Abstract
Objective
We evaluated the current evidence on the association between migraine including aura status and cervical artery dissection.
Methods
We performed a systematic review and meta-analysis of studies investigating the association between migraine or migraine subtypes (e.g. migraine with aura) and cervical artery dissection published until October 2010.
Results
We identified five case-control studies investigating the association between migraine and cervical artery dissection. In pooled analysis, migraine doubled the risk of cervical artery dissection (pooled odds ratio [OR]=2.06, 95% CI 1.33–3.19). All studies allowed evaluation of migraine aura status. While the effect estimate for migraine without aura (pooled OR=1.94, 95% CI 1.21–3.10) was similar to overall migraine, the association was weaker for migraine with aura (pooled OR=1.50, 95% CI 0.76–2.96) However, there is no evidence that aura status significantly modifies the association between migraine and cervical artery dissection (meta-regression on aura status p=0.58). The risk does not appear to differ between women and men; however, only few studies presented gender-specific data. Heterogeneity among studies was low to moderate.
Conclusion
In this meta-analysis migraine is associated with a two-fold increased risk of cervical artery dissection. This risk does not appear to significantly differ by migraine aura status or gender.
Keywords: migraine, migraine aura, cervical artery dissection, carotid artery dissection, vertebral artery dissection, meta-analysis
Introduction
Migraine is a common disorder affecting approximately 18% of the female population and 6% of the male population (1). Clinically it presents with severe headache attacks and vegetative symptoms. About one-third of migraineurs experience transient neurological symptoms mostly involving the visual system prior to or during the migraine attack, known as migraine aura (2).
Migraine physiology is incompletely understood. While the condition is mainly considered to be a functional disorder of the brain, there are clear links to the vascular system. For example, endothelial dysfunction and hypercoagulability (3) as well as altered vascular reactivity (4) are among the findings in patients with migraine. In addition, migraine, especially migraine with aura, has been shown to increase the risk of ischemic stroke (5). All these associations are particularly strong among younger people and women.
The mechanisms behind the increased risk of ischemic stroke among patients with migraine are not fully understood. One plausible link may be cervical artery dissection (CAD),(6) since CAD is one of the leading causes of ischemic stroke in the young (7). The association between migraine and CAD has been suggested by case series (8, 9) and also in some case-control studies (10–13), while another study did not find such an association (14). Further, it is unclear if the risk differs by migraine aura status. The association between migraine and many co-morbidities, for example cardiovascular events and depression, is particularly strong among those suffering from migraine with aura (15). However, in studies looking at the association between migraine and CAD, the number of patients with migraine with aura was small obviating firm conclusions (11–14).
The aim of this study was to summarize the current evidence on the association of migraine and migraine aura status with CAD by systematically reviewing the literature and performing a meta-analysis.
Methods
To perform this meta-analysis, we used the guidelines published by the MOOSE group for the design, performance, and reporting of meta-analyses of observational studies (16).
Data Sources and searches
Two investigators (P.M.R., M.S.) independently searched MEDLINE, EMBASE, and Science Citation Index from their inceptions through October 2010 using the terms “headache”, “migraine disorders” or “migraine” combined with the terms “vertebral artery dissection”, “carotid artery dissection”, “cervical artery dissection”, “artery dissection” or “carotid artery injury”. The “explode” feature was used where applicable and no language restrictions were applied. We also manually searched the reference lists of all primary and review articles.
Study selection
A priori, we established the following inclusion criteria. First, the studies must have a case-control or cohort design. Second, the studies must investigate patients with cervical artery dissection and control subjects without dissection. Third, migraine must be diagnosed according to the criteria established by the International Headache Society (IHS) (17, 18). Fourth, in their analyses the authors must use a multivariable model or matching procedure that controls for potential confounding. Fifth, the study must provide effect estimates with 95% confidence intervals or enough data to calculate these. Finally, if studies had overlapping cases and/or controls, the largest study with extractable data meeting all the other inclusion criteria was included.
To determine which studies to include, two investigators (P.M.R., M.S.) screened the title and abstracts of all studies identified in the literature search and by consensus excluded all studies that did not meet any of the pre-specified criteria. The same investigators then reviewed the full articles of the remaining studies and excluded any studies not meeting our inclusion criteria.
Data Extraction
Two investigators (P.M.R., M.S.) independently extracted data and entered them into a customized database. All disagreements were resolved by consensus. The extracted data included the authors and title of the study, country the study was performed in, year of publication, study design, source population, study size, age and gender distribution of participants, method of diagnosing CAD, criteria used for migraine diagnosis, migraine status including aura, number of migraineurs among patients with dissections and controls, and effect estimates and 95% confidence intervals.
Statistical Analysis
We included all studies regardless of their gender and age distributions. We performed an overall analysis of the association between migraine and CAD. We also performed stratified analyses by migraine aura status (migraine with aura and migraine without aura) and by gender.
