.0 | 4.2 | 51.8 (22.5) |
| Regular Cheerios | 45.0/165.6 | 3.7 | 42.4 (21.7) |
Note: Food palatability was assessed on a numerical rating scale from 0–100. See Methods.
Measures
Anthropometrics
Height and weight were measured in light clothing using a balance beam scale with height rod. BMI was calculated as: weight(kg)/height2(m).
Delay Discounting
Delay discounting for monetary rewards was measured using a computerized choice task adapted from other sources (27). In a series of 161 choice trials, participants were asked to choose whether they would prefer to receive a fixed hypothetical reward of $100.00 at one of seven different delay intervals (1 day, 7 days, 30 days, 90 days, 180 days, 1 years, or 5 years), or a different amount of money available “right now.” Twenty-three immediate monetary rewards were offered at each delay interval: $0.10, $2.50, $5.00, $10.00, $15.00, $20.00, $25.00, $30.00, $35.00, $40.00, $45.00, $50.00, $55.00, $60.00, $65.00, $70.00, $75.00, $80.00, $85.00, $90.00, $95.00, $100.00, $105.00. Trials were administered in a randomized order with respect to both delay interval and the value of immediate reward offered.
“Indifference points,” the amount of money at which immediate rewards became preferred over the delayed reward, were computed for each subject at each delay interval. For example, a participant who chose to receive $70 or more right now rather than $100 after a 30-day delay, but preferred the delayed reward of $100 to receiving $65 or less right now, would have an indifference point of $67.50 at the 30-day delay interval. Fifty-six percent of indifference points were discrete in that participants always preferred the delayed reward below a certain value of immediate reward, and preferred all higher values of immediate reward to the delayed reward. Similar to prior studies (31), the remaining 44% of indifference points were not discrete, with preference for the delayed reward alternating across several values of immediate reward. In these instances, the indifference points were defined as the choice of a delayed reward over the two highest consecutive values of immediate reward, not necessarily by the lowest value immediate reward chosen (31).
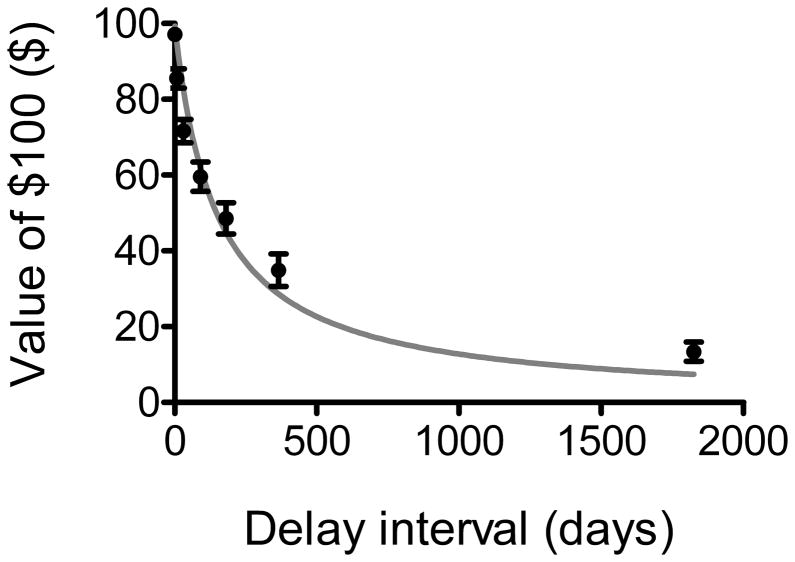
Participants’ indifference points were plotted at each delay interval, and the area under the curve (AUCDD) was calculated as a metric of delay discounting (32). AUCDD is an atheoretical metric of discounting that is normally distributed and commonly used in other studies [e.g., (22,33,34)]. AUCDD values have a range from 0 (greatest possible discounting) to 1 (no discounting). For descriptive purposes only, curve-fitting software (GraphPad Prism 5, Graphpad Software, Inc., LaJolla, CA, USA) was used to fit the hyperbolic discounting function V=A/(1+kD) to participants’ indifference points (Figure 1). In this function, V represents the reward value (i.e., the indifference point), A is the amount of the immediate award, D is the delay interval, and k is a constant which reflects the steepness at which indifference points (the value of delayed rewards) decrease in value with increasing temporal delay (35).
