Abstract
Background
Cigarette smoking is the leading preventable cause of death. Unfortunately, the majority of smokers who attempt to quit smoking relapse within weeks. Abnormal dorsal anterior cingulate cortex (dACC) function may contribute to tobacco smoking relapse vulnerability. Growing evidence suggests that glutamate neurotransmission is involved in mediating nicotine dependence. We hypothesized that prior to a cessation attempt, dACC glutamate levels would be lower in relapse vulnerable smokers.
Methods
Proton magnetic resonance spectra (MRS) were obtained from dACC and a control region, the parieto-occipital cortex (POC), using two-dimensional J-resolved MRS at 4 Tesla and analyzed using LCModel. Nine nicotine-dependent women were scanned prior to making a quit attempt. Subjects then were divided into two groups; those able to maintain subsequent abstinence aided by nicotine replacement therapy (NRT) and those who slipped while on NRT (smoked any part of a cigarette after attaining at least 24 hours of abstinence).
Results
Slip subjects exhibited significantly reduced dACC MRS glutamate (Glu/Cr) levels (p<0.03) compared to abstinent subjects. This effect was not observed in the POC control region.
Conclusions
Our preliminary findings suggest that dACC Glu levels as measured with MRS may help identify and/or be a biomarker for relapse vulnerable smokers. Future research following up on these findings may help clarify the role of dACC Glu in smoking dependence that may lead to new treatment strategies.
Keywords: dorsal anterior cingulate cortex, glutamate, relapse, smoking, spectroscopy, nicotine
1. Introduction
Tobacco-derived nicotine dependence accounts for nearly 450,000 yearly deaths in the US (DeVita, 2005). Despite the existence of pharmacotherapies that substantially improve smoking cessation rates when combined with behavioral therapy (Gonzales et al., 2006: Jorenby et al., 2006), most smokers who quit eventually relapse (Hughes et al., 2003). Relapse vulnerability and nicotine dependence are associated with disrupted functional connectivity between the dorsal anterior cingulate cortex (dACC) and brain regions implicated in reward and smoking behavior (Janes et al., 2010; Hong et al., 2009). Additionally, smokers exhibit increased dACC activation when resisting craving to smoking cues (Brody et al., 2007), which may reflect greater effort to exert cognitive control over craving (Kerns et al., 2004). Thus, a disconnect between the dACC and reward-related brain regions could reflect a dysfunctional cognitive control network, and when present could make it more difficult for vulnerable smokers to resist craving, leading to higher relapse vulnerability. Identifying neurobiological markers of dACC dysfunction may help lead to novel smoking cessation and relapse prevention treatments.
In this preliminary study, we hypothesized that relapse vulnerable smokers would have decreased glutamate (Glu) proton metabolite levels in the dACC prior to a cessation attempt. This hypothesis is based on previous work indicating that chronic substance abusers have reduced Glu levels in the ACC. For instance, ACC Glu levels, as measured with proton magnetic resonance spectroscopy (MRS), are decreased in chronic cocaine (Yang et al., 2009) and chronic opiate users (Yücel et al., 2007). Additionally, ACC Glu levels are decreased in individuals with attention deficit hyperactivity disorder (Perlov et al. 2007), a condition associated with cognitive control dysfunction. Moreover, glutamate has been implicated as playing a role in cognitive control and in coping with cognitive challenges (Cull-Candy et al., 2001). Collectively, these findings suggest a possible link between ACC Glu, cognitive control, and substance abuse. Given that our group previously reported reduced functional connectivity of the dACC in relapse-vulnerable smokers, and that the dACC mediates cognitive control (Carter et al., 1999; Botvinick et al., 2004), the present study focused on whether dACC Glu abnormalities may play a role in smoking relapse vulnerability. In our study, following pretreatment MRS measurements, all subjects attempted to quit smoking aided by nicotine replacement therapy (NRT). Glutamate levels, expressed as metabolite ratios over total creatine (Glu/Cr) then were compared between smokers who subsequently were able to maintain abstinence vs. those who could not. Relapse vulnerability was defined as a slip (smoking any part of a cigarette after attaining at least 24 hours of abstinence), which has been shown to predict future smoking relapse (Brandon et al., 1990; Shiffman et al., 1996).