For each study we weighted the log of the odds ratio (OR) by the inverse of its variance to obtain pooled relative risk estimates. For studies that did not present ORs and 95% confidence intervals (CI) (11, 14, 19), we calculated these based on the numbers of migraineurs and non-migraineurs among patients with and without CAD. We decided to run random effect models, since they include assumptions about potential differences between studies as opposed to fixed effects models. We performed the DerSimonin and Laird Q test for heterogeneity. Additionally, we also calculated the I2 statistic (20). To visually examine the impact of individual studies on the overall homogeneity of the test statistic, we constructed Galbraith plots (21). Meta-regression was used to statistically evaluate the extent of heterogeneity due to migraine aura status and reported method of diagnosing CAD. Finally, to test for small study effects, e.g. publication bias, we used statistical methods described by Begg and Mazumdar (22) and Egger (23).
A two-tailed P value<0.05 was considered statistically significant and all analyses were carried out using SAS v.9.1 (SAS Institute Inc., Cary, NC, USA) and STATA v.10.1 (Stata, College Station, TX, USA).
Results
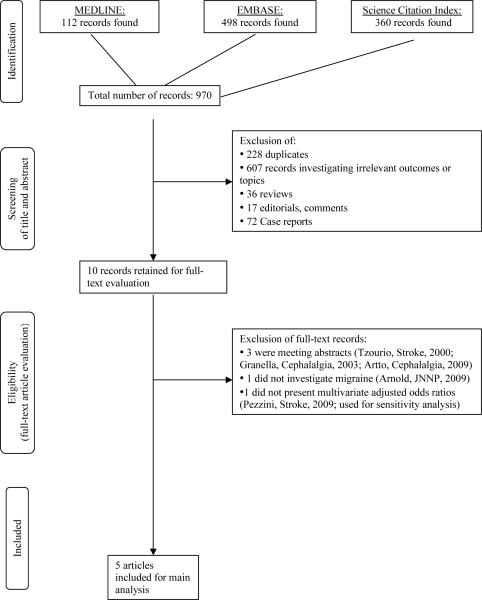
Figure 1 summarizes how studies were selected for inclusion in this meta-analysis. The electronic search identified 970 references. After reviewing the titles and abstracts and excluding those not meeting our inclusion criteria, we were left with 10 references. Of these, after evaluating the full references, three were excluded because they were conference abstracts and later published as full papers (24–26), and one was excluded because it did not investigate migraine (27). One additional paper was excluded from the main analysis because the authors did not present adjusted odds ratios and patients with CAD were not matched to controls (19); however, we used it for sensitivity analysis. No additional articles were identified when we manually searched the reference lists of all included articles leaving a total of five articles for inclusion in this meta-analysis (10–14).
Figure 1.
Process of selecting studies
Study Characteristics and individual study results
The characteristics of all the studies that were included in this review are summarized in Table 1. All of the studies were case-control studies and all studies investigated the association of any migraine with CAD (10–14). In addition, all studies either presented or allowed us to calculate ORs for migraine with aura and migraine without aura (10–14). Only two studies presented results stratified by gender (10, 11). Only one study specified the temporal relationship between CAD diagnosis and migraine diagnosis (10). The results from each of the included studies are summarized in Table 2.
Table 1.
Characteristics of case-control studies investigating the association between migraine and cervical artery dissection
| Author, Year | Country | Source population | # of cases (women)/# of controls (women) | Mean age [years] | Gender | CAD diagnosis | Migraine diagnosis/time from diagnosis of CAD | Migraine status investigated |
|---|---|---|---|---|---|---|---|---|
| D'Anglejan-Chatillon 1989 (11) | France | cases: clinic-based controls: spouses and age- and sex-matched friends | 50 (25)/100 (50) | cases: 42.1±8.6; controls: 43.6±9.1 | mixed, women, men | angiography | IHS 1; direct interview/NS | Any migraine, MA, MO |
|
| ||||||||
| Tzourio 2002 (13) | France | cases: clinic-based controls: clinic-based (patients with 'cerebral ischemic events' from same hospital without CAD; matched by sex and age ±5yrs) | 47 (21)/52 (22) | cases: 44.8 (SD 7.4); controls: 45.8 (SD 8.5) | mixed | duplex and MRI+MRA and/or conventional angiography | IHS 1; direct interview/NS | Any migraine, MA, MO |
|
| ||||||||
| Akova-Öztürk 2003 (14) | Germany | cases: clinic-based controls: family members, friend, and clinic-based (matched by gender and age ±1yr) | 148 (60)/148 (60) | cases: 42.6±10.7; controls: 42.7±10.8 | mixed | methods not specified | IHS 1; questionnaire based/NS | Any migraine, MA, MO |
|
| ||||||||
| Pezzini 2005 (12) | Italy | cases: clinic-based controls: clinic-based (stroke patients from same hospital without CAD; matched by sex and age) | 72 (30)/72 (30) | cases: 43.6±11.1; controls: 42.9±11.1 | mixed | duplex+MRI and MRA and/or conventional angiography | IHS 1; direct interview/NS | Any migraine, MA, MO |
| cases: clinic-based controls: hospital staff (matched by age and sex)† | 72 (30)/72 (30) | cases: 43.6±11.1; controls: 43.0±10.8 | mixed | duplex+MRI and MRA and/or conventional angiography | IHS 1; direct interview/NS | Any migraine, MA, MO | ||
|
| ||||||||
| Pezzini 2007 (19)* | Italy | cases: clinic-based controls: clinic-based (hospital staff with no known history of vascular disease aged ≤ 45 years) | 106 (54)/187 (88) | cases: 42.9±10.2; controls: 36.4±7.5 | mixed | duplex+MRI and MRA and/or conventional angiography | IHS 2; direct interview/NS | Any migraine, MA, MO |
|
| ||||||||
| Artto 2010 (10) | Finland | cases: clinic-based controls: from population registry; age- and sex matched | 313 (105)/313 (105) | cases: 46.1 (range: 15–78); controls: 45.8 (range: 21–74) | mixed, women, men | methods not specified | IHS 2; direct interview/average of 3.5 years | Any migraine, MA, MO |
CAD: cervical artery dissection; IHS: International Headache Society; IHS 1: diagnostic criteria according to the IHS from 1988; IHS 2: diagnostic criteria according to the IHS from 2004; MA: migraine with aura; MO: migraine without aura; MRI: magnetic resonance imaging; MRA: magnetic resonance angiography; NS: not specified.