Figure 1.
Hyperbolic delay discounting function fit to the mean indifference points in the current sample. Bars represent standard errors of the means. This figure is shown for descriptive purposes only. All analyses utilized area under the curve (AUCDD) as a measure of delay discounting.
Food reward sensitivity
The Power of Food Scale (PFS) is a measure of individual differences in the appetitive drive to consume highly palatable food, independent of homeostatic hunger (36,37). The scale has 15 items that assess the influence of food on behavior and cognition when it is available but not present, present but not tasted, and tasted. Participants rate their agreement with statements about their responses to food on a 5-point scale from “I don’t agree at all” to “I strongly agree.” Representative items are, “When I’m around a fattening food I love, it’s hard to stop myself from at least tasting it,” and “I find myself thinking about food even when I’m not physically hungry.” A summary score was calculated as arithmetic mean of responses to all 15 items. Internal consistency was excellent in the current sample (Cronbach α=.92).
Palatability
As part of the taste test, participants were asked to indicate how much they “like the taste” of each food consumed on a numerical rating scale from 0 (Not at all) to 100 (Extremely).
Food Intake
Each portion of food was weighed (in grams) before consumption. The food remaining after the experimental session was weighed again, and the difference between the pre-session and post-session food weight was calculated and then converted from grams to kilocalories using nutrition information from food labels.
Data Analysis
Our primary analyses tested the hypothesis that food reward sensitivity would be predictive of food intake in the absence of hunger only when accompanied by deficient inhibitory control. Two separate linear regression models were used to predict intake of palatable and bland food items from PFS scores, AUCDD, and their interaction term while controlling for BMI and the amount of oatmeal preload consumed. All independent variables were centered at their mean. Significant interactions were probed in accordance with recommendations from Aiken and West (38). Briefly, following the identification of a significant interaction, the simple slopes of PFS on food intake were tested for significance at both 1 SD above and 1 SD below the mean of AUCDD. This required the formation of two additional models which include PFS scores, conditional values of AUCDD at either 1 SD above or 1 SD below the variable mean, the interaction of PFS and the conditional values of AUCDD, and the same control variables from the original model. The coefficients associated with PFS score in these models reflect the simple slopes of PFS on energy intake at 1 SD above and 1 SD below the mean of AUCDD. Eta-squared (η2), representing the percentage of total variance in the dependent variable explained by a predictor variable, is reported as a measure of effect size.
Results
Preliminary analyses
Sample characteristics are shown in Table 2. Participants were generally well educated and ethnically diverse, with 62.9% of the sample self-identifying as an ethnic minority group and over half possessing a 4-year college degree or higher. Delay discounting data were lost for one participant due to a computer error, and energy intake data for one participant were excluded due to experimenter error in administering the taste task (certain food items were not presented). Altogether, data from 60 out of 62 participants were included in final models.
Table 2.
Sample characteristics (N=62).
| M | SD | |
|---|---|---|
| Age (y) | 31.0 | 7.7 |
| BMI | 31.5 | 3.4 |
| AUCDD | 0.306 | 0.245 |
| k | 0.022 | 0.057 |
| Power of Food Scale (1–5 scale) | 2.5 | 0.9 |
| Food intake (kcals) | ||
| Oatmeal preload | 73.0 | 47.5 |
| Palatable items | 223.0 | 121.5 |
| Bland items | 26.1 | 28.4 |
| Total test meal intake | 249.0 | 125.3 |
|
| ||
| N | % | |
|
| ||
| Ethnicity | ||
| Asian | 3 | 4.8 |
| Black/African-American | 20 | 32.3 |
| Hispanic | 13 | 21.0 |
| Non-Hispanic, White | 23 | 37.1 |
| Other/Multi-ethnic | 3 | 4.8 |
| Education level | ||
| High school or equivalent | 2 | 3.2 |
| Some college | 16 | 25.8 |
| 2-year degree or technical degree | 11 | 17.7 |
| 4-year degree | 25 | 40.3 |
| Masters degree | 5 | 8.1 |
| Doctorate, legal, professional degree | 3 | 4.8 |
| Household income (USD) | ||
| $0–$14,999 | 8 | 12.9 |
| $15,000–$29,999 | 11 | 17.7 |
| $30,000–$44,999 | 14 | 22.6 |
| $45,000–$59,999 | 11 | 17.7 |
| $60,000–$74,999 | 6 | 9.7 |
| $75,000–$89,999 | 6 | 9.7 |
| $90,000 and above | 6 | 9.7 |
Note: BMI=body mass index; AUCDD=area under the delay discounting curve; k=constant from hyperbolic delay discounting function.