2. Methods and Materials
2.1. Subjects
Smokers involved in a smoking cessation clinical trial at Massachusetts General Hospital (MGH; NCT00218465) were referred to this optional neuroimaging study at McLean Hospital. Not all neuroimaging subjects completed MRS components. Participants in the present study were those who volunteered to undergo MRS and who were able to maintain at least 24 hours of abstinence after their quit date as part of the MGH trial. These subjects were a small subset of smokers who also participated in our prior fMRI study, in which we showed reduced functional connectivity between the dACC and brain regions involved in smoking behavior and smoking cue reactivity (Janes et al., 2010). Subjects enrolled in the study met DSM-IV criteria for current nicotine dependence, smoked ≥10 cigarettes/day in the previous six months, and had expired air carbon monoxide >10 ppmv at screening. Smokers with current unstable medical illness, pregnancy, recent drug and alcohol use (QuickTox 11 Panel Drug Test Card, Branan Medical, Irvine, California; Alco-Sensory IV, Intoximeters, St. Louis, Missouri), major depressive disorder, alcohol use disorder (prior 6 months), current psychotropic drug use, or lifetime diagnosis of organic mental or psychotic disorders were excluded. Women were exclusively enrolled because the parent clinical trial involved an investigational medication not yet FDA approved for men. The MGH and McLean Hospital Institutional Review Boards approved this study. Subjects provided written informed consent and were compensated for participation.
2.2. Assessment procedure
Baseline (pre-quit) smoking behavior was characterized by recording tobacco smoking pack-years, average number of cigarettes smoked per day, measuring end-expiratory CO levels (Bedfont Micro IV Smokerlyzer, Bedfont Scientific, Kent, England), and by administering the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al., 1991; Table 1). After baseline assessments and MRS, subjects quit smoking and began the 8-week NRT smoking cessation phase of the clinical trial. Interventions involved nicotine patch (20 mg/day for 4 weeks, 14 mg/day for 2 weeks, 7 mg/day for 2 weeks), and 2-mg nicotine polacrilex gum or lozenge, up to 12 mg/day as needed, and weekly manualized individual behavioral interventions. Only subjects who quit smoking for at least 24 hours were included in this analysis. Subjects who smoked any cigarettes during NRT were classified as high risk for relapse (slip group) while those who remained abstinent were classified at low relapse risk (abstinence group). This classification was based on a Society for Research on Nicotine and Tobacco’s working group definition of a slip as smoking any amount following at least 24 hours of abstinence (Hughes et al., 2003).
Table 1. Demographic information.
Age, carbon monoxide levels, and number of cigarettes smoked between awakening and shortly before the baseline spectroscopy scan. FTND, Ham-D, average number of cigarettes smoked per day, and pack-years were assessed at screening before the MRS scan day. Days on NRT are the total number of days subjects were treated with NRT during their quit attempt.
| Group | Eventual Slip Subjects (n=4) |
Abstinence Subjects (n=4) |
|---|---|---|
| Age (years) | 47.8 ± 11.8 | 49.3 ± 14.5 |
| Carbon monoxide (ppmv) | 16.8 ± 10.8 | 16.3 ± 9.5 |
| Cigarettes smoked prior to MRS | 4.4 ± 2.2 | 2.8 ± 2.2 |
| FTND | 6.0 ± 0.7 | 3.3 ± 1.5 * |
| Ham-D | 2.8 ± 2.7 | 0.5 ± 0.6 |
| Cigarettes smoked per day | 19.9 ± 9.3 | 14.9 ± 3.7 |
| Pack-years | 31.3 ± 27.5 | 25.4 ± 18.1 |
| Days on NRT | 43.0 ± 17.6 | 41.5 ± 15.5 |
MRS, magnetic resonance spectroscopy; FTND, Fagerstrom Test for Nicotine Dependence; Ham-D, Hamilton Depression Rating Scale; NRT, nicotine replacement therapy.
Data are expressed as means ± SD.
p<0.01
Group (slip vs. abstinence) differences in demographics were assessed with 2-sided two-sample Student’s t tests while differences in baseline MRS metabolite levels were assessed with 1-sided two-sample Student’s t tests. Data were analyzed using GraphPad Prism (Prism Version 5.0b, GraphPad Software, San Diego, California) with a statistical significance threshold of p<0.05.