Overlapping population with Pezzini et al. 2005(12), but larger. Adjusted odds ratios for this study are not available. Study only used for sensitivity analysis.
results for this control group are not used for the pooled analysis.
Table 2.
Association between migraine and cervical artery dissection in individual studies
| CAD | Controls | ||||
|---|---|---|---|---|---|
| Study | Gender | Migraine status | Migraineurs/total (%) | Migraineurs/total (%) | OR (95% CI) |
| D'Anglejan-Chatillon 1989 (11) | women and men | no migraine | 30/50 (60) | 76/100 (76) | Reference |
| any migraine | 20/50 (40) | 24/100 (24) | 2.11 (1.02–4.38) | ||
| MA | 4/50 (8) | 3/100 (3) | 3.38 (0.71–16.00) | ||
| MO | 16/50 (32) | 21/100 (21) | 1.93 (0.89–4.19) | ||
|
|
|||||
| women | any migraine | 12/25 (48) | 18/50 (36) | 1.64 (0.62–4.35) | |
|
|
|||||
| men | any migraine | 8/25 (32) | 6/50 (12) | 3.45 (1.04–11.43) | |
|
| |||||
| Tzourio 2002 (13) | women and men | no migraine | 24/47 (51.1) | 41/52 (78.8) | Reference |
| any migraine | 23/47 (48.9) | 11/52 (21.2) | 3.6 (1.5–8.6) | ||
| MA | 6/47 (12.8) | 4/52 (7.7) | 1.7 (0.4–6.5) | ||
| MO | 17/47 (36.2) | 7/52 (13.5) | 3.2 (1.2–9.0) | ||
|
| |||||
| Akova-Öztürk 2003 (14) | women and men | no migraine | 127/148 (85.8) | 125/148 (84.5) | Reference |
| any migraine | 21/148 (14.2) | 23/148 (15.5) | 0.90 (0.47–1.71) | ||
| MA | 5/148 (3.4) | 6/148 (4.1) | 0.82 (0.24–2.76) | ||
| MO | 16/148 (10.8) | 17/148 (11.5) | 0.93 (0.45–1.92) | ||
|
| |||||
| Pezzini 2005 (12) | women and men | no migraine | 29/72 (40.3) | 50/72 (69.4) | Reference |
| any migraine | 43/72 (59.7) | 22/72 (30.6) | 3.14 (1.41–7.01) | ||
| MA | 6/72 (8.3) | 9/72 (12.5) | 0.48 (0.13–1.75) | ||
| MO | 41/72 (56.9) | 18/72 (25) | 3.91 (1.71–8.90) | ||
|
|
|||||
| no migraine | 29/72 (40.3) | 59/72 (81.9) | Reference | ||
| any migraine | 43/72 (59.7) | 13/72 (18.1) | 7.41 (3.11–17.64) | ||
| MA | 6/72 (8.3) | 6/72 (8.3) | 0.97 (0.25–3.77) | ||
| MO | 41/72 (56.9) | 9/72 (12.5) | 9.84 (3.85–25.16) | ||
|
| |||||
| Pezzini 2007 (19)* | women and men | no migraine | 49/106 (46.2) | 153/187 (81.2) | Reference |
| any migraine | 57/106 (53.8) | 34/187 (18.2) | 5.24 (3.07–8.92) | ||
| MA | 13/106 (12.3) | 9/187 (4.8) | 4.51 (1.82–11.19) | ||
| MO | 44/106 (41.5) | 25/187 (13.4) | 5.50 (3.06–9.88) | ||
|
| |||||
| Artto 2010 (10) | women and men | no migraine | 199/313 (64) | 242/313 (77) | Reference |
| any migraine | 114/313 (36) | 71/313 (23) | 2.15 (1.48–3.14) | ||
| MA | 71/313 (23) | 39/313 (12) | 2.41 (1.53–3.80) | ||
| MO | 43/313 (14) | 32/313 (10) | 1.64 (0.98–2.76) | ||
|
|
|||||
| women | no migraine | 48/105 (46) | 68/105 (65) | Reference | |
| any migraine | 57/105 (54) | 37/105 (35) | 2.30 (1.28–4.13) | ||
| MA | 37/105 (35) | 19/105 (18) | 2.79 (1.40–5.59) | ||
| MO | 20/105 (19) | 18/105 (17) | 1.60 (0.74–3.49) | ||
|
|
|||||
| men | no migraine | 151/208 (73) | 174/208 (84) | Reference | |
| any migraine | 57/208 (27) | 34/208 (16) | 2.02 (1.23–3.31) | ||
| MA | 34/208 (16) | 20/208 (10) | 2.21 (1.19–4.11) | ||
| MO | 23/208 (11) | 14/208 (7) | 1.75 (0.84–3.65) | ||
CAD: cervical artery dissection; MA: migraine with aura; MO: migraine without aura; OR: odds ratio; CI; confidence interval.