Pearson correlations tested the associations among key study variables (Table 3). Higher education level was associated with both higher PFS score (r(62)=.27, p=.03) and reduced discounting of delayed rewards (AUCDD: r(61)=.32, p=.01; k: r(61)=−.29, p=.02), but was not associated with intake of either palatable or bland foods. Including education level in statistical models did not alter the significance of our findings, so results from models excluding education level are reported for simplicity. Household income level was not associated with delay discounting, PFS scores, or food intake. Higher BMI was correlated with greater intake of palatable food (r(61)=.34, p<.01), but not with intake of bland food, PFS scores, or delay discounting.
Table 3.
Pearson correlations among key variables.
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|
| 1. BMI | −.17 | −.13 | .20 | −.04 | −.04 | .34** | −.01 |
| 2. PFS | - | .14 | −.07 | .27* | −.10 | .12 | −.04 |
| 3. AUCDD | - | −.38** | .32* | .00 | .01 | −.02 | |
| 4. k | - | −.29* | −.19 | −.18 | −.13 | ||
| 5. Education level | - | .07 | .04 | −.14 | |||
| 6. Household income | - | −.09 | −.22 | ||||
| 7. Palatable food intake | - | .02 | |||||
| 8. Bland food intake | - | ||||||
Note: BMI=body mass index; PFS=Power of Food Scale; AUCDD=area under the delay discounting curve; k=constant from hyperbolic delay discounting function.
p<.05
p<.01.
Participants consumed an average of 164.7 g (SD=109.7 g) of oatmeal preload, which corresponds to roughly 73.0 kcals (SD=47.5 kcals). Total energy intake during the laboratory taste test ranged from 40.7 kcals to 665.5 kcals (M=249.0, SD=125.3), with 89.7% of the total energy consumed coming from the four palatable items (M=223.0 kcals, SD=121.5 kcals). As shown in Table 1, palatability ratings for the four palatable items (M=65.8) were substantially higher than those for the two bland items (M=47.1, which supports the decision to analyze these foods separately. As would be expected, palatability ratings of foods collected during the taste test were positively correlated with intake of the respective food item (r’s between .22 and .66). However, none of the palatability ratings were associated with BMI, PFS, or AUCDD (all r’s <|.20|), so palatability ratings were not controlled for in subsequent analyses.
Primary analyses
Palatable food intake
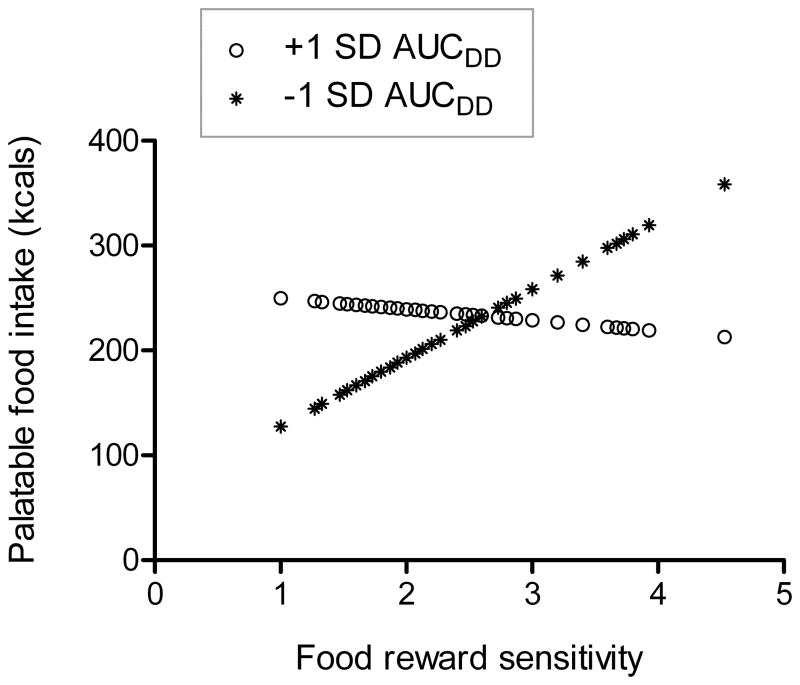
Results of regression models are summarized in Table 4. The interaction term between PFS and AUCDD emerged as a significant predictor in a model predicting palatable food intake while adjusting for BMI and oatmeal preload consumed (β=−.28, t(54)=−2.06, p=.04, η2=.04; Figure 2). The simple effects of PFS on palatable food intake at 1 SD above and below the mean of AUCDD were then tested. At 1 SD above the mean of AUCDD, which represents reduced discounting of delayed rewards and higher inhibitory control, PFS showed no association with palatable food intake (β=−.07, t(54)=−.44, p=.66, η2=.00). However, at 1 SD below the mean of AUCDD, which indicates steeper discounting of delayed rewards and reduced inhibitory control, PFS was positively associated with palatable food intake (β=.46, t(54)=2.58, p=.01, η2=.07). As mentioned previously, findings were unchanged when controlling for education level in these models.