2.3. Imaging and Spectroscopy
Smoking was allowed until shortly before scanning. Subjects were scanned on a Varian Unity INOVA 4 Tesla whole-body MR system (Varian, Palo Alto, CA) using a volumetric head coil. The unsuppressed water signal was used to manually shim the global water signal. Subsequently, T1-weighted sagittal and axial structural images were obtained: echo time/repetition time (TE/TR)=6.2s/11.4ms, field-of-views=22×22×8 and 16 cm sagittal and axial, (respectively), in-plane matrix sizes=128×256×16 (sagittal) and 256×256×64 (axial).
These images guided voxel placements in dACC (33×23×22 mm) and parieto-occipital cortex (POC) (33×23×22 mm). Manual voxel shimming resulted in global water line widths of 9–12 Hz. Proton spectroscopy utilized a 2D-JPRESS approach collecting 24 TE-stepped spectra (30–260 ms, 10-ms increments), providing 100 Hz J-resolved bandwidth, sufficient to resolve Glu from its metabolic predecessor glutamine (Gln), as well as other metabolites (Jensen et al., 2009). Additional acquisition parameters were: TR=2 s, f1 bandwidth=50 Hz, spectral bandwidth=2 kHz, readout duration=1024 ms, NEX=16, total scan duration=13 min.
2.4. Spectral processing
MRS data analyses were undertaken using code written on-site and commercial fitting software (LCModel; Provencher, 1993). To quantify Glu, γ-Aminobutyric acid (GABA), Gln, N-acetylaspartate (NAA), creatine (Cr), and choline (Cho), free-induction decay series were zero-filled to 64 points, Gaussian-filtered to minimize residual ringing from NAA and Cr signals, and Fourier-transformed in the TE dimension. This produced 64 J-resolved spectra. Using GAMMA-simulated J-resolved basis sets, J-resolved spectral extractions were fit with LCModel templates (Jensen et al., 2009). Integrated areas under entire 2D surfaces for each metabolite were calculated by summing raw peak areas across all 64 J-resolved extractions, and were corrected for T2 relaxation. Metabolite ratios using total creatine (Cr, sum of creatine and phosphocreatine raw integrals) as the denominator are reported, since there were no group (slip vs. abstinence) Cr differences. Two POC spectra from subjects who later slipped were excluded due to low signal-to-noise and/or spectral resolution.
3. Results
3.1. Behavioral assessment and outcome data
Of nine smokers who underwent MRS, five slipped while on NRT. Slips occurred on average 19 (range: 3–49) days following an initial 24 hours of abstinence. Slip subjects had higher baseline FTND scores (t7 = 3.67, p<0.01; Table 1; scores ≥6 indicate high nicotine dependence), but did not differ on any other demographic variable.
3.2. MRS results
Figure 1 illustrates the main finding that slip subjects exhibited reduced baseline dACC Glu/Cr ratios (t7 = 2.36, p<0.03) relative to smokers who remained abstinent during NRT. A correlation analysis revealed no association between FTND scores and dACC Glu/Cr ratios (r = −0.21, p>0.58). Slip subjects also exhibited reduced baseline GABA/Cr (t7 = 2.89, p<0.01) and Cho/Cr ratios (t7 = 3.30, p<0.01). By contrast, no metabolites differed by group in POC.
Figure 1.