Overlapping population with Pezzini et al. 2005(12), but larger. Adjusted odds ratios for this study are not available. Study only used for sensitivity analysis.
The study by D'Anglejan-Chatillon et al. enrolled 25 men and 25 women with extracranial internal carotid and vertebral artery dissection unrelated to obvious neck trauma (11). The control group consisted of the 50 spouses of the patients and 50 friends of the same age and sex as the patients. The authors found a significant difference in the proportion of patients who experience migraine compared to the controls. We calculated ORs based on the data provided in the manuscript.
Tzourio et al. performed a hospital-based case-control study in two neurology departments in France (13). A total of 47 patients with CAD were consecutively recruited as were 52 control patients who were hospitalized in the same centers during the same time period for an ischemic stroke or a TIA unrelated to CAD and were comparable to case subjects on sex and age (±5 years). They found that patients with any migraine or migraine without aura were at significantly increased risk of CAD. Patients with migraine with aura also appeared to have an increase in their risk, but this increase was not statistically significant.
Akova-Öztürk performed a case-control study at a University hospital in Germany (14). They recruited 148 patients with CAD and matched them by age and gender to individuals free of CAD who were family members, friends, and clinic staff. Neither the provided ORs for any migraine nor our calculated ORs for migraine subtypes suggested an association with CAD.
Pezzini et al. also performed a hospital-based case-control study in which they enrolled 72 consecutively admitted patients with spontaneous CAD (12). Two separate control groups were selected. One consisted of age- and sex-matched patients with ischemic stroke unrelated to CAD consecutively admitted during the same time period as the cases. The second control group was age- and sex-matched hospital staff with no known history of vascular disease. When comparing the cases to either control group, a significant increase in risk of CAD was seen for those with any migraine and migraine without aura, but a non-significant decrease in risk was seen for those who experience migraine with aura. For our analysis we included the results comparing patients with spontaneous CAD to patients with ischemic stroke unrelated to CAD for the following reasons: First, this approach resembles the study design by Tzourio et al. (13). Second, stroke patients without CAD may have a higher prevalence of migraine than healthy hospital staff; hence, estimates for the migraine-CAD association are more conservative. Third, stroke patients with and without CAD are more likely to originate from the same catchment area around the hospitals than patients and hospital staff.
The same group presented data on a larger number of cases and controls as part of another analysis (19) which allowed us to calculate the crude association between migraine and CAD. However, since we could not determine ORs adjusted for potential confounders, we only used this study for sensitivity analysis.
Using a Finnish database for CAD patients, Artto et al. enrolled 313 patients for whom information on migraine status could be obtained (10). Control subjects were recruited randomly from the Finnish Population Register Center and were frequency-matched to the cases on age (5-year intervals) and sex. Among CAD patients, the average follow-up time before the interview to establish migraine diagnosis was 3.5 years. The authors observed a statistically significant increase in the risk of CAD for those who experience migraine with aura. The increase in risk among those with migraine without aura was not statistically significant.