Table 4.
Results of regression analyses predicting palatable food intake.
| β | t | df | P | η2 | |
|---|---|---|---|---|---|
| Model 1: Overall model | |||||
| BMI | .50 | 3.85 | 54 | <.001 | .16 |
| Oatmeal preload | .41 | 3.44 | 54 | .001 | .12 |
| PFS | .19 | 1.67 | 54 | .10 | .03 |
| AUCDD | .05 | .45 | 54 | .65 | .00 |
| PFS X AUCDD | −.28 | −2.06 | 54 | .04 | .04 |
| Model 2: Simple effect of PFS at 1 SD above mean of AUCDD (high inhibitory control) | |||||
| BMI | .50 | 3.85 | 54 | <.001 | .16 |
| Oatmeal preload | .41 | 3.44 | 54 | .001 | .12 |
| PFS | −.07 | −.44 | 54 | .66 | .00 |
| AUCDD (at 1SD above mean) | .05 | .45 | 54 | .65 | .00 |
| PFS X AUCDD (1SD above) | −.38 | −2.06 | 54 | .04 | .04 |
| Model 3: Simple effect of PFS at 1 SD below mean of AUCDD (low inhibitory control) | |||||
| BMI | .50 | 3.85 | 54 | <.001 | .16 |
| Oatmeal preload | .41 | 3.44 | 54 | .001 | .12 |
| PFS | .46 | 2.58 | 54 | .01 | .07 |
| AUCDD (at 1SD below mean) | .05 | .45 | 54 | .65 | .00 |
| PFS X AUCDD (1SD below) | −.38 | −2.06 | 54 | .04 | .04 |
Note: BMI=body mass index; AUCDD=area under the delay discounting curve. Model 1 shows the overall interaction between PFS and AUCDD predicting palatable food intake. Model 2 shows no effect of PFS (p=.66) at high levels of inhibitory control (1 SD above the mean of AUCDD). In model 3, higher PFS was associated with greater palatable food intake (p=.01) at low levels of inhibitory control (1 SD below the mean of AUCDD). Models 2 and 3 provide estimates of the effect of PFS when inhibitory control is fixed at specific values, so only the parameter estimates for PFS differ between models.
Figure 2.
Interaction between food reward sensitivity and inhibitory control. Predicted values for palatable food intake are plotted against food reward sensitivity at one standard deviation above and below the mean of AUCDD. Lower AUCDD reflects greater discounting of delayed rewards and lower inhibitory control. Food reward sensitivity was measured with the Power of Food Scale.
We also performed an exploratory analysis to determine whether the interactive effect of PFS and AUCDD on palatable food intake varied across the range of body mass in this sample. In a regression model containing all lower-order terms and the covariates from the previous analyses, the three-way interaction between PFS, AUCDD, and BMI was found to be a non-significant predictor of palatable food intake (t(50)=−1.14, p=.26). The current sample size provided very limited statistical power to detect even a large three-way interaction effect in this sample.