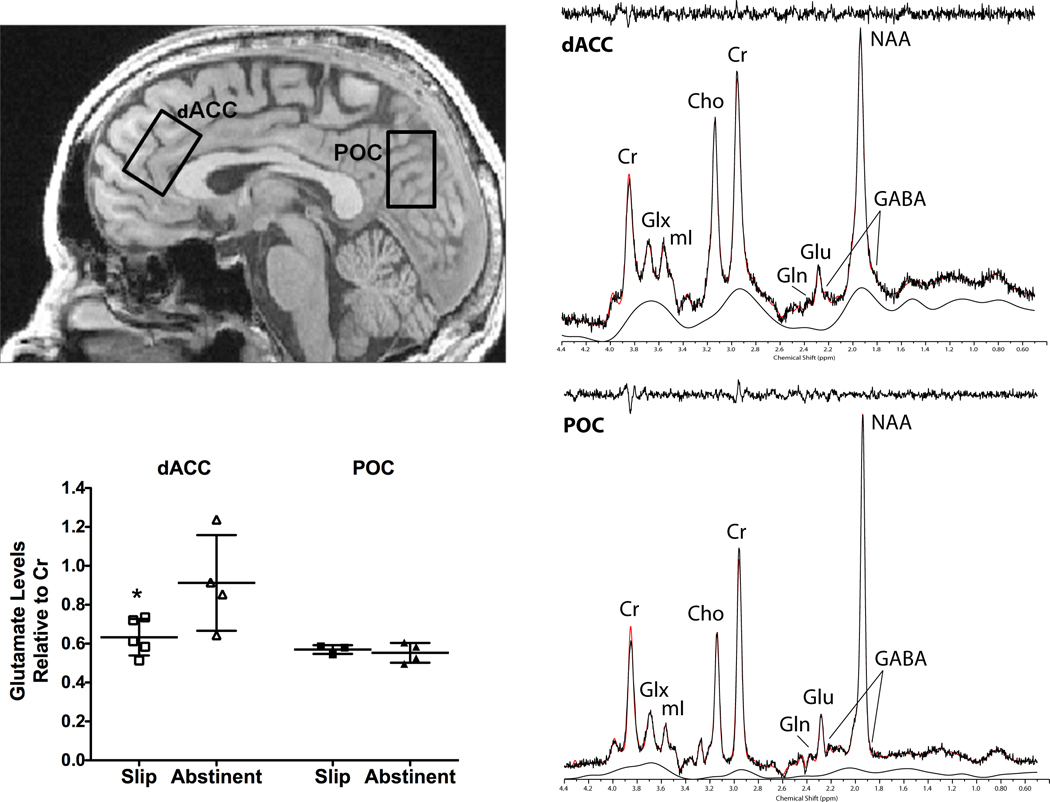
Sagittal (top, left panel) view of a representative T1-weighted image illustrating voxel placements in the dorsal anterior cingulate cortex (dACC) and parieto-occipital cortex (POC). The J=0.0Hz spectral extraction is shown for dACC (top, right panel) and POC (bottom, right panel) voxels. Both spectra are displayed with no filtering and LCModel fit with residual. Note: our results for metabolite measures are based on the LCModel serial fitting method, as described by Jensen and colleagues (2009), which fits all 64 J-resolved spectra over a bandwidth of 50Hz in f1. Highly coupled metabolites such as glutamine (Gln) and gamma-aminobutyric acid (GABA) are not readily apparent in the J=0.0Hz spectrum due to either suppression or overlap by dominant resonances such as creatine (Cr) and N-acetylaspartate (NAA). Nonetheless, we label the remnant peaks of Gln and GABA that are partially visible in the J=0.0Hz spectrum for clarity and completion. Glutamate (Glu)/Cr ratios in the dACC and POC voxels in slip and abstinent groups are also shown (bottom, left panel). Values are the means ± SD. * p<0.03 relative to abstinent group dACC Glu/Cr ratios.
Cho, choline; Glx, combined Glu-Gln; mI, myo-inositol.
4. Discussion
4.1. Overview of findings
We found lower dACC Glu/Cr ratios in smokers who would later slip following initial abstinence aided by NRT versus those who would remain abstinent. Thus, our present finding is consistent with our a priori hypothesis. Though slip subjects also exhibited reduced dACC GABA/Cr and Cho/Cr ratios, we did not have a priori hypotheses for other metabolite ratios, and these effects did not survive Bonferroni multiple comparisons corrections. Thus, they will not be discussed further. Slip subjects also reported higher levels of nicotine dependence as measured by the FTND.
4.2. Interpretation of findings
Our finding of reduced dACC Glu/Cr ratios in smokers who slipped relative to those who remained abstinent is consistent with prior MRS studies in people with substance use disorders. Reductions in ACC Glu have been reported in those with chronic cocaine (Yang et al., 2009) and chronic opiate (Yücel et al., 2007) use relative to healthy normal controls. Our preliminary finding of low ACC Glu/Cr ratios in smokers who slipped versus those who remained abstinent is consistent with the finding of low ACC Glu levels in people with different substance dependence disorders (Yücel et al., 2007; Yang et al., 2009). Although we did not include healthy nonsmoking controls in this study, historical data from our group (Licata et al., 2009) using a similar 4T J-resolved single voxel MRS technique documented ACC Glu/Cr ratios in healthy nonsmoking controls (0.87 ± 0.03, N=19) that are higher in magnitude than ratios we observed presently in our slip group (0.63 ± 0.09, N=5) but similar to ratios we found in our smokers who remained abstinent (0.91 ± 0.25, N=4). We intend to perform additional studies to compare healthy nonsmoking controls to smokers of different relapse vulnerabilities to see whether we can replicate the effect we observed in slip subjects.