Results from the pooled analysis
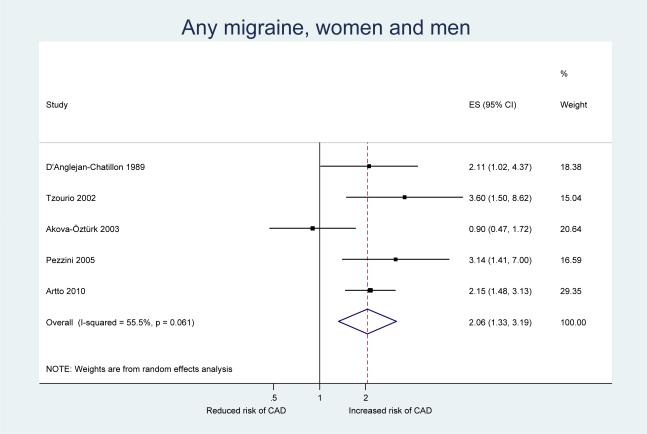
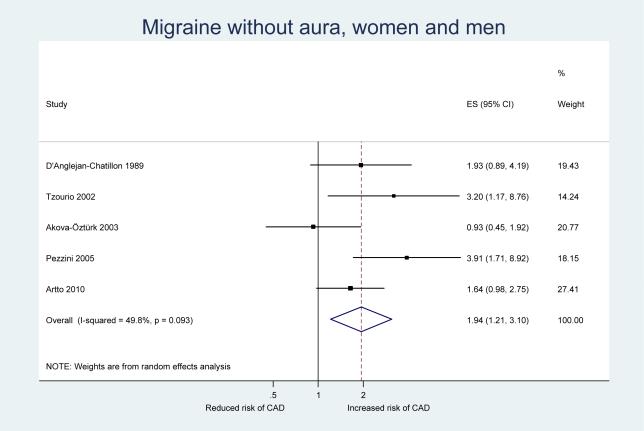
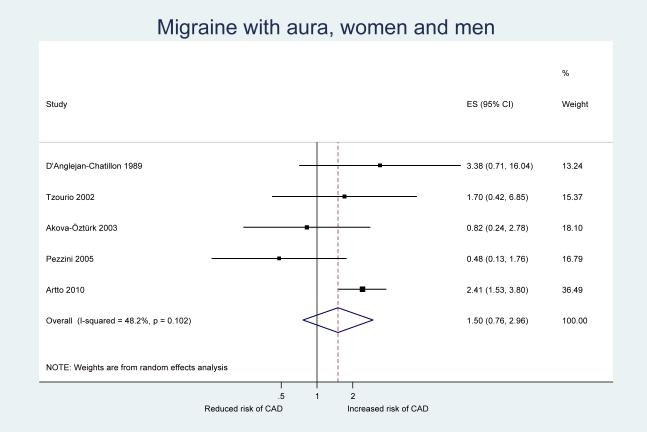
Table 3 shows the results from our pooled analysis along with measures of heterogeneity and small study effects. After pooling the results from our five included studies, we observed a statistically significant increase in the risk of CAD among participants who experience any migraine (pooled OR=2.06, 95% CI 1.33–3.19) (Figure 2). When we stratified our analysis by migraine aura status, a significant increase in the risk of CAD was only apparent for migraine without aura (pooled OR=1.94, 95% CI 1.21–3.10) (Figure 3) While the pooled results also suggested an increased risk for CAD among migraineurs with aura, this was not significant (pooled OR=1.50, 95% CI 0.76–2.96) (Figure 4). However, meta-regression did not identify aura status as a significant source of heterogeneity (p=0.58); hence, there is no indication that aura status significantly modifies the association between migraine and CAD.
Table 3.
Association between migraine and CAD from random effects model, heterogeneity, and small study effects
| Migraine status | No. of studies | Pooled effect estimates | Heterogeneity | Small study effects p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | Q | df | p-value | I2 in % | Begg test | Egger's test | ||
| Any migraine | |||||||||
| All studies | 5 (10–14) | 2.06 (1.33–3.19) | 0.001 | 9.0 | 4 | 0.06 | 55.5 | 0.14 | 0.81 |
| Women | 2 (10, 11) | 2.10 (1.27–3.47) | 0.004 | 0.3 | 1 | 0.56 | 0.0 | 0.32 | --- |
| Men | 2 (10, 11) | 2.18 (1.38–3.45) | 0.001 | 0.7 | 1 | 0.42 | 0.0 | 0.32 | --- |
|
| |||||||||
| Migraine with aura | |||||||||
| All studies | 5 (10–14) | 1.50 (0.76–2.96) | 0.24 | 7.7 | 4 | 0.10 | 48.2 | 1.00 | 0.31 |
| Women | 1 (10) | 2.79 (1.40–5.59) | --- | --- | --- | --- | --- | --- | --- |
| Men | 1 (10) | 2.21 (1.19–4.11) | --- | --- | --- | --- | --- | --- | --- |
|
| |||||||||
| Migraine without aura | |||||||||
| All studies | 5 (10–14) | 1.94 (1.21–3.10) | 0.006 | 8.0 | 4 | 0.09 | 49.8 | 0.14 | 0.40 |
| Women | 1 (10) | 1.60 (0.74–3.49) | --- | --- | --- | --- | --- | --- | --- |
| Men | 1 (10) | 1.75 (0.84–3.65) | --- | --- | --- | --- | --- | --- | --- |
OR: odds ratio; df: degrees of freedom.
Figure 2.
Odds ratios for cervical artery dissection among patients with any migraine from the individual studies and from the pooled analysis
Figure 3.
Odds ratios for cervical artery dissection among patients with migraine without aura from the individual studies and from the pooled analysis
Figure 4.
Odds ratios for cervical artery dissection among patients with migraine with aura from individual studies and from the pooled analysis
Gender stratified data are available from only two studies and the pooled analyses do not suggest a different effect for the association between any migraine and CAD by gender (women: pooled OR=2.10, 95% CI 1.27–3.47; men: pooled OR=2.18, 95% CI 1.38–3.45).
Heterogeneity among the included studies for all analyses was low to moderate and there was no evidence for small study effects in formal investigations with Begg and Egger's tests (all p-values > 0.14). Meta-regression showed that type of CAD diagnosis (duplex+MRI and MRA and/or conventional angiography versus no details given) was not a significant source of heterogeneity in any of our analyses (P-values > 0.24)
Sensitivity Analyses
For our first sensitivity analysis, we included the larger study by Pezzini et al. from 2007 (19), which did not allow us to determine adjusted ORs, and excluded the smaller study from 2005 (12). We observed results similar to our main analysis, the pooled effect estimates being somewhat higher (any migraine: pooled OR=2.38, 95% CI 1.34–4.21; migraine with aura: pooled OR=2.37, 95% CI 1.46–3.84; migraine without aura: pooled OR=2.19, 95% CI 1.16–4.13). These results further support that the migraine-CAD association does not differ according to migraine aura status.