Bland food intake
It was expected that food reward sensitivity and inhibitory control would be predictive of intake of palatable foods, but not bland foods. To test this hypothesis, a model was formed predicting intake of bland foods which was structurally identical to the one formed for palatable food intake. None of the predictors in this model, including BMI and the amount of oatmeal preload consumed, were predictive of bland food intake (all p’s >.40, all η2’s≤.01).
Discussion
This study tested the hypothesis that palatable food intake among overweight and obese women is influenced by an interaction between food reward sensitivity and inhibitory control. Consistent with this hypothesis, it was found that greater food reward sensitivity was associated with increased palatable food intake only among those who demonstrated diminished inhibitory control on the delay discounting task. There was no association between food reward sensitivity and palatable food intake in the context of adequate inhibitory control. Importantly, food intake was assessed after participants had consumed a bland preload of plain oatmeal, so the observed findings can be presumed to be independent of homeostatic energy needs (1). Additionally, there was no evidence of an interaction or main effect of food reward or inhibitory control when examining intake of bland foods. This study lends further support for the potential involvement of inhibitory control of reward-driven eating in weight maintenance (12), and extends prior research that has supported this model in non-obese populations (20–22).
Inhibitory control may be relevant to several other behavioral processes relevant to obesity. For example, Nederkoorn et al. (39) found that physiological (homeostatic) hunger was associated with both greater snack intake in a laboratory taste test and greater purchasing of calories from snack foods in a virtual food shopping task, but only when coupled with ineffective inhibitory control. A similar interaction with inhibitory control would be expected with reward-driven, “hedonic hunger.” Inhibitory control of food reward may also predict weight gain. Among predominantly normal weight university students, greater food reward sensitivity was associated with more weight gain over one year, but only among those who showed diminished inhibitory control on a behavioral response inhibition task (21). No association was observed between inhibitory control of food reward and subsequent weight gain in a sample of overweight adolescent girls (N=29), but this study did not have adequate statistical power to detect medium or small effects (19).
Limitations
A general limitation of the delay discounting task is that it only reflects inhibitory control in relation to the rewards offered, which potentially limits its value as a general measure of inhibitory control. Like most prior studies of delay discounting in humans (40), the task used in this study assessed inhibitory control with respect to monetary rewards. Money can be exchanged for virtually any desired reward in modern society (with only a few exceptions). Therefore, in most populations, money is relatively uniform in terms of its reward value across individuals and delay discounting tasks involving monetary rewards probably provide a relatively general measure of inhibitory control across individuals (33). It is important to note that in the present study, potential influences on the reward value of money were either found to be unrelated to delay discounting (household income) or were controlled for in statistical models (education level). Accordingly, we interpret our findings as reflecting an interaction between food reward sensitivity and a relatively general capacity for inhibitory control in predicting palatable food intake. However, there is clearly a need to develop tasks which specifically measure inhibitory control in the context of food rewards. One such task, which required participants to inhibit behavioral responses (button presses) when presented with appetizing food cues, was recently described by Batterink et al. (19). Tasks assessing discounting of delayed food rewards have been developed (33,34), but the fact that such tasks can feature only one particular class of food reward at a time (e.g., “juice,” “candy,” “your favorite dish”) limits their ability to provide a general measure of inhibitory control with respect to all palatable food rewards.
An additional limitation associated with the delay discounting task used in this study was that it featured hypothetical, rather than actual, monetary rewards. Most delay discounting studies with humans utilize hypothetical rewards, and studies have shown that comparable estimates of delay discounting are obtained with actual and hypothetical rewards (40).
The measure of sensitivity to food reward used in this study, the Power of Food Scale, was recently developed and has been subjected to only a few validation studies (e.g., references 36 and 37). These studies have demonstrated acceptable convergence between the PFS and relevant measures of eating behavior, but associations between PFS and general measures of reward sensitivity have not yet been tested. Such tests are needed to establish that the PFS reflects an aspect of eating behavior associated with reward processing.