In contrast to our present findings, a prior study did not observe ACC glutamate metabolite differences when comparing cohorts of current smokers, former smokers, and never-smokers (Gallinat and Schubert, 2007). The apparent discrepancy between our results and those of Gallinat and Schubert (2007) could be due to the fact that we studied a small, heavy smoking cohort composed only of women smokers and compared findings based on subsequent smoking cessation outcomes. However, methodological differences also could contribute to this apparent discrepancy; Gallinat and Schubert (2007) used a 3T scanner and the PRESS (point-resolved spectroscopy) pulse sequence, whereas we used a 4T scanner and the 2D-JPRESS pulse sequence. Our methods are optimized for measuring Glu as greater spectral resolution at higher magnetic field strengths enhances sensitivity to measure Glu, as does our use of the 2D-JPRESS sequence (Jensen et al., 2009; Henry et al., 2011). Thus, cohort and/or methodological differences may contribute to study differences.
It is important to note that the smokers in our study were drawn from a slightly larger cohort of subjects in which slip subjects were found to exhibit reduced functional connectivity between the dACC and brain regions involved in smoking behavior and smoking cue reactivity (Janes et al., 2010). We interpreted those functional connectivity findings to reflect reduced top-down cognitive control over reactivity to smoking-related cues (Janes et al., 2010), leading to enhanced relapse vulnerability (Garavan and Hester, 2007; Goldstein and Volkow, 2002). Collectively, our past and present work suggests that both functional and neurochemical changes in the dACC may enhance relapse vulnerability, possibly as a consequence of decreased cognitive control.
There were no differences between slip and abstinent groups with regard to the average numbers of cigarettes smoked per day or in numbers smoked on the scan day prior to the MRS scan, suggesting that it is unlikely that the dACC Glu/Cr ratio is moderated by group differences in these measures. Group differences in nicotine dependence severity may have contributed to increased slip vulnerability as well as to the differences in dACC Glu/Cr ratios between the groups. Smokers who slipped in the present study reported FTND scores that reflect greater severity of nicotine dependence relative to smokers who remained abstinent during the smoking cessation phase. A subsequent correlation analysis did not reveal significant associations between Glu/Cr ratios and FTND scores. Due to our small sample size, we cannot further clarify relationships between FTND scores and other variables in our present sample, but we aim to study relationships between dACC Glu and FTND scores in future studies.
4.3. Limitations and Future Directions
Given our small sample size that included only female smokers, these findings require replication. Further study in larger cohorts including male smokers and healthy controls may help to clarify how dACC Glu levels are related to dACC functional connectivity, FTND scores, and smoking cessation outcomes. Future studies should also control for menstrual cycle phase, which can influence smoking cue reactivity (Gray et al., 2010), as well as smoking cue-induced craving (Franklin et al., 2004) and cessation outcomes (Franklin et al., 2008; Mazure et al., 2010). In addition, with the MRS technique we used, we are unable to resolve whether dACC Glu metabolite differences in participants who slipped relative to those who maintained abstinence reflect differences in neurotransmission, metabolism, or both. This limitation in proton MRS studies could be addressed by using other MRS methods including carbon-13 spectroscopy, which can differentiate between neurotransmitter and bioenergetic pools of glutamate (Gruetter et al., 1998).
5. Conclusion
Our findings suggest that the dACC Glu/Cr ratio may be a neurobiological marker of glutamatergic dysfunction in relapse-vulnerable smokers. We believe our pilot results warrant additional studies to replicate and extend these findings in larger cohorts of smokers, including men, to more definitively establish how dACC Glu/Cr measurements relate to smoking histories, smoking dependence severity, functional connectivity, and treatment outcomes. Characterizing relationships between these measures may lead to novel smoking cessation therapies with potential to improve treatment outcomes.
Research Highlights.
-
*
Used MRS to assess smoker dACC Glu/Cr ratios based on smoking cessation outcome.
-
*
Lower dACC Glu/Cr in relapse-vulnerable smokers vs. abstinent smokers.
-
*
Control region POC Glu/Cr ratios did not differ between groups.
-
*
dACC Glu/Cr may be a neurobiological marker of smoking relapse vulnerability.