We further used Galbraith plots to identify individual studies that may be potential sources of heterogeneity. We excluded any study that fell outside the margin set by two standard deviations of the z-score and re-ran our analysis. For the association between any migraine and CAD, the study by Akova-Öztürk (14) was identified and exclusion of this study yielded higher pooled effect estimates than our main analysis (pooled OR=2.39, 95% CI 1.78–3.91). With regard to the association of migraine with aura and CAD, the study by Pezzini et al. (12) was identified as a potential source of heterogeneity. Excluding this study gave results similar to the main analysis (pooled OR=2.12, 95% CI 1.40–3.20). For the association between migraine without aura and CAD, Galbraith plots did not suggest any of the studies as sources of heterogeneity.
Discussion
This meta-analysis shows that any migraine increases the risk of CAD by two-fold. There is no strong evidence that migraine aura status significantly modifies this association. Results do not appear to differ between women and men; however, the number of studies with gender stratified data was small.
Most (8–13), but not all (14) available studies have suggested that migraine increases the risk for CAD. Furthermore, the study results were inconsistent with regard to potential modifying effects of migraine aura status or gender (Table 2). In particular the association between migraine with aura and CAD could not be reliably assessed due to the low number of migraineurs with aura in individual studies. Some of the inconsistencies in the results may derive from differences among control groups (Table 1). Population-based controls, matched to CAD patients by age and gender, may be considered the most appropriate control group (6). In contrast for example, stroke patients without CAD may have a higher prevalence of migraine than healthy hospital staff or population-based controls and may also differ with respect to other vascular risk factors. This may result in an underestimation of the migraine-CAD association. Furthermore, hospital staff may differ with respect to their health status from CAD patients and may not be representative of the catchment area from which patients originate. This may result in an overestimation of the migraine-CAD association. Differences in results in the study by Pezzini et al. (12) for the two control groups illustrate these issues. However, differences in the control groups do not introduce high overall heterogeneity (Table 3).
Our pooled results firmly suggest that the risk for CAD is doubled for any migraine, a finding supported by low to medium heterogeneity among the studies (Table 3) and robustness in sensitivity analyses. The associations were driven by migraine without aura, the largest subgroup in all but one study (10). The results for migraine with aura also show an increased risk of CAD. Although this was not statistically significant in the main analysis, meta-regression did not identify aura status as a significant source of heterogeneity and sensitivity analyses do indicate a statistically significant association with the same order of magnitude as seen for migraine without aura.
The exact mechanisms behind the association between migraine and CAD are unclear, but vessel pathologies are plausible linking factors. First, endothelial dysfunction, which may in part be genetically determined, for example by the insertion/deletion polymorphism in the angiotension-converting enzyme gene, has been reported in migraineurs (3) and may account for the altered systemic vascular reactivity among migraine patients.(4) Such vessel wall pathologies are also among the most important risk factors for CAD. For example, an increased aortic root diameter and increased relative diameter change of the common carotid artery during the cardiac cycle increase the odds of CAD by 14- and 10-fold respectively (6). Second, biological evidence is provided by studies suggesting that migraine and CAD share common genetic susceptibility factors such as the 677C>T variant in the gene coding for the methylenetetrahydrofolate reductase (19, 28, 29). Third, the activity of serum elastase, a metalloendopeptidase involved in the extracellular matrix degradation, is increased among migraineurs (30) providing one physiological explanation for the increased risk of CAD we observed.
The association between migraine and CAD may be one explanation for the increased risk of cerebral ischemic events especially among young migraineurs. A recent meta-analysis has firmly established a link between migraine and cardiovascular events especially ischemic stroke (5), an association that appears to be independent of classical cardiovascular risk factors (31) and particularly strong among people without most cardiovascular risk factors (32). This association may extend to sub-clinical brain lesions. Magnetic resonance imaging studies have reported an increased risk for sub-clinical brain lesions in areas supplied by the posterior (33, 34) and anterior (35) circulation, mostly for migraine with aura (34, 35).
The following limitations should be considered. First, two included studies did not specify the methods used to diagnose CAD in their participants (10, 14), leaving the potential for misclassification. However, meta-regression did not identify this as a source of heterogeneity. Second, the clinical spectrum of migraine is wide and it is possible that some participants may have been misdiagnosed. However, all studies used standardized methods for migraine diagnosis strictly adhering to the criteria established by the IHS (17, 36). Third, we only used data that were extractable from the papers and did not contact authors for additional data. All studies allowed us to determine measures of association for any migraine as well as migraine subtypes and CAD. Only two studies presented gender-stratified results (10, 11); hence, our pooled analysis leaves some remaining uncertainties. However, there is no a priori reason to believe that the biological link between migraine and CAD differs between women and men and results from the available studies do not suggest a differential effect by gender (Tables 3 and 4). Finally, the available studies do not definitively establish the direction of association. Most studies did not specify the temporal relationship between CAD diagnosis and migraine diagnosis and one study reported an average follow-up time of 3.5 years after CAD diagnosis before the interview to establish migraine diagnosis (10). Headache and neck pain with various characteristics may be the initial and only signs of CAD in about 8% of patients (37, 38). Among those, some patients may have migraine-like headaches; however, by three months after CAD patients are typically pain free (37, 38). Given this small prevalence of CAD patients with migraine-like headaches and since diagnosis of migraine according to IHS criteria requires multiple attacks separated by pain-free episodes (36), it is unlikely that a migraine-like headache will be misdiagnosed as an overt migraine.