Another limitation of the current study is that our sample was restricted to women with a BMI between 25 and 39.9 kg/m2, which limits the generalization of findings to women with a BMI over 40 kg/m2 and men. Also, the food items offered during the taste test were limited in number and variety and may not represent the foods that are commonly overconsumed in naturalistic settings. Though there are also methodological drawbacks to studying intake of participants’ self-identified preferred foods, which can vary substantially in both palatability and energy density, it is important to replicate the current findings using naturalistic measures of food intake.
Future directions
The current findings suggest several interesting areas for future study. For example, multicomponent interventions which simultaneously reduce sensitivity to food reward and increase inhibitory control might be more effective than interventions targeting either of these factors alone. Similar proposals have been made regarding new treatments for drug addiction (24). Research is also needed to identify novel situational factors that produce transient vulnerability to overeating by reducing inhibitory control or increasing food reward. Similarly, future studies should investigate whether the effects of known “disinhibitors” on eating (e.g., stress) are mediated by changes in the balance between reward sensitivity and inhibitory control. Finally, it would be valuable to identify the neural mechanisms underlying the interaction between food reward and inhibitory control. This knowledge could provide additional insight into the interaction between reward and inhibition at the behavioral level and identify novel targets for pharmacological obesity treatments.
The results of the current study indicate that food reward sensitivity is associated with vulnerability to overconsuming palatable food only when coupled with ineffective inhibitory control. If findings are replicated, the role of inhibitory control of reward should be incorporated into existing models of vulnerability to weight gain, which have primarily emphasized individual differences in food reward processing and the abundance of highly palatable food in the environment.
Acknowledgments
This work was supported by National Cancer Institute grant R03CA139857 to Dr. Appelhans. The first author (BMA) has consulted for Merck.
Footnotes
All other authors report no potential conflicts of interest.
References
- 1.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiology & Behavior. 2007;91:432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Zheng H, Lenard NR, Shin AC, et al. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33 (Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge KC, Ho CY, Richard JM, et al. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Davis C, Patte K, Levitan R, et al. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Epstein LH, Temple JL, Neaderhiser BJ, et al. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 8.Temple JL, Legierski CM, Giacomelli AM, et al. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulton S. Appetite and reward. Front Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Stice E, Spoor S, Bohon C, et al. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 12.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity (Silver Spring, Md) 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- 14.van den Bos R, de Ridder D. Evolved to satisfy our immediate needs: self-control and the rewarding properties of food. Appetite. 2006;47:24–29. doi: 10.1016/j.appet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Le DS, Pannacciulli N, Chen K, et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006;84:725–731. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- 16.Le DS, Pannacciulli N, Chen K, et al. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small DM, Zatorre RJ, Dagher A, et al. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 18.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (London) 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 19.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 21.Nederkoorn C, Houben K, Hofmann W, et al. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29:389–393. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- 22.Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 24.Bickel WK, Miller ML, Yi R, et al. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90 (Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka SC, Doya K, Okada G, et al. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 26.McClure SM, Laibson DI, Loewenstein G, et al. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AR, Brown SM, Williamson DE, et al. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein LH, Salvy SJ, Carr KA, et al. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis C, Patte K, Curtis C, et al. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54:208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Weller RE, Cook EW, III, Avsar KB, et al. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 32.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estle SJ, Green L, Myerson J, et al. Discounting of monetary and directly consumable rewards. Psychol Sci. 2007;18:58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 34.Odum AL, Baumann AA, Rimington DD. Discounting of delayed hypothetical money and food: effects of amount. Behav Processes. 2006;73:278–284. doi: 10.1016/j.beproc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analysis of Behavior, Vol 5 The Effects of Delay and of Intervening Events on Reinforcement Value. Erlbaum; New Jersey: 1987. pp. 55–73. [Google Scholar]
- 36.Cappelleri JC, Bushmakin AG, Gerber RA, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes (Lond) 2009;33:913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- 37.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage Publications; Thousand Oaks, CA: 1991. [Google Scholar]
- 39.Nederkoorn C, Guerrieri R, Havermans RC, et al. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. Int J Obes (Lond) 2009;33:905–912. doi: 10.1038/ijo.2009.98. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]