Acknowledgments
Author Disclosures
Role of Funding Source
This study was funded in part by National Institute of Health grants U01DA019378, R01DA022276, R01DA014764; R01DA09448; K02DA017324, T32DA015036, and S10RR13938; by funding from the Counter-Drug Technology Assessment Center (CTAC), an office within the Office of National Drug Control Policy (ONDCP) via Army Contracting Agency Contracts DABT63-99-C and DABK39-03-C-0075; and by research support from GlaxoSmithKline (GSK). The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsement should be inferred. Funding sponsors had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the research report; or in the decision to submit the manuscript for publication.
Abbreviations
- 2D
2-dimensional
- Cr
creatine
- Cho
choline
- dACC
dorsal anterior cingulate cortex
- FTND
Fagerstrom Test for Nicotine Dependence
- GABA
γ-Aminobutyric acid
- Gln
glutamine
- Glu
glutamate
- JPRESS
J-resolved spectroscopy
- MGH
Massachusetts General Hospital
- MRS
magnetic resonance spectroscopy
- NAA
N-acetylaspartate
- NEX
number of excitations
- NMDA
N-methyl-D-aspartate
- NRT
nicotine replacement therapy
- POC
parieto-occipital cortex
- PRESS
point-resolved spectroscopy
- TE
echo time
- TR
repetition time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Yasmin Mashhoon, Dr. Amy C. Janes, Dr. Perry F. Renshaw, Dr. Maurizio Fava, Dr. A. Eden Evins, and Dr. Marc J. Kaufman conceptualized the study. Dr. J. Eric Jensen processed the spectroscopy data and created images for publication. Dr. Mashhoon and Dr. Jensen analyzed the data. Dr. Andrew P. Prescot created the experimental protocol. Dr. Gladys Pachas and Dr. A. Eden Evins oversaw the clinical trial. Dr. Mashhoon drafted most of the manuscript and undertook consolidating edits from coauthors, and Dr. Kaufman and Dr. Janes critically reviewed and revised manuscript drafts. All authors edited and approved of the final manuscript.
Conflict of Interest
The following authors have no conflict disclosures: Dr. Y. Mashhoon, Dr. A.C. Janes, Dr. J.E. Jensen, Dr. A.P. Prescot, and Dr. G. Pachas. The following authors have conflict of interest disclosures: Dr. P.F. Renshaw, Dr. M. Fava, Dr. A.E. Evins, and Dr. M.J. Kaufman received research support from GSK. Dr. Renshaw and Dr. Fava have also consulted for GSK, and Dr. Fava has been involved in speaking/publishing on behalf of GSK.
References
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. (Regul Ed) 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addict. Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev. Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- DeVita VT. The framework convention on tobacco control. Nat. Clin. Pract. Oncol. 2005;2:177. doi: 10.1038/ncponc0133. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O’Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob. Res. 2004;6:171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J. Womens Health. 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–67. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol. Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupriopion and placebo for smoking cessation: a randomized controlled trial. J.A.M.A. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe SD, Upadhyaya HP. Menstrual cycle and cue reactivity in women smokers. Nicotine Tob. Res. 2010;12:174–178. doi: 10.1093/ntr/ntp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Kim S, Ugurbil K. Localized in vivo 13C-NMR of glutamate metabolism in the human brain: initial results at 4 tesla. Dev. Neurosci. 1998;20:380–388. doi: 10.1159/000017334. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. J. Magn. Reson. 2011;208:210–218. doi: 10.1016/j.jmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch. Gen. Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob. Res. 2003;5:13–25. [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BD, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, Renshaw PF. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. N.M.R. Biomed. 2009;22:762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupriopion for smoking cessation: a randomized controlled trial. J.A.M.A. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Mazure CM, Toll B, Mckee S, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2010.07.024. doi:10.1016/j.drugalcdep.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Hesslinger B, Buechert M, Ahrendts J, Feige B, Bubl E, Hennig J, Ebert D, Tebartz van Elst L. Reduced cingulate glutamate/glutamine-to-creatine ratios in adult patients with attention deficit/hyperactivity disorder -- a magnet resonance spectroscopy study. J. Psychiatr. Res. 2007;41:934–941. doi: 10.1016/j.jpsychires.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J. Consult. Clin. Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi Z-X, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009;174:171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, Roffel K, Clarke K, Wood SJ, Forman SD, Pantelis C. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol. Psychiatry. 2007;12:691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]