In summary, the increased risk for CAD among migraineurs appears to be well-supported by available evidence. This association does not appear to differ by migraine aura status or gender.
Acknowledgments
Full Disclosures for the last 5 years P. Rist is supported by a T-32 training grant from the National Institutes of Health (NIH). Dr. Diener has received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Addex Pharma, Allergan, Almirall, AstraZeneca, Bayer Vital, Berlin Chemie, Coherex, CoLucid, Böhringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Grünenthal, Janssen-Cilag, Lilly, La Roche, 3M Medica, Medtronic, Minster, MSD, Novartis, Johnson & Johnson, Pierre Fabre, Pfizer, Schaper and Brümmer, SanofiAventis, Weber & Weber. Financial support for research projects was provided by Allergan, Almirall, AstraZeneca, Bayer, GSK, Janssen-Cilag, Pfizer. Headache research at the Department of Neurology in Essen is supported by the German Research Council (DFG), the German Ministry of Education and Research (BMBF) and the European Union. He has no ownership interest and does not own stocks of any pharmaceutical company.
Dr. Kurth has received investigator-initiated research funding from the National Institutes of Health, McNeil Consumer & Specialty Pharmaceuticals, Merck, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety and World Health Information Science Consultants, LLC, and he has received honoraria from the American Academy of Neurology, Genzyme, Merck, and Pfizer for educational lectures.
Dr. Schürks has received investigator-initiated research grants from the German Research Foundation and the Migraine Research Foundation. He has received honoraria from L.E.K. Consulting for telephone surveys and from the American Academy of Neurology for educational material.
Footnotes
Funding and Support There was no specific funding for this study.
References
- 1.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321–34. doi: 10.1016/j.ncl.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Silberstein SD. Migraine. Lancet. 2004;363:381–91. doi: 10.1016/S0140-6736(04)15440-8. [DOI] [PubMed] [Google Scholar]
- 3.Tietjen GE. Migraine as a systemic disorder. Neurology. 2007;68:1555–6. doi: 10.1212/01.wnl.0000265415.18382.fb. [DOI] [PubMed] [Google Scholar]
- 4.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68:1563–70. doi: 10.1212/01.wnl.0000260964.28393.ed. [DOI] [PubMed] [Google Scholar]
- 5.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systemic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005;36:1575–80. doi: 10.1161/01.STR.0000169919.73219.30. [DOI] [PubMed] [Google Scholar]
- 7.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195–203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 8.Metso TM, Metso AJ, Salonen O, Haapaniemi E, Putaala J, Artto V, et al. Adult cervicocerebral artery dissection: a single-center study of 301 Finnish patients. Eur J Neurol. 2009;16:656–61. doi: 10.1111/j.1468-1331.2009.02535.x. [DOI] [PubMed] [Google Scholar]
- 9.Pieri A, Spitz M, Valiente RA, Avelar WM, Silva GS, Massaro AR. Disseccao espontanea das arterias carotidas e vertebrais em uma populacao multietnica. Arquivos de Neuro-Psiquiatria. 2007;65:1050–5. doi: 10.1590/s0004-282x2007000600029. [DOI] [PubMed] [Google Scholar]
- 10.Artto V, Metso TM, Metso AJ, Putaala J, Haapaniemi E, Wessman M, et al. Migraine with aura is a risk factor for cervical artery dissection: a case-control study. Cerebrovasc Dis. 2010;30:36–40. doi: 10.1159/000313608. [DOI] [PubMed] [Google Scholar]
- 11.D'Anglejan-Chatillon J, Ribeiro V, Mas JL, Youl BD, Bousser MG. Migraine--a risk factor for dissection of cervical arteries. Headache. 1989;29:560–1. doi: 10.1111/j.1526-4610.1989.hed2909560.x. [DOI] [PubMed] [Google Scholar]
- 12.Pezzini A, Granella F, Grassi M, Bertolino C, Del Zotto E, Immovilli P, et al. History of migraine and the risk of spontaneous cervical artery dissection. Cephalalgia. 2005;25:575–80. doi: 10.1111/j.1468-2982.2005.00919.x. [DOI] [PubMed] [Google Scholar]
- 13.Tzourio C, Benslamia L, Guillon B, Aidi S, Bertrand M, Berthet K, Bousser MG. Migraine and the risk of cervical artery dissection: a case-control study. Neurology. 2002;59:435–7. doi: 10.1212/wnl.59.3.435. [DOI] [PubMed] [Google Scholar]
- 14.Akova-Öztürk E. Migräne – Risikofaktor für Sinusvenenthrombosen und Dissektionen? Shaker Verlag; Aachen: 2003. [Google Scholar]
- 15.Schürks M, Buse DC, Wang S-J. Comorbidities of headache disorders. In: Martelletti P, Steiner TJ, editors. Handbook of Headache - Practical Management. Springer-Verlag; Heidelberg: 2010. in press. [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- 18.Olesen J, Steiner TJ. The International classification of headache disorders, 2nd edn (ICDH-II) J Neurol Neurosurg Psychiatry. 2004;75:808–11. doi: 10.1136/jnnp.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzini A, Grassi M, Del Zotto E, Giossi A, Monastero R, Dalla Volta G, et al. Migraine mediates the influence of C677T MTHFR genotypes on ischemic stroke risk with a stroke-subtype effect. Stroke. 2007;38:3145–51. doi: 10.1161/STROKEAHA.107.491506. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–94. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artto V, Metso T, Metso A, Putaala J, Haapaniemi E, Farkkila M, et al. Migraine with aura is a risk factor for cervical artery dissection: a case control study. Cephalalgia. 2009;29:114. doi: 10.1159/000313608. [DOI] [PubMed] [Google Scholar]
- 25.Granella F, Pezzini A, Zanferrari C, Del Zotto E, Bertolino C, Bazzoli E. Migraine without aura is a major risk factor for cervical artery dissection. A case-control study. Cephalalgia. 2003;23:571. [Google Scholar]
- 26.Tzourio C, Benslamia L, Guillon B, Aidi S, Lucas C, Berthet K, Bousser M. Migraine and the risk of cervical artery dissection. A prospective case-control study. Stroke. 2000;31:197. doi: 10.1212/wnl.59.3.435. [DOI] [PubMed] [Google Scholar]
- 27.Arnold M, Pannier B, Chabriat H, Nedeltchev K, Stapf C, Buffon F, et al. Vascular risk factors and morphometric data in cervical artery dissection: a case-control study. J Neurol Neurosurg Psychiatry. 2009;80:232–4. doi: 10.1136/jnnp.2008.151324. [DOI] [PubMed] [Google Scholar]
- 28.Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40:e459–66. doi: 10.1161/STROKEAHA.108.534669. [DOI] [PubMed] [Google Scholar]
- 29.Schürks M, Rist PM, Kurth T. MTHFR 677C>T and ACE D/I polymorphisms in migraine: a systematic review and meta-analysis. Headache. 2010;50:588–99. doi: 10.1111/j.1526-4610.2009.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzourio C, El Amrani M, Robert L, Alperovitch A. Serum elastase activity is elevated in migraine. Ann Neurol. 2000;47:648–51. [PubMed] [Google Scholar]
- 31.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–91. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 32.Kurth T, Schürks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. doi: 10.1136/bmj.a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–34. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 34.Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–70. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurth T, Mohamed S, Maillard P, Zhu YC, Chabriat H, Mazoyer B, et al. Headache, migraine, and structural brain lesions and function: the population-based EVA MRI study. BMJ. 2010 doi: 10.1136/bmj.c7357. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 37.Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77:1021–4. doi: 10.1136/jnnp.2006.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biousse V, D'Anglejan-Chatillon J, Massiou H, Bousser MG. Head pain in non-traumatic carotid artery dissection: a series of 65 patients. Cephalalgia. 1994;14:33–6. doi: 10.1046/j.1468-2982.1994.1401033.x. [DOI] [PubMed] [Google Scholar]
Top 5 references
- 1.D'Anglejan-Chatillon J, Ribeiro V, Mas JL, Youl BD, Bousser MG. Migraine--a risk factor for dissection of cervical arteries. Headache. 1989;29:560–1. doi: 10.1111/j.1526-4610.1989.hed2909560.x. [DOI] [PubMed] [Google Scholar]; [mg.bousser@lrb.aphp.fr]
- 2.Tzourio C, Benslamia L, Guillon B, Aidi S, Bertrand M, Berthet K, Bousser MG. Migraine and the risk of cervical artery dissection: a case-control study. Neurology. 2002;59:435–7. doi: 10.1212/wnl.59.3.435. [DOI] [PubMed] [Google Scholar]; [mg.bousser@lrb.aphp.fr]
- 3.Pezzini A, Granella F, Grassi M, Bertolino C, Del Zotto E, Immovilli P, et al. History of migraine and the risk of spontaneous cervical artery dissection. Cephalalgia. 2005;25:575–80. doi: 10.1111/j.1468-2982.2005.00919.x. [DOI] [PubMed] [Google Scholar]; [ale_pezzini@hotmail.com]
- 4.Artto V, Metso TM, Metso AJ, Putaala J, Haapaniemi E, Wessman M, et al. Migraine with aura is a risk factor for cervical artery dissection: a case-control study. Cerebrovasc Dis. 2010;30:36–40. doi: 10.1159/000313608. [DOI] [PubMed] [Google Scholar]; [turgut.tatlisumak@hus.fi]
- 5.Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005;36:1575–80. doi: 10.1161/01.STR.0000169919.73219.30. [DOI] [PubMed] [Google Scholar]; [Haldemanmd@aol.com